Abstract
Dyskeratosis congenita (DC) is a bone marrow failure disorder characterized by shortened telomeres, defective stem cell maintenance, and highly heterogeneous phenotypes affecting predominantly tissues that require high rates of turnover. Here we present a mutant zebrafish line with decreased expression of nop10, one of the known H/ACA RNP complex genes with mutations linked to DC. We demonstrate that this nop10 loss results in 18S rRNA processing defects and collapse of the small ribosomal subunit, coupled to stabilization of the p53 tumor suppressor protein through small ribosomal proteins binding to Mdm2. These mutants also display a hematopoietic stem cell deficiency that is reversible on loss of p53 function. However, we detect no changes in telomere length in nop10 mutants. Our data support a model of DC whereupon in early development mutations involved in the H/ACA complex contribute to bone marrow failure through p53 deregulation and loss of initial stem cell numbers while their role in telomere maintenance does not contribute to DC until later in life.
Introduction
Dyskeratosis congenita (DC) is a congenital disease that in one comprehensive population-based study was found to represent up to 5% of all bone marrow failure disorders.1 Patients with this disease typically present in the first decade of life with a classic triad of phenotypes, including nail dystrophy, oral leukopathies, and hyperpigmentation of the skin.2 More than 90% of all DC patients will experience cytopenia before the age of 20.3 Other phenotypes reminiscent of aging may develop later in life, including premature graying of the hair, alopecia, taurodant teeth, and osteoporosis.4-6 Although the majority of DC patients die from bone marrow failure, an increased risk of malignancy also contributes to DC mortality.7
The genotypes of DC vary as widely as the phenotypes and encompass genes that are, with one exception, involved in the H/ACA RNP complex and/or telomere maintenance. Dyskerin (DKC1) was the first H/ACA complex gene linked to DC, the X-linked form of which has been implicated in the more severe cases of DC.8 DKC1 mutations have also been linked to Hoyeraal-Hreidarsson syndrome,9 which is now considered to be an extreme form of DC with an earlier onset and a shorter life expectancy. Mutations in 2 other H/ACA RNP complex genes, NHP25 and NOP10,10 have also been linked to DC patients. Two subunits of the telomerase complex, telomerase reverse transcriptase (TERT) and the telomerase RNA component (TERC), together function in the de novo synthesis of telomere ends,11 and mutations in each component have been described in DC patients, although in general the phenotypes of these patients are relatively milder than those linked to DKC1 mutations.12-14 Other mutations in genes coding for proteins that either protect telomere ends or assist in the trafficking of telomerase to Cajal bodies, TINF2 and TCAB1, respectively, have also been identified.15,16 One protein of unknown function, C16orf57, has also recently been linked to DC, and these are the only patients that do not display shortened telomere lengths.17 Importantly, although most of the genotypes of DC identified to date have been single amino acid substitutions, it has recently been shown that a decrease in expression of DKC1, in lieu of any gene mutations, is also linked to DC.18
NOP10 is a short, 64-amino acid protein that shares approximately 60% homology with its yeast analog, Nop10p, originally identified by affinity purification of Gar1p (also part of the H/ACA complex).19 The H/ACA complex, in addition to its role in telomere maintenance, also functions in guiding the pseudo-uridinylation of rRNAs, ribosome biogenesis, splicing of small nuclear RNAs, and select microRNA processing.20-22 Loss of mature 18S rRNA results from dkc1 hypomorphic mutations in mice,23 and the loss of snoRNA components required for normal 18S rRNA processing is evident in yeast cells lacking Nop10p.19 The homozygous R34W mutation in human NOP10 was identified in a large consanguineous family with a history of classic DC.10 This mutation was shown to result in shorter telomeres in DC-affected family members, although shortened telomeres were also identified in healthy heterozygous carriers of R34W.10 The homozygous R34W mutation was also shown to result in decreased levels of TERC in DC patients, and knockdown of NOP10 in a transient system was able to successfully recapitulate the lowered levels of TERC observed in DC patient cells.10 Taken together with the recent DKC1 patient data,18 these results suggest that decreasing the expression of NOP10 in a model system is an efficient method for studying the mechanism of DC.
Although there is broad consensus that DC results from stem cell renewal failure, and incontrovertible evidence that telomeres play an important role, there remains a considerable amount of debate over the mechanisms. One area of contention is over the contribution of ribosome biogenesis defects in DC. An example of this is hypomorphic dkc1 mice that do not exhibit telomere shortening until the fourth generation, whereas significant rRNA defects are apparent in the first generation.23 However, other studies have been unable to detect differences in rRNA processing in immortalized DC patient cell lines.24 Alternately, it has been recently postulated that the failure of IRES-mediated translation of certain mRNAs, including that of the p53 tumor suppressor, occurs when cells are subject to dkc1 knockdown.25-27 In contrast, other studies have demonstrated stabilization of p53 in mouse hepatocyte cells induced to delete dkc1.28
The p53 tumor suppressor is the most studied protein in biology today because the p53 gene is mutated in the majority of human cancers and p53 protein is regarded as one of the most important defenses in protecting cells against damage. p53 is typically kept at low levels in normal cells through a physical interaction with the E3 ubiquitin ligase Mdm2, which targets p53 for proteasomal degradation.29 Stabilization of p53 occurs under a myriad of cellular stresses, including but not limited to DNA damage, oncogene presence, hypoxia, and rRNA synthesis inhibition.30 DNA damage stabilizes p53 through phosphorylation events that uncouple p53 from Mdm2, whereas the defective rRNA synthesis (using RNA polymerase inhibitor drugs, such as actinomycin D, for example) can result in the binding of ribosomal proteins (rps), including but not limited to L5, L11, and S7 to Mdm2 and subsequent p53 stabilization.31-33 Stabilized p53 directly up-regulates the expression of genes that (depending on the level of cellular stress and DNA damage) will either arrest the cell cycle and activate DNA repair enzymes or initiate apoptosis.30
The mutant zebrafish line hi2578 was generated in a large-scale screen for genes required for embryonic development in the laboratory of Dr Nancy Hopkins.34 The hi2578 line carries a viral insertion in the first intron of the nop10 gene that results in a decrease of nop10 transcript that is lethal to homozygous embryos by 5 days post fertilization (dpf; described by submission to http://zfin.org and on the Hopkins laboratory website, http://web.mit.edu/hopkins/index.html). Here we demonstrate with these mutants that nop10 loss results in a failure of 18S rRNA to be properly processed, which in turn results in a collapse of the 40S small ribosomal subunit. Furthermore, we demonstrate that widespread p53-specific apoptosis is coupled to the increased binding of Mdm2 to the small ribosomal protein S7. This results in increased degradation of rpS7, but not of large ribosomal proteins. The nop10 mutant embryos also fail to form hematopoietic stem cells (HSCs), a phenotype that is rescued by introducing a loss-of-function p53 background. Interestingly, the mutants display no telomere shortening by 4 dpf. Our results suggest that one mechanism of the cytopenia phenotype of DC resulting from loss of the H/ACA complex is driven by ribosome biogenesis defects, resulting in p53-mediated apoptosis in HSCs during early development, caused at least partly by the association of rpS7 with Mdm2.
Methods
Fish maintenance
Zebrafish were raised and embryos obtained through natural spawning as previously described35 in accordance with all Dutch regulations and guidelines. The hi2578 and hi1034b mutant lines were a generous gift from the laboratory of Dr Nancy Hopkins (Massachusetts Institute of Technology, Cambridge, MA).
Imaging
In situ and whole-mount images were obtained using a Zeiss Axioplan Stereomicroscope (Carl Zeiss) equipped with a Leica digital camera using 5×, 10×, or 20× magnifications.
Northern blot analysis
For rRNA processing blots, total RNA was isolated from 5 embryos at 4 dpf with Trizol (Invitrogen), run on a 1% formaldehyde gel, and probed as previously described36 using prehybridization and hybridization solution (Clontech) at 65°C and 0.1% SDS/0.2× saline sodium citrate wash buffer also at 65°C. For p53 blots, total RNA isolated from 20 embryos was probed with full-length p53 as previously described.37 Imaging of the blots was done using phosphor-imaging screens (Molecular Dynamics) followed by scanning using a Typhoon Scanner (GE Healthcare). Quantifications were performed using Quantity One 1D analysis software (Bio-Rad).
In situ hybridizations
TUNEL assay
TUNEL assays were performed using a kit (Roche Diagnostics) according to the manufacturer's specifications.
o-Dianisidine staining
Embryos were anesthetized with Tricane at 36 hpf and stained with 1 mg/mL o-dianisidine (Sigma-Aldrich) in staining buffer (40% ethanol, 10mM NaAc, 0.675% H2O2) for 15 minutes in the dark.
RT-PCR
cDNA was constructed using the Cloned AMV First-Strand cDNA Synthesis Kit (Invitrogen) and RNA isolated using Trizol (Invitrogen) from 4-dpf embryos. Zebrafish nop10 was amplified using primer pairs 5′-AGTGGACCCCAGTGGTCAG-3′ and 5′-GTCAGCAGGAGTCCGAATCT-3′. Zebrafish βactin-2 was amplified using primer pairs 5′-GGACCTGTATGCCAACACTG-3′ and 5′-TGATCTCCTTCTGCATCCTG-3′.
Western blot analysis
Embryos were subject to 25 Gy of γ-irradiation, mechanically de-yolked 6 hours later using a P200 pipette, lysed, and subjected to analysis as previously described using 5 embryos/sample.37 Cycloheximide (Sigma-Aldrich) was added to DMEM plus 10% FCS (Invitrogen) at a concentration of 100 μg/mL to embryos that had first been treated with 5 mg/mL dispase (Sigma-Aldrich), 2.5% trypsin (Invitrogen), 5% DNAse RQ1 (Promega) in DMEM, plus 10% FCS (Invitrogen) for 20 minutes at 30°C to obtain single-cell suspensions. The incubation of cycloheximide-treated cells was done in a 28°C incubator plus 5% CO2. Antibodies used were αp53 (16H12 supernatant diluted 1:1 in blocking solution),37 αrpS7 (1:200; #100834, Santa Cruz Biotechnology), αrpL11 (1:200; #25931, Santa Cruz Biotechnology), αrpL5 (1:200; #103865 Santa Cruz Biotechnology), and αactin (1:1000; #1616, Santa Cruz Biotechnology). Quantifications of Western blot bands were done using a GS-800 densitometer (Bio-Rad) and Quantity One software (Bio-Rad).
Coimmunoprecipitation
The 4-dpf embryos were de-yolked and lysed as for Western blotting, protein concentrations and volumes normalized to 1 mg in 200 μL lysis buffer, and incubated overnight at 4°C with either 2 μL αrpS7-IP antibody or 2 μL of preimmune serum (both kind gifts from Dr Carol Prives, previously described).33 A total of 50 μg of lysate was removed from each sample before antibody additions for 5% input analysis. A total of 40 μL of Protein A/G Plus agarose beads (Santa Cruz Biotechnology) was added and incubated for 2 hours at 4°C with gentle turning of the tubes. The agarose beads were spun down at 1500g for 5 minutes and washed 3 times with ice-cold lysis buffer. After the final wash, the beads were resuspended in 20 μL of 2× Laemmli buffer (Bio-Rad) plus 1 μL β-mercaptoethanol (Sigma-Aldrich), incubated for 5 minutes at 95°C, and spun down at 1500g for 5 minutes. Samples for αMdm2 (#55470; AnaSpec) blotting (including 5% input samples) were run on an 8% SDS-PAGE gel, while technical duplicate samples for αrpS7 (#135372; Santa Cruz Biotechnology) blotting were run on a 15% gel (including 5% input samples). Blotting was performed as described in “Western blot analysis,” using dilutions of 1:500 for αMdm2 and 1:200 for αrpS7.
Polysomal profiling
Fifty zebrafish embryos were homogenized with a Dounce tissue grinder (Wheaton) on ice in 500 μL of polysome lysis buffer (0.11M potassium acetate, 20mM magnesium acetate, 10mM HEPES, pH 7.6, 100mM potassium chloride, 10mM magnesium chloride, 0.1% NP-40, 2mM DTT, and 40 U/mL RNase inhibitor; Promega). Nuclei and debris were removed from the lysates by centrifuging at 1100g for 10 minutes at 4°C in a tabletop centrifuge (Eppendorf 5415 R). Lysates were layered on 11 mL of a 17% to 50% sucrose gradient and centrifuged at 27 000g at 4°C for 2 hours in an SW40 Ti rotor (Beckman) and a Kontron T-1080 ultracentrifuge. Samples were displaced in a fractionator (Brandell) using a 60% sucrose solution, and the RNA content read by a UA-6 absorbance reader (Teledyne ISCO) at 280 UV.
Real-time PCR analysis
RNA was isolated from each individual peak using Trizol LS (Invitrogen). RNA was used to create cDNA using Cloned-AMV First Strand cDNA Synthesis Kit (Invitrogen) according to the manufacturer's protocol, using an Oligo(dT) primer. Gene expression was analyzed using a MyiQ Single Color Real-Time PCR Detection System on a Bio-Rad iCycler. A total of 1 μL cDNA was used in combination with FastStart High Fidelity PCR reagents (Roche Diagnostics) and 1 μL 3.75 × SYBR Green (Sigma-Aldrich) in a volume of 25 μL total, except for the no-template control (neg). Primers for zebrafish p53 used were 5′-TTAAGTGATGTGGTGCCTGCCT-3′ and 5′-AGCTTCTTTCCCTGTTTGGGCT-3′. Primers for zebrafish βactin-2 used were 5′-GGACCTGTATGCCAACACTG-3′ and 5′-TGATCTCCTTCTGCATCCTG-3′. All reactions were performed in triplicate for 45 cycles. Data output was obtained using MyiQ Version 1.0+ software and analyzed with Microsoft Office Excel 2007. The average Ct was determined, and ΔCt was calculated by subtracting Ct target from Ct-neg. Next, the relative amount of p53 compared with the total amount of RNA (represented by actin) was calculated (ΔCt[p53]ΔCt[actin]). To visualize the difference between the wild-type and mutant samples, the relative amounts of p53 mRNA were calculated as a ratio of mutant/wild-type, wherein the amount in the wild-type was set to 1. These were performed with biologic duplicates.
Telomere length assay
Telomere assay was done using the Telomere PNA Kit/FITC for Flow Cytometry (Dako). Control cells used were a human lymphoblast cell line obtained from Dr Hanna Gazda. Single-cell suspensions of 4 dpf zebrafish embryos were obtained by incubating approximately 200 embryos at 30°C for 1 hour in an Eppendorf Thermoshaker at 500 rpm in 750 μL of 1 × PBS with 0.25 mg/mL dispase I (Sigma-Aldrich), 0.125% trypsin (Invitrogen), 50 U RQ1 RNase-Free DNase (Promega), and 10% FCS. Cells were then pulled through a 0.6-mm needle and filtered through a 35-μm nylon mesh cell strainer cap (Falcon).
Results
A viral insertion in the hi2578 zebrafish line results in impaired 18S rRNA processing and 40S ribosomal subunit collapse
A viral insertion in the first intron of the nop10 gene in the hi2578 zebrafish line results in the decreased expression of nop10 in homozygous embryos by 4 dpf (Figure 1A). Homozygous embryos are distinguishable at 1 dpf by a slight dent in the mid-hindbrain barrier, and by 4 dpf have a smaller head and eyes, an underdeveloped liver and gut, and frequent pericardial edema (Figure 1B). Their swim bladders do not inflate, they exhibit a phlegmatic touch response, and most die by 5 dpf. The heterozygous nop10 embryos develop into normal adults and to date have not been shown to have a predisposition to malignancy or other gross phenotypes.
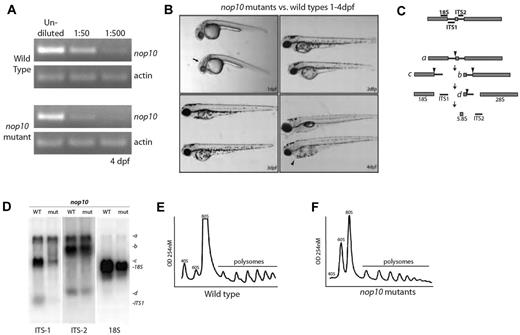
nop10 loss results in ribosome biogenesis defects affecting the small ribosomal subunit. (A) Semiquantitative PCR analysis on wild-type versus nop10 mutants at 4 dpf using nop10 or actin primers. (B) Gross morphology of the nop10 mutant compared with a wild-type sibling from 1 to 4 dpf. Arrow indicates dent in mid-hind brain barrier; and arrowhead, pericardial edema. (C) A contemporary schematic illustrating the major pathway of rRNA processing in zebrafish. Hybridization sites of ITS-1, ITS-2, and 18S probes are indicated on top. Arrowheads indicate major cleavage sites. (D) Northern blot analysis of total RNA isolated from nop10 mutants compared with wild-type siblings at 4 dpf using probes against ITS-1, ITS-2, and 18S. (E-F) Polysome profiles of wild-type siblings (E) compared with nop10 mutants (F) at 4 dpf. The 40S, 60S, 80S, and polysomal peaks are indicated.
nop10 loss results in ribosome biogenesis defects affecting the small ribosomal subunit. (A) Semiquantitative PCR analysis on wild-type versus nop10 mutants at 4 dpf using nop10 or actin primers. (B) Gross morphology of the nop10 mutant compared with a wild-type sibling from 1 to 4 dpf. Arrow indicates dent in mid-hind brain barrier; and arrowhead, pericardial edema. (C) A contemporary schematic illustrating the major pathway of rRNA processing in zebrafish. Hybridization sites of ITS-1, ITS-2, and 18S probes are indicated on top. Arrowheads indicate major cleavage sites. (D) Northern blot analysis of total RNA isolated from nop10 mutants compared with wild-type siblings at 4 dpf using probes against ITS-1, ITS-2, and 18S. (E-F) Polysome profiles of wild-type siblings (E) compared with nop10 mutants (F) at 4 dpf. The 40S, 60S, 80S, and polysomal peaks are indicated.
Because it has been previously demonstrated in yeast that Nop10p loss results in impairment of 18S rRNA pseudo-uridinylation and processing,19 we performed northern blot analysis to determine whether similar effects are evident in zebrafish embryos. Figure 1C shows a basic schematic of rRNA processing in zebrafish embryos derived from the results in our laboratory as well as those previously reported.36 Using probes designed to bind to the internally transcribed sequence 1 (ITS1), internally transcribed sequence 2 (ITS2), or 18S rRNA, we were able to observe an impairment of 18S rRNA processing in nop10 mutants (Figure 1D). A significant loss of the precursor rRNA strand that ultimately makes up the 18S strand is visible in the lanes probed with ITS1; moreover, a significant decrease in the total amount of 18S rRNA is evident in lanes probed with 18S. In contrast, lanes probed with ITS2 appear to be relatively similar between wild-types and mutants, suggesting that there is little impairment of 28S or 5.8S rRNA processing in nop10 mutants. Polysome profiling reveals an almost complete loss of the 40S peak that represents the small ribosome subunit in the nop10 mutants compared with wild-types (Figure 1E-F), which is consistent with an 18S rRNA processing impairment.
Telomere shortening does not occur on nop10 loss
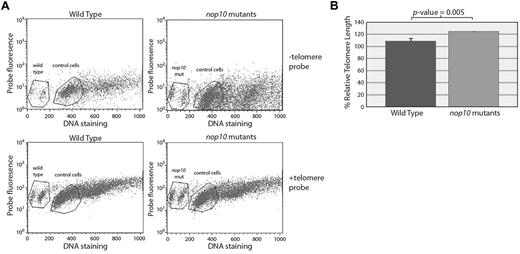
To determine whether the loss of nop10 could result in shorter telomeres in our mutants, we measured the relative telomere lengths of 4-dpf embryonic cells in G0/G1 with or without a fluorescent telomere sequence-specific probe compared with control cells (a normal human tissue culture cell line). Both control cells and zebrafish embryonic cells show an upward shift in fluorescent intensity when incubated with the telomere probe; however, there was no difference detected between the shift of the wild-type cells compared with nop10 mutant cells (Figure 2), which would have indicated a difference in telomere length.
Loss of nop10 results in no detectable telomere shortening. (A) No evidence of a shift difference is detectable in wild-type versus nop10 mutant cells at 4 dpf on hybridization of a telomere-specific probe. Gated cells represent those in G0/1. (B) Quantification of panel A, calculating the percentage relative telomere length compared with control cells.
Loss of nop10 results in no detectable telomere shortening. (A) No evidence of a shift difference is detectable in wild-type versus nop10 mutant cells at 4 dpf on hybridization of a telomere-specific probe. Gated cells represent those in G0/1. (B) Quantification of panel A, calculating the percentage relative telomere length compared with control cells.
Loss of nop10 increases p53
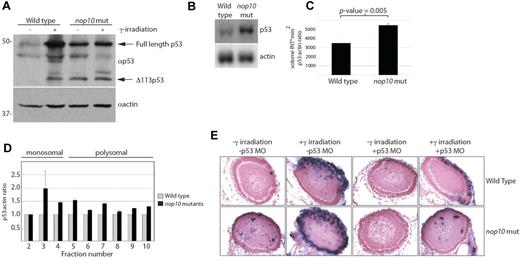
To evaluate the consequences of the loss of small ribosome subunit formation, we examined the status of the p53 tumor suppressor. Typically, the induction of DNA damage in zebrafish embryos by γ-irradiation results in p53 stabilization.37 This is observed by Western blotting with a zebrafish p53-specific antibody in 4 dpf wild-type embryos treated with 25 Gy of γ-irradiation after 6 hours (Figure 3A). nop10 mutant embryos at 4 dpf in the absence of DNA damage also reveal p53 stabilization, a result that is consistent with rRNA synthesis deficiencies.30 No further increase of p53 stabilization is evident in nop10 mutants on γ-irradiation. Expression of the Δ113p53 isoform is evident in both the mutants and irradiated wild-types (Figure 3A). Transcription of p53 is also increased significantly in nop10 mutants, shown by northern blot analysis (Figure 3B-C). Moreover, using real-time PCR analysis of mRNA isolated from polysomal fractions, we were able to demonstrate that this increase in cellular p53 mRNA in nop10 mutants is coupled to an increase in the amount of p53 mRNA that is loaded onto polysomes (Figure 3D). Injection of a translation-blocking p53 morpholino (MO) into embryos at the one cell stage followed by TUNEL analysis at 4 dpf reveals a significant reduction in the number of TUNEL-positive cells on histologic slides of p53 MO-injected nop10 embryos (Figure 3E). These results indicate that the rRNA synthesis defect of the nop10 mutants initiates cellular apoptosis in a p53-dependent manner and that a decrease of p53 mRNA translation does not occur.
nop10 loss results in p53-dependent apoptosis. (A) Western blot analysis of p53 protein stabilization in 4-dpf embryos either untreated or treated with 25 Gy γ-irradiation and incubated for 6 hours. (B) Northern blot analysis of p53 mRNA levels in nop10 mutant embryos compared with wild-type siblings at 4 dpf. (C) Quantification of 3 independent p53 Northern blots comparing the level of p53:actin mRNA by densitometer analysis. (D) Real-time PCR analysis indicating the relative ratios of p53:actin mRNA in individual fractions isolated from polysome profiles of wild-type or nop10 mutants at 4 dpf. Fractions 2 to 4 represent the monosomal portion of the profile, and fractions 5 to 10 represent the polysomal portion. (E) TUNEL assay (focusing on the zebrafish eye) indicating cells undergoing apoptosis in embryos untreated, treated with 25 Gy γ-irradiation and incubated for 6 hours, injected at the 1-cell stage with a p53-MO, or both. Images were obtained using a Nikon Eclipse E600 microscope with 40× Nikon Plan Fluor, 0.75 NA dry objective. A Leica DFC 500 camera was used with Leica Application Suite Version 3.6.0 software.
nop10 loss results in p53-dependent apoptosis. (A) Western blot analysis of p53 protein stabilization in 4-dpf embryos either untreated or treated with 25 Gy γ-irradiation and incubated for 6 hours. (B) Northern blot analysis of p53 mRNA levels in nop10 mutant embryos compared with wild-type siblings at 4 dpf. (C) Quantification of 3 independent p53 Northern blots comparing the level of p53:actin mRNA by densitometer analysis. (D) Real-time PCR analysis indicating the relative ratios of p53:actin mRNA in individual fractions isolated from polysome profiles of wild-type or nop10 mutants at 4 dpf. Fractions 2 to 4 represent the monosomal portion of the profile, and fractions 5 to 10 represent the polysomal portion. (E) TUNEL assay (focusing on the zebrafish eye) indicating cells undergoing apoptosis in embryos untreated, treated with 25 Gy γ-irradiation and incubated for 6 hours, injected at the 1-cell stage with a p53-MO, or both. Images were obtained using a Nikon Eclipse E600 microscope with 40× Nikon Plan Fluor, 0.75 NA dry objective. A Leica DFC 500 camera was used with Leica Application Suite Version 3.6.0 software.
nop10 loss results in Mdm2 binding to and degradation of rpS7
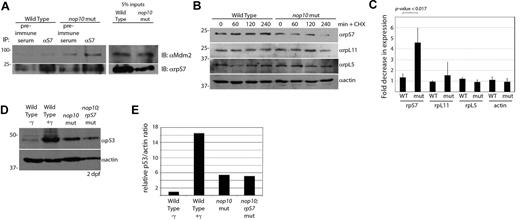
We surmised that nop10 loss would result in a pool of free small rps, unable to incorporate into a ribosome subunit because of the loss of 18S rRNA, now available to bind other proteins, such as the E3 ubiquitin ligase Mdm2. rpS7 is one small subunit rp that has been shown in vitro to be able to bind to MDM2 on actinomycin D treatment.33,40 Coimmunoprecipitation of rpS7 and blotting with a zebrafish specific Mdm2 antibody reveal an increased amount of Mdm2 binding to rpS7 in nop10 mutants compared with wild-types (Figure 4A). It has furthermore been demonstrated in vitro that the rpS7-MDM2 association results in the E3 ubiquitin ligase activity of MDM2 promoting the degradation of rpS7.33 To test this, we subjected zebrafish embryo cells to cycloheximide treatment and then determined the expression levels of rpS7, rpL5, and rpL11. Figure 4B-C illustrates that, by 4 hours after cycloheximide exposure, the expression of rpS7 in nop10 mutant cells is substantially decreased compared with rpS7 in wild-type cells. This is in contrast to an absent or mild reduction of expression of large ribosomal subunit proteins L5 and L11, respectively. To assess whether rpS7 is the only small rp binding Mdm2 and causing p53 stabilization, we crossed the hi1034b line (carrying a viral insertion in the rpS7 gene)41 with the hi2578 line to create double nop10;rpS7 mutants. These double mutants do not show a reduction of p53 stabilization compared with the nop10 mutant alone at the same age (Figure 4D-E), suggesting that rpS7 is most likely not the only protein binding Mdm2 and decoupling it from p53. (These blots were performed at 2 dpf because of the earlier lethality of the double mutants). This is not surprising given the other rps known to bind Mdm2, including rpS3.42 These data strongly suggest that the collapse of the small ribosomal subunit in nop10 embryos results in increased binding of small rps with Mdm2 followed by the subsequent degradation of rpS7
nop10 loss causes rpS7 binding to Mdm2 and increased degradation of rpS7. (A; left) Immunoprecipitation of embryo lysates at 4 dpf with preimmune serum or αrpS7 followed by blotting with αMdm2 (top) or αrpS7 (bottom). (Right) A total of 50 μg (5%) of total lysate used for immunoprecipitations blotted with either αrpS7 or αMdm2 (note: these samples are on the same blots as the immunoprecipitations but are displayed separately because of different exposure requirements). (B) Western blot analysis of levels of rpS7, rpL11, rpL5, and actin in wild-type and nop10 mutant embryos treated with cycloheximide for 0 to 4 hours. (C) Quantification of 3 independent experiments indicating the protein with the largest fold decrease of expression in 4 hours after cycloheximide treatment is rpS7 in nop10 mutant embryos. (D) Western blot analysis of 2 dpf wild-type embryos untreated or treated with 25 Gy γ-irradiation, nop10 mutants, or nop10;rpS7 mutants. (E) Densitometer quantification of expression levels in panel D comparing relative p53/actin ratios, setting the wild-type γ-ratio to 1.
nop10 loss causes rpS7 binding to Mdm2 and increased degradation of rpS7. (A; left) Immunoprecipitation of embryo lysates at 4 dpf with preimmune serum or αrpS7 followed by blotting with αMdm2 (top) or αrpS7 (bottom). (Right) A total of 50 μg (5%) of total lysate used for immunoprecipitations blotted with either αrpS7 or αMdm2 (note: these samples are on the same blots as the immunoprecipitations but are displayed separately because of different exposure requirements). (B) Western blot analysis of levels of rpS7, rpL11, rpL5, and actin in wild-type and nop10 mutant embryos treated with cycloheximide for 0 to 4 hours. (C) Quantification of 3 independent experiments indicating the protein with the largest fold decrease of expression in 4 hours after cycloheximide treatment is rpS7 in nop10 mutant embryos. (D) Western blot analysis of 2 dpf wild-type embryos untreated or treated with 25 Gy γ-irradiation, nop10 mutants, or nop10;rpS7 mutants. (E) Densitometer quantification of expression levels in panel D comparing relative p53/actin ratios, setting the wild-type γ-ratio to 1.
Loss of nop10 results in a p53-dependent loss of HSC formation
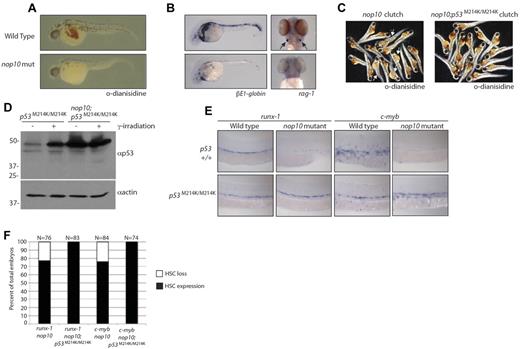
One of the hallmarks of DC is cytopenia, which may be because of a loss of HSC formation in early development. To determine the effect of nop10 loss on HSC development, we first performed whole-mount hemoglobin staining with o-dianisidine. Figure 5A demonstrates that at 36 hpf hemoglobin-expressing RBCs are clearly visible in the yolk and heart of wild-type embryos in contrast to very few visible in nop10 mutants. This suggests that the nop10 mutants, similar to DC patients, experience erythrocyte loss. We next performed in situ hybridizations to measure the levels of βE1-globin and rag-1 in 36-hpf and 4-dpf embryos, respectively. The βE1-globin gene is known in zebrafish embryos to be up-regulated in erythroid cells during early stages of primitive hematopoiesis.43 Figure 5B illustrates a significant decrease in staining of βE1-globin in nop10 mutants compared with wild-type embryos at 36 hpf, suggesting a link between nop10 loss and an impairment of primitive hematopoiesis. Figure 5B additionally shows a complete absence of rag-1 transcripts in nop10 mutant embryos at 4 dpf, indicating a loss of differentiated thymic T cells that is also consistent with the DC anemia phenotype. To ascertain whether this blood cell loss was the result of deregulated p53 stabilization, we introduced a homozygous loss-of-function p53M214K/M214K allele44 to the nop10 mutant background. At 1 dpf, unlike the nop10 mutants, the nop10;p53M214K/M214K mutants were indistinguishable from clutch siblings (data not shown). However, by 2 dpf, the gross nop10 mutant phenotype was evident; and by 4 to 5 dpf, the nop10;p53M214K/M214K mutants looked very similar to the nop10 mutants alone (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). O-Dianisidine staining of nop10;p53M214K/M214K mutants revealed a complete rescue of hemoglobin-producing RBCs compared with nop10 mutants, illustrated in representative clutch samples in Figure 5C. Western blot analysis of the nop10;p53M214K/M214K mutants in Figure 5D compared with p53M214K/M214K mutants alone indicates hyperstabilization of p53 protein in the double mutants with or without γ-irradiation and also loss of the Δ113p53 protein (consistent with recent evidence that wild-type p53 is required for induction of Δ113p53).45 Next, we tested levels of c-myb and runx-1 transcripts that are expressed in emerging definitive hematopoietic stem and progenitor cells by in situ hybridization. In wild-type zebrafish embryos at 36 hpf, these markers are evident in cells within the dorsal aorta (Figure 5E). In contrast, very little expression of c-myb or runx-1 is detected in nop10 mutants at 36 hpf, suggesting a loss of definitive HSC formation. Substantial rescue of runx-1 and c-myb expression is also revealed in the nop10;p53M214K/M214K mutants compared with nop10 mutants alone (Figure 5E-F). Taken together, these data suggest that the loss of HSCs observed in nop10 mutants is p53-dependent.
nop10 loss results in p53-dependent defects in HSC formation. (A) O-Dianisidine staining of whole RBCs at 36 hpf in nop10 mutant embryos or wild-type siblings. (B) In situ hybridization analysis of βE1-globin (36 hpf) or rag-1 (4 dpf) mRNA levels. (C) O-Dianisidine staining in a sampling from 36-hpf clutches of either nop10 mutants (left) and wild-type siblings (arrows indicate mutants) or nop10;p53M214K/M214K mutants and p53M214K/M214K siblings (right). (D) Western blot analysis of p53 stabilization in nop10;p53M214K/M214K embryos compared with p53M214K/M214K embryos in the presence or absence of 25 Gy γ-irradiation. (E) In situ hybridization analysis of runx-1 mRNA (left) or c-myb mRNA (right) in 36-hpf nop10 mutant embryos compared with clutch siblings either in a p53 wild-type background (top) or p53M214K/M214K mutant background (bottom). Embryos were mounted in methylcellulose and images were obtained using a Zeiss Axioplan microscope with a 10× Zeiss Neofluor, 0.3 NA dry objective. A Leica DFC 480 camera was used with Leica Application Suite Version 3.5.0 software. (F) Quantification of in situ hybridization results showing percentages of the HSC-loss phenotype in total clutches of embryos.
nop10 loss results in p53-dependent defects in HSC formation. (A) O-Dianisidine staining of whole RBCs at 36 hpf in nop10 mutant embryos or wild-type siblings. (B) In situ hybridization analysis of βE1-globin (36 hpf) or rag-1 (4 dpf) mRNA levels. (C) O-Dianisidine staining in a sampling from 36-hpf clutches of either nop10 mutants (left) and wild-type siblings (arrows indicate mutants) or nop10;p53M214K/M214K mutants and p53M214K/M214K siblings (right). (D) Western blot analysis of p53 stabilization in nop10;p53M214K/M214K embryos compared with p53M214K/M214K embryos in the presence or absence of 25 Gy γ-irradiation. (E) In situ hybridization analysis of runx-1 mRNA (left) or c-myb mRNA (right) in 36-hpf nop10 mutant embryos compared with clutch siblings either in a p53 wild-type background (top) or p53M214K/M214K mutant background (bottom). Embryos were mounted in methylcellulose and images were obtained using a Zeiss Axioplan microscope with a 10× Zeiss Neofluor, 0.3 NA dry objective. A Leica DFC 480 camera was used with Leica Application Suite Version 3.5.0 software. (F) Quantification of in situ hybridization results showing percentages of the HSC-loss phenotype in total clutches of embryos.
Discussion
The impaired proliferative and renewal capacity of stem cells is a generally accepted mechanism underlying the phenotypes of DC. This could occur in 2 ways: one would be an initial reduction of total stem cell numbers during early development, and another would be the gradual loss of stem cell function over time because of critically short telomeres inducing senescence. Our zebrafish model of DC supports the previous hypomorphic Dkc1 murine model,23 suggesting that mutations affecting the H/ACA RNP complex in early development result in phenotypes because of ribosome biogenesis defects before the onset of telomere shortening.
The ribosome biogenesis defects in our zebrafish model drive p53-dependent apoptosis of HSCs (and perhaps other stem cell types) and suggest similar defects may play a role in the DC cytopenia phenotype through a reduction of initial stem cell numbers. After development is complete, the shortening of telomeres over time and senescence then probably plays a second role in stem cell failure, probably including a further decrease of blood cell formation. This model may explain why DC patients carrying dkc1 mutations tend to exhibit some of the most deleterious phenotypes, as both an initial stem cell reduction and gradual stem cell failure are presumably affecting these patients. It is also consistent with recent studies that mice with homozygous mutations in either TERT or TERC do not show any evidence of p53 activation under basal conditions,46 or any overt phenotypes for several generations,47 whereas first-generation mice with hypomorphic dkc1 mutations exhibit both bone marrow and skin phenotypes reminiscent of DC.23 Taken together, these data argue against the idea that H/ACA complex gene mutations, such as dkc1 and nop10 in DC, elicit stem cell failure strictly through telomere shortening and, instead, suggest that p53 stabilization in early development reduces the total number of stem cells because of small rps binding Mdm2. It seems unlikely that mutations in TERT or TERC would result in a similar phenomenon, having no known role in rRNA processing, although these mutations may drive stem cell failure later through their well-established role in telomere maintenance and the links between critically short telomeres, senescence, and apoptosis.48 The data also suggest that perhaps DC linked to mutations in C16orf57, which do not result in telomere shortening, may be instead the result predominantly of p53 deregulation in early development.
Although it is interesting that the introduction of the p53M214K/M214K alleles to the nop10 mutants does not seem to result in a significant rescue beyond very early development, this is most probably the result of the induction of other methods of programmed cell death. p53-independent mechanisms of cell death can occur in other ribosome biogenesis mutants; for example, the lethality of rpL35 loss in zebrafish embryos is not rescued by the p53M214K/M214K alleles, although there is a slight amelioration of the mutant phenotype in very early development similar to what we observe with the nop10;p53M214K/M214K mutants (A. Amsterdam and A.W.M., unpublished results, January 2008). Nevertheless, it is clear that, within that timeframe of early development (within 36 hpf), the slight rescue effect of p53M214K/M214K is enough for HSC development to proceed in nop10 mutants.
We present in this work a vertebrate model of nop10 loss and a novel zebrafish model of DC. Our results with this model bring together how impairment of rRNA processing by nop10 loss can result in the cytopenia phenotype common in DC through the interaction of rpS7 with Mdm2 and stabilization of p53 in HSCs. Although it remains to be determined whether this phenomenon may be attributed to all stem cell types, our zebrafish model suggests the existence of 2 mechanisms of stem cell failure in DC patients depending on the type of mutation. Dysfunction of the H/ACA complex in our zebrafish model results in rp-mediated p53 stabilization in early development, whereas other DC mutations in genes involved solely in telomere maintenance more probably cause stem cell proliferation defects through the shortening of telomeres over time.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank J. Korving and H. Begthel of the histology laboratory and S. Holwerda for excellent technical assistance; the animal facility caretakers of the Hubrecht Institute; the group of S. Schulte-Merker for the in situ hybridization probes; and R. Ketting, S. Schulte-Merker, and D. Guardavaccaro for helpful discussions and critical readings of the manuscript.
This work was supported by the Koninklijke Nederlandse Akademie van Wetenschappen (KNAW) and the Hubrecht Institute (A.W.M.).
Authorship
Contribution: A.W.M. conceived of and designed the experiments and wrote the manuscript, and all authors performed the experiments and collected and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for A.B. is Department of Rheumatology, Erasmus Medical Centre, Rotterdam, The Netherlands.
Correspondence: Alyson W. MacInnes, Hubrecht Institute, KNAW and Universitair Medisch Centrum Utrecht, Uppsalalaan 8, Utrecht, 3584 CT, The Netherlands; e-mail: a.macinnes@hubrecht.eu.
References
Author notes
T.C.P. and L.J.v.W. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal