Abstract
Mucorales-specific T cells were investigated in 28 hematologic patients during the course of their treatment. Three developed proven invasive mucormycosis (IM), 17 had infections of known origin but other than IM, and 8 never had fever during the period of observation. Mucorales-specific T cells could be detected only in patients with IM, both at diagnosis and throughout the entire course of the IM, but neither before nor for long after resolution of the infection. Such T cells predominantly produced IL-4, IFN-γ, IL-10, and to a lesser extent IL-17 and belonged to either CD4+ or CD8+ subsets. The specific T cells that produced IFN-γ were able to directly induce damage to Mucorales hyphae. None of the 25 patients without IM had Mucorales-specific T cells. Specific T cells contribute to human immune responses against fungi of the order Mucorales and could be evaluated as a surrogate diagnostic marker of IM.
Introduction
Invasive mucormycosis (IM), the second most common cause of invasive mold infections in hematologic patients, has mortality rates approaching 70% of affected individuals because of difficulties in obtaining an early definite diagnosis.1-5 In fact, a definitive diagnosis of IM relies exclusively on both histopathologic demonstration and cultural isolation of the pathogen from the involved organs.6 However, the obtainment of tissue specimens in hematologic patients is too often hampered by the presence of several comorbidities, and histologically proven IM may fail to grow in culture in at least one third of cases.7 Furthermore, neither serologic nor antigenic diagnostic methods exist, and the use of polymerase chain reaction has been limited almost exclusively to the identification and discrimination of fungal species.8,9
Adaptive immunity has been reported to play a crucial role in the defense of the host against fungi, at least in the case of invasive aspergillosis and invasive candidiasis,10,11 and the recognition and enumeration of antigen-specific T cells has been demonstrated to be a useful tool for the diagnosis of definite infectious diseases, particularly of either active or latent tuberculosis.12 We therefore explored the possibility that Mucorales-specific T cells are elicited in patients with IM and that their detection may be of value in the diagnosis of active disease.
Methods
Twenty-eight hematologic patients were studied. Patients 1-3 had disseminated (pulmonary and splenic), tracheobronchial, and cerebral proven IM, respectively (supplemental Figures 1-2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The antifungal treatments of the 3 patients with proven IM are described in detail in supplemental Methods.
The remaining 25 cases included 17 patients who presented with infectious complications of proven origin on the basis of cultural or histologic examinations, but other than IM, and 8 who did not develop infectious complications during the course of their induction chemotherapy. Patients' clinical characteristics are given in supplemental Table 1. Informed consent was obtained in accordance with the Declaration of Helsinki, and the study was approved by the University of Modena and Reggio Emilia ethics committee.
An enzyme-linked immunospot (ELISpot) assay was performed to detect either Mucorales- or Aspergillus-specific T cells, as reported previously13 and described in detail in supplemental Methods, on 80 peripheral blood samples (range 2-6 per patient). Time points analyzed were as follows: in patient 1, at the beginning of induction chemotherapy, 20 days before the pulmonary biopsy, at the histologic and cultural identification of Rhizomucor pusillus infection, at the beginning of consolidation chemotherapy, and 16 weeks after resolution of the infection; in patient 2, on the day of cultural and histologic demonstration of Rhizopus oryzae infection and until death on 4 further occasions during the course of IM; in patient 3, on the day of histologic and molecular demonstration of Absydia corymbifera infection, on 3 occasions during the course of IM, and on complete resolution of the infection. All other patients were analyzed at least twice during the course of their infections or chemotherapeutic treatments (supplemental Table 1).
The phenotypic and functional characterization of Mucorales-specific T cells has been performed with a cytokine secretion assay, as reported previously14 and described in detail in supplemental Methods. Molecular studies, micromanipulation, and single-cell PCR to identify Mucorales species (supplemental Figure 2) were performed as reported previously15 and described in detail in supplemental Methods. Anti-Mucorales activity of specific T cells was assessed as reported in supplemental Methods.
Results
Identification of Mucorales-specific T cells
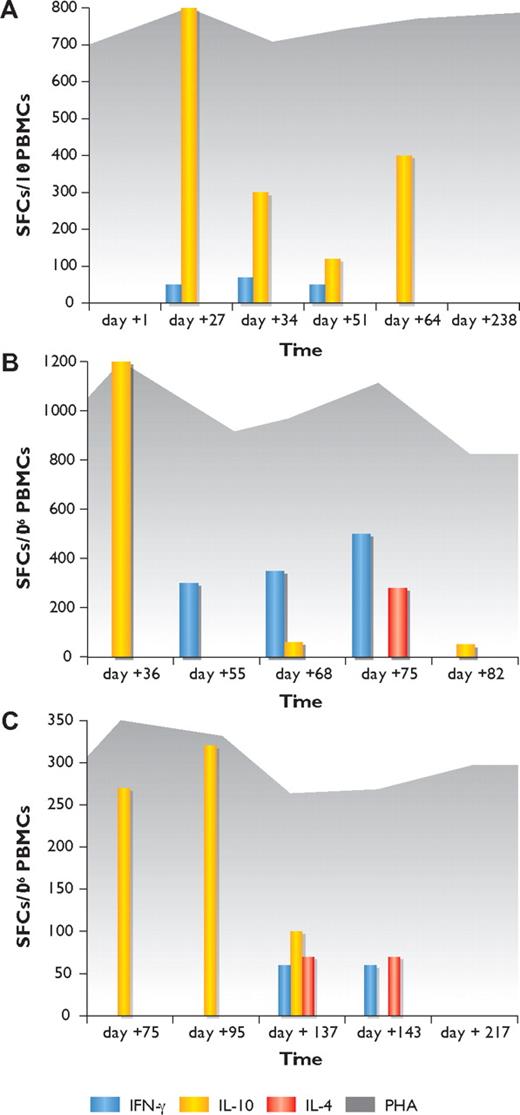
In patient 1, the ELISpot was positive for the presence of Mucorales-specific T cells producing IL-10 in the second, third, fourth, and fifth samples and Mucorales-specific T cells producing IFN-γ in the second, third, and fourth samples. In contrast, no Mucorales-specific T cells could be detected before the occurrence of the infection (at day +1 of induction chemotherapy) or long after its resolution (day +238; Figure 1A).
Kinetics of Mucorales-specific T-cell responses by IFN-γ, IL-10, and IL-4 ELISpot assay in the 3 patients with invasive mucormycosis. (A) Patient 1. (B) Patient 2. (C) Patient 3. Yellow columns represent the number of Mucorales-specific T cells producing IL-10; blue columns, the number of Mucorales-specific T cells producing IFN-γ; red columns, the number of Mucorales-specific T cells producing IL-4; and dark gray background, T-cell responses in wells with phytohemagglutinin (PHA). Vertical axis shows the number of spot-forming cells (SFCs) per million peripheral blood mononuclear cells (PBMCs); horizontal axis indicates the time (in days) from the beginning of induction chemotherapy.
Kinetics of Mucorales-specific T-cell responses by IFN-γ, IL-10, and IL-4 ELISpot assay in the 3 patients with invasive mucormycosis. (A) Patient 1. (B) Patient 2. (C) Patient 3. Yellow columns represent the number of Mucorales-specific T cells producing IL-10; blue columns, the number of Mucorales-specific T cells producing IFN-γ; red columns, the number of Mucorales-specific T cells producing IL-4; and dark gray background, T-cell responses in wells with phytohemagglutinin (PHA). Vertical axis shows the number of spot-forming cells (SFCs) per million peripheral blood mononuclear cells (PBMCs); horizontal axis indicates the time (in days) from the beginning of induction chemotherapy.
In patients 2 and 3, the ELISpot showed the sole presence of Mucorales-specific T cells producing IL-10 in the first sample (on the day of cultural and histologic demonstration of IM) in both patients; increasing numbers of Mucorales-specific T cells producing IFN-γ in the second, third, and fourth samples in patient 2 and in the third and fourth samples in patient 3; and the occurrence of Mucorales-specific T cells producing IL-4 in the fourth sample in patient 2 and in the third and fourth samples in patient 3. The last examination demonstrated the sole presence of Mucorales-specific T cells producing IL-10 in patient 2, close to the time of the patient's death, and the absence of specific responses in patient 3 at the time of complete resolution of the infection (Figure 1B,C).
The differences in the median frequencies of Mucorales-specific T cells producing IL-10, IFN-γ, and IL-4 were not statistically significant in the 3 patients (P = .3), even when the results of the first 2 patients with more disseminated diseases were compared with those of the third patient, who had a more limited infection (P = .5). In the 25 control patients, the ELISpot never showed the presence of Mucorales-specific T cells. None of the analyzed patients demonstrated the occurrence of Aspergillus-specific T cells at any time point (supplemental Table 1).
Phenotypic and functional characterization of Mucorales-specific T cells
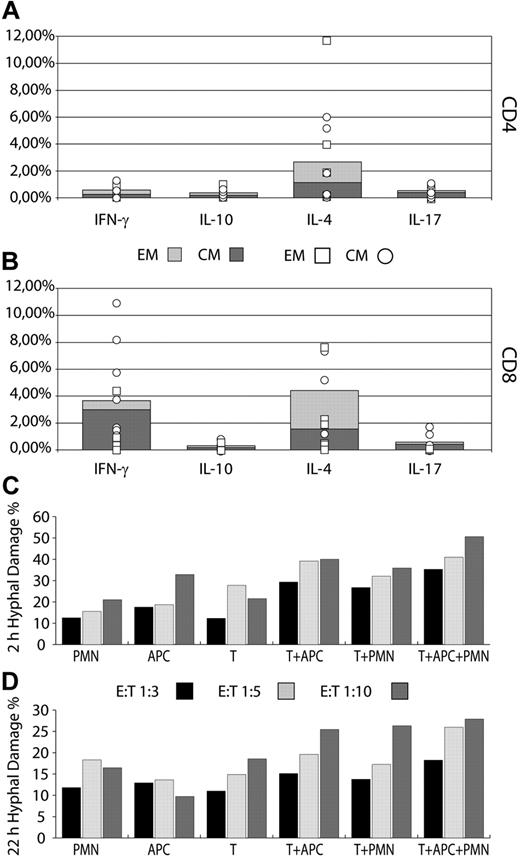
In patients 1-3, Mucorales-specific T cells were (1) predominantly CD8+ T cells (mean CD8+/CD4+ frequencies 3.62%/0.57%) of the CM (central memory) phenotype, producing IFN-γ; (2) predominantly CD8+ T cells (mean CD8+/CD4+ frequencies 4.35%/2.60%) of the EM (effector memory) phenotype, producing IL-4; or (3) either CD4+ or CD8+ T cells (mean CD4+/CD8+ frequencies 0.32%/0.26%), the former of either the CM or EM phenotype, and the latter mainly of the CM phenotype, producing IL-10. Mucorales-specific T cells producing IL-17 were also detectable, which were either CD4+ or CD8+ (mean frequency 0.44% and 0.56%, respectively), and exhibited predominantly the CM phenotype (Figure 2A-B).
Cytokine production profile and lytic activities of Mucorales-specific T cells. (A-B) The frequencies of Mucorales-specific T cells producing IFNγ, IL-10, IL-4, or IL-17, either as EM (light gray) or CM (dark gray), are shown as the mean percentage of positive cells, computed for the 3 patients with IM. Results are expressed as percentages of either CD4+ T cells (A) or CD8+ T cells (B). Mean frequencies of specific cytokine-producing T cells for individual patients are reported in each column, either as EM (■) or CM (○). (C-D) Hyphal damage at 2 (C) and 22 (D) hours to Rhizomucor pusillus and Rhizopus oryzae hyphae induced by anti-Mucorales T cells (T), polymorphonuclear leukocytes (PMNs), and antigen-presenting cells (APCs), alone or in combination, derived from patients 1 and 2 during the course of IM. E:T indicates effector/target cell ratio.
Cytokine production profile and lytic activities of Mucorales-specific T cells. (A-B) The frequencies of Mucorales-specific T cells producing IFNγ, IL-10, IL-4, or IL-17, either as EM (light gray) or CM (dark gray), are shown as the mean percentage of positive cells, computed for the 3 patients with IM. Results are expressed as percentages of either CD4+ T cells (A) or CD8+ T cells (B). Mean frequencies of specific cytokine-producing T cells for individual patients are reported in each column, either as EM (■) or CM (○). (C-D) Hyphal damage at 2 (C) and 22 (D) hours to Rhizomucor pusillus and Rhizopus oryzae hyphae induced by anti-Mucorales T cells (T), polymorphonuclear leukocytes (PMNs), and antigen-presenting cells (APCs), alone or in combination, derived from patients 1 and 2 during the course of IM. E:T indicates effector/target cell ratio.
Lytic activity of Mucorales-specific T cells
Mucorales-specific T cells from patients 1-3 were able to induce direct damage to the hyphae of the 2 clinical isolates, similar to that of either polymorphonuclear leukocytes or APCs. Only the combination of all 3 cell types resulted in significantly greater damage to the hyphae (P < .05; Figure 2C-D).
Discussion
We have shown for the first time that Mucorales-specific T cells may occur during the course of infection in patients with IM and that they exhibit direct antifungal activity comparable, at least in vitro, to that of either polymorphonuclear leukocytes or APCs. The contribution of T cells to host defenses against these moulds could only be suspected on the basis of the enhanced fungicidal activity against Mucorales of polymorphonuclear leukocytes exposed to IFN-γ,16 but this has not yet been demonstrated formally.
The presence of Mucorales-specific T cells only during the course of IM and neither before infection nor after resolution of the infection in patients 1-3, as well as their absence in patients without infections or with infections other than IM, suggests that they are closely related to the occurrence of IM and may be a marker of overt disease. Of note, the presence of Mucorales-specific T cells was the only proof of IM in patient 1, before obtainment of the biopsy. The lower frequencies of specific T cells in patient 3 appear to suggest that a more confined IM could be associated with responses of an inferior magnitude; however, no statistically significant differences were observed in the median numbers of Mucorales-specific T cells in the 3 patients in the present study. Unfortunately, because all the samples were collected either when the patients were undergoing antifungal treatment or after withdrawal of the drug, no interaction between antifungal therapy and the occurrence of Mucorales-specific T cells could be determined in the present study.
The cytokine production profile of Mucorales-specific T cells in the present study was partially in line with what observed either in mice affected by invasive aspergillosis or in human T-cell clones stimulated with different Aspergillus antigens in vitro.17-19 The demonstration that CD8+Mucorales-specific T cells may produce either IL-4 or IL-10, predominantly in the late phase of the infection, is reminiscent of the type 2 cytokine shift of CD8+ lymphocytes, thus far reported only in patients with the cavitary phase of tuberculosis and in the late phase of HIV infection.20,21
In conclusion, Mucorales-specific T cells emerge in the course of IM and contribute to human immune responses against Mucorales. The detection of Mucorales-specific T cells may be evaluated as a surrogate diagnostic marker of IM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the Associazione Italiana per la Ricerca sul Cancro, Milan, Italy (M.L.); the European Commission's FP6 Life Science Health Program (INCA project; LSHC-CT-2005-018704) (M.L.); the Associazione Italiana Lotta alle Leucemie, Linfoma e Mieloma-Sezione “Luciano Pavarotti”-Modena-ONLUS (L.P. and F.F.); the Programma di ricerca Regione-Università 2007-2009, Regione Emilia Romagna (M.L. and F.N.); and the Società Italiana di Ematologia Sperimentale (“Piero Martino” award, L.P.).
Authorship
Contribution: L.P. and M.L. conceived and designed the study and wrote the manuscript; D.V., P.B., G. Riva, E.Z., C.Q., G. Rossi, F.R., and M.P. performed the ELISpot analysis, the cytokine secretion assay analysis, the XTT assays, the histologic examination, and the molecular characterization of fungi and interpreted the data; F.F., A.C., J.M., M. Morselli, M.C., A.P., M. Maccaferri, R.M., and F.N. provided well-characterized patient samples and critically revised the manuscript; C.D.G. and R.D. performed the statistical analysis and interpreted the data; and F.C. performed the radiologic studies and critically revised the manuscript.
Conflict-of-interest disclosure: M.L. received research funds from and serves on advisory boards for Merck Sharp & Dohme and Gilead Sciences and received honoraria from these 2 pharmaceutical companies and from Pfizer and Nanogen; L.P. serves on an advisory board for Merck Sharp & Dohme; A.C. serves on an advisory board for Merck Sharp & Dohme and received funds by Merck Sharp & Dohme, Gilead Sciences, and Pfizer; L.P., P.B., and M.L. have applied for a European patent regarding clinical applications of the ELISpot assay for the diagnosis of Aspergillus infection (PCT Nos. WO2008/075395A3, EP2094295, IT2007/000867); and L.P., D.V., P.B., F.F., and M.L. have applied for an Italian patent regarding clinical applications of the ELISpot assay for the diagnosis of Mucorales infection (No. MI2010A002224). The remaining authors declare no competing financial interests.
Correspondence: Mario Luppi, MD, PhD, Professor of Hematology, Chief, Division of Hematology, Department of Oncology, Hematology and Respiratory Diseases, University of Modena and Reggio Emilia, Azienda Ospedaliero-Universitaria, Policlinico, Modena, Italy; e-mail: mario.luppi@unimore.it.
References
Author notes
L.P., D.V., P.B., G.R., and F.F. contributed equally to the study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal