Abstract
CD8+ CTLs are thought to play a role in the control of follicular lymphoma (FL). Yet, the link between CTL tissue distribution, activation status, ability to kill FL cells in vivo, and disease progression is still elusive. Pretreatment lymph nodes from FL patients were analyzed by IHC (n = 80) or by 3-color confocal microscopy (n = 10). IHC revealed a rich infiltrate of CD8+ granzyme B+ (GrzB) cells in FL interfollicular spaces. Accordingly, confocal microscopy showed an increased number of CD3+CD8+GrzB+ CTLs and a brighter GrzB staining in individual CTL in FL samples compared with reactive lymph nodes. CTLs did not penetrate tumor nodules. In 3-dimensional (3-D) image reconstructions, CTLs were detected at the FL follicle border where they formed lytic synapse-like structures with FL B cells and with apoptotic cells, suggesting an in situ cytotoxic function. Finally, although GrzB expression in CTLs did not correlate with risk factors, high GrzB content correlated with prolonged progression free-survival (PFS) after rituximab-combined chemotherapy. Our results show the recruitment of armed CTLs with a tumor-controlling potential into FL lymph nodes and suggest that CTL-associated GrzB expression could influence PFS in FL patients having received rituximab-combined chemotherapy.
Introduction
Follicular lymphoma (FL) is the second most frequent B-cell lymphoma in adults. It is based on the tumoral proliferation of B lymphocytes that are organized in neoplastic nodules with a follicular structure. It is considered an “indolent” lymphoma, based on nonaggressive initial presentation. The introduction of therapeutic strategies combining chemotherapy and rituximab, a mAb directed against the B-cell membrane-associated CD20 Ag, has significantly improved both response rate and progression free-survival in large phase 3 studies.1-3 However, despite the indolent course and evident therapeutic benefits, FL is still considered an incurable disease because patients invariably relapse and ultimately die, particularly in the aggressive forms of the disease, characterized by high tumor burden correlated with risk factors as measured by the Follicular Lymphoma International Prognostic Index (FLIPI)4 or the Follicular Lymphoma Study Group (Groupe d'Etude du Lymphome Folliculaire [GELF]) scores.5
Although the mechanisms of relapse and tumor progression are likely to be manifold, several lines of evidences indicate a role for the antitumor immune response in FL clinical outcome. IHC and gene-expression studies have contributed to establish the notion that, in FL, immune cell infiltration strongly influences the clinical behavior, including FL transformation into aggressive lymphomas6 and patient survival6 (reviewed in Relander et al7 ). A predominance of T cells or of T cell–related genes correlates with good prognosis, whereas predominance of accessory cells (dendritic cells and monocytes) is associated with aggressive course.8-14 The T-cell infiltrate in FL displays heterogeneous phenotypic markers including CD4, CD8, CD57, and Foxp3, and the activation marker CD69, suggesting an intense functional interaction between various T-cell populations and neoplastic B cells.6-14 Among T-cell populations, it is generally considered that CD8+ T cells play an essential role in antitumor immune response as described in other tumor models.15 Accordingly, an increased CD8+ cell infiltrate was found to correlate with a better FL prognosis.8,14,16
CD8+ cytotoxic T cells (CTLs) are important actors of the immune response against virally infected and tumor cells. They are activated by the engagement of their Ag receptors (TCR) with complexes formed between antigenic peptides and MHC class I molecules displayed on the surface of target cells. TCR signaling leads to the rapid secretion of the pore-forming protein perforin, granzyme B, and other proteases stocked in CTL cytoplasmic granules (named lytic granules) at the CTL/target cell contact site.17,18 Penetration of granzyme B in target cells triggers an apoptotic cascade ultimately leading to target cell annihilation.19
Several important questions remain unresolved concerning the role of CD8+ T cells in FL, including 3-dimensional (3-D) tissue distribution, cytotoxic equipment, lytic capacity, ability to form functional contacts with FL tumor cells as well as the relationship between these parameters and the clinical outcome.
In this study we used conventional IHC and 3-D confocal microscopy to investigate, in FL lymph nodes, the number and location of infiltrating CTLs as well as the cytolytic potential of individual CTL. Our results show that CTLs are enriched in interfollicular spaces in FL, express—at the individual cell level—high amounts of GrzB, form lytic synapse-like structures in contact with FL B cells, and exhibit lytic potential. Our study also indicates that GrzB expression correlates with prolonged PFS in FL patients treated with immunochemotherapy.
Methods
Patients
For IHC analysis, 80 FL patients diagnosed within 2 institutions (Department of Hematology, CHU Toulouse, France; and Department of Hematology, Catholic University, Rome, Italy) were included in this study between 2000 and 2009. All patient clinical data are described in Table 1. All patients were treated with immunochemotherapy (R-chemo) by combining rituximab with either CVP (cyclophosphamide, vincristine, and prednisone) (n = 15) or CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone; n = 58) or fludarabine mitoxantrone (n = 7). R-chemo was given as 6 (RCHOP) or 8 (RCVP) cycles. Patients treated with fludarabine and mitoxantrone received 6 cycles when aged < 60 years (n = 5) or 4 cycles when aged > 60 years (n = 2). Twelve patients received rituximab maintenance therapy (RCHOP + R or fludarabine mitoxantrone + R) in the context of the PRIMA study.20 Response to treatment was assessed according to the Cheson 1999 criteria.21
Clinical and histological features of the 80 FL cases
| Variables . | No. (%) . | PFS . | P value for PFS . | ||
|---|---|---|---|---|---|
| Median, mo . | 2-year survival, % (95 CI%) . | Univariate analysis . | Multivariate analysis . | ||
| Center | .0396 | .657 | |||
| French | 50 (63) | 62 | 73 (58-84) | ||
| Italian | 30 (37) | 85 | 85 (65-94) | ||
| Age, y | NC | NC | .18 | .179 | |
| Median | 58 | ||||
| Range | 26-83 | ||||
| Hazard ratio | 1.02 | ||||
| Sex | .0742 | .003 | |||
| Male | 39 (49) | 40 | 70 (53-82) | ||
| Female | 41 (51) | 76 | 86 (69-94) | ||
| Histologic grade | .8519 | ||||
| 1 | 42 (53) | 62 | 79 (62-89) | ||
| 2 | 24 (30) | NR | 86 (62-95) | ||
| 3 | 14 (17) | 76 | 63 (33-83) | ||
| Architectural pattern | .9560 | ||||
| Follicular | 71 (90) | 70 | 76 (64-85) | ||
| Follicular and diffuse | 6 (8) | NR | 83 (27-97) | ||
| Diffuse | 3 (2) | 62 | 100 | ||
| Stage | .7422 | ||||
| I | 2 (2) | 19 | 50 (1-91) | ||
| II | 7 (9) | NR | 83 (27-97) | ||
| III | 16 (20) | 76 | 66 (37-84) | ||
| IV | 55 (69) | 70 | 82 (68-90) | ||
| B symptoms | .0982 | .252 | |||
| Present | 28 (35) | 70 | 68 (47-83) | ||
| Absent | 52 (65) | 85 | 83 (69-91) | ||
| BM involvement | .9270 | ||||
| Present | 48 (60) | 70 | 79 (64-89) | ||
| Absent | 32 (40) | 76 | 76 (56-88) | ||
| FLIPI* | .9797 | ||||
| Low risk (0-1) | 15 (19) | 62 | 86 (54-96) | ||
| Intermediate risk (2) | 26 (33) | NR | 79 (57-91) | ||
| High (≥ 3) | 38 (48) | 70 | 74 (55-85) | ||
| GELF | .3138 | ||||
| GELF = 0 | 14 (18) | 62 | 85 (52-96) | ||
| GELF > 0 | 65 (82) | 70 | 76 (62-85) | ||
| Primary treatment | .2685 | .015 | |||
| R-chemotherapy | 68 (85) | 70 | 77 (64-86) | ||
| R-chemotherapy + R | 12 (15) | NR | 82 (45-95) | ||
| Treatment response* | .0015 | .004 | |||
| CR or CRu† | 61 (76) | 70 | 87 (74-93) | ||
| PR‡ | 18 (23) | 28 | 50 (26-70) | ||
| CD8 | .0202 | ||||
| < 10% | 4 (5) | 12 | 50 (6-84) | — | |
| 10%-30% | 39 (49) | 40 | 69 (50-81) | .627 | |
| ≥ 30% | 37 (46) | 85 | 91 (75-97) | .300 | |
| GrzB score | .0008 | < .0001 | |||
| Low | 32 (40) | 40 | 59 (40-74) | ||
| High | 48 (60) | 85 | 93 (79-98) | ||
| Variables . | No. (%) . | PFS . | P value for PFS . | ||
|---|---|---|---|---|---|
| Median, mo . | 2-year survival, % (95 CI%) . | Univariate analysis . | Multivariate analysis . | ||
| Center | .0396 | .657 | |||
| French | 50 (63) | 62 | 73 (58-84) | ||
| Italian | 30 (37) | 85 | 85 (65-94) | ||
| Age, y | NC | NC | .18 | .179 | |
| Median | 58 | ||||
| Range | 26-83 | ||||
| Hazard ratio | 1.02 | ||||
| Sex | .0742 | .003 | |||
| Male | 39 (49) | 40 | 70 (53-82) | ||
| Female | 41 (51) | 76 | 86 (69-94) | ||
| Histologic grade | .8519 | ||||
| 1 | 42 (53) | 62 | 79 (62-89) | ||
| 2 | 24 (30) | NR | 86 (62-95) | ||
| 3 | 14 (17) | 76 | 63 (33-83) | ||
| Architectural pattern | .9560 | ||||
| Follicular | 71 (90) | 70 | 76 (64-85) | ||
| Follicular and diffuse | 6 (8) | NR | 83 (27-97) | ||
| Diffuse | 3 (2) | 62 | 100 | ||
| Stage | .7422 | ||||
| I | 2 (2) | 19 | 50 (1-91) | ||
| II | 7 (9) | NR | 83 (27-97) | ||
| III | 16 (20) | 76 | 66 (37-84) | ||
| IV | 55 (69) | 70 | 82 (68-90) | ||
| B symptoms | .0982 | .252 | |||
| Present | 28 (35) | 70 | 68 (47-83) | ||
| Absent | 52 (65) | 85 | 83 (69-91) | ||
| BM involvement | .9270 | ||||
| Present | 48 (60) | 70 | 79 (64-89) | ||
| Absent | 32 (40) | 76 | 76 (56-88) | ||
| FLIPI* | .9797 | ||||
| Low risk (0-1) | 15 (19) | 62 | 86 (54-96) | ||
| Intermediate risk (2) | 26 (33) | NR | 79 (57-91) | ||
| High (≥ 3) | 38 (48) | 70 | 74 (55-85) | ||
| GELF | .3138 | ||||
| GELF = 0 | 14 (18) | 62 | 85 (52-96) | ||
| GELF > 0 | 65 (82) | 70 | 76 (62-85) | ||
| Primary treatment | .2685 | .015 | |||
| R-chemotherapy | 68 (85) | 70 | 77 (64-86) | ||
| R-chemotherapy + R | 12 (15) | NR | 82 (45-95) | ||
| Treatment response* | .0015 | .004 | |||
| CR or CRu† | 61 (76) | 70 | 87 (74-93) | ||
| PR‡ | 18 (23) | 28 | 50 (26-70) | ||
| CD8 | .0202 | ||||
| < 10% | 4 (5) | 12 | 50 (6-84) | — | |
| 10%-30% | 39 (49) | 40 | 69 (50-81) | .627 | |
| ≥ 30% | 37 (46) | 85 | 91 (75-97) | .300 | |
| GrzB score | .0008 | < .0001 | |||
| Low | 32 (40) | 40 | 59 (40-74) | ||
| High | 48 (60) | 85 | 93 (79-98) | ||
Final cox model contains score, treatment response, primary treatment, and sex.
FL indicates follicular lymphoma; PFS, progression free-survival; CI, confidence interval; NC, not calculated; NR, not reached; FLIPI, Follicular Lymphoma International Prognostic Index; GELF, Groupe d'Etude des Lymphomes Folliculaires; CR, complete response; PR, partial response; CRu, CR unconfirmed; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CVP, rituximab, cyclophosphamide, vincristine, and prednisone; R-CHOP + R, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone and rituximab maintenance; R-Fludarabine-mitoxantrone + R, rituximab, fludarabine, mitoxantrone, rituximab maintenance; and GrzB, granzyme B.
One patient had no remission after treatment.
Complete response and complete response undefined (according to Cheson criteria [18]).
Partial response.
For confocal microscopy analysis, 10 additional FL patients diagnosed between 2008 and 2010 in 2 institutions (n = 8 in Department of Hematology, CHU Toulouse and n = 2 in Department of Hematology, Catholic University, Rome) were included in this study. The median age was 62 years (ranged from 42 to 74 years). The male/female ratio was 1/1. According to Ann Arbor staging, 8 patients (80%) were in advanced stages (stage III-IV).
All patients, included in both IHC and confocal microscopy analysis, were studied at the time of diagnosis. Institutional ethical approval from Inserm U1043 and informed consent were obtained in compliance with the Helsinki protocol.
IHC
For IHC examination, 3-μm-thick sections from paraffin-embedded whole tissue of 80 FL lymph nodes fixed in 10% formalin or Dubosq-Brazil (alcohol-based Bouin) were tested using either a Ventana Benchmark XT immunostainer (Ventana) or a TechMate (DAKO). In parallel, 3-μm-thick sections from the same patients were H&E stained. For IHC, the panel included Abs directed against CD3 (A452 Poly, dilution 1:25; DAKO), CD8 (clone C8/144b, dilution 1:10; DAKO), and GrzB (clone GrB-7, dilution 1:30; DAKO). To characterize CD8+GrzB+ cells, double staining was performed with anti-CD8 and anti-GrzB Abs. For GrzB staining, samples were grouped according to their scores as indicated in Table 2. GrzB staining was scored by 3 pathologists (C.L., P.B., and L.M.L.) in a blinded fashion. Interrater agreement was estimated by Cohen κ coefficient (κ), according to Landis and Koch magnitude guidelines.22 Agreement for the GrzB score was almost “perfect” (κ = 0.84, P < .0001). However, agreement for intensity staining and percentage of GrzB+ cells were “substantial” (κ = 0.74, P < .0001 and κ = 0.6616, P < .0001, respectively).
Clinical features and IHC score for CD8 and GrzB staining of the 80 FL cases
| Variable . | Immunohistochemical analysis . | P . | |
|---|---|---|---|
| Group A . | Group B . | ||
| High GrzB . | Low GrzB . | ||
| % [95% CI], N = 48 . | % [95% CI], N = 32 . | ||
| Median age (range), y | 57.5 (26-83) | 58 (33-78) | .8742* |
| Sex | .121† | ||
| Male | 58 (n = 28) [43-72] | 41 (n = 13) (24-59] | |
| Female | 42 (n = 20) [28-57] | 59 (n = 19) [41-76] | |
| Stage | .628‡ | ||
| I | 4 (n = 2) [1-14] | 0 (0-11] | |
| II | 8 (n = 4) [1-20] | 9 (n = 3) [2-25] | |
| III | 17 (n = 8) [7-30] | 25 (n = 8) [11-43] | |
| IV | 71 (n = 34) [56-83] | 66 (n = 21) [47-81] | |
| FLIPI | .183† | ||
| Low risk (0-1) | 26 (n = 12) [14-40] | 9 (n = 3) [2-25] | |
| intermediate risk (2) | 32 (n = 15) [19-47] | 35 (n = 11) [19-53] | |
| High (≥ 3) | 42 (n = 20) [28-58] | 56 (n = 18) [38-74] | |
| GELF | .109* | ||
| GELF = 0 | 23 (n = 11) [12-38] | 9 (n = 3) [2-25] | |
| GELF > 0 | 77 (n = 36) [62-88] | 91 (n = 29) [75-98] | |
| BM involvement | 58 (n = 28) [43-72] | 63 (n = 20) [44-79] | .748* |
| Treatment | .592‡ | ||
| R-CHOP | 65 (n = 31) [49-78] | 50 (n = 16) [32-68] | |
| R-CVP | 17 (n = 8) [7-30] | 22 (n = 7) [9-40] | |
| R Fludarabine-mitoxantrone | 6 (n = 3) [13-17] | 9 (n = 3) [2-25] | |
| R-CHOP + R | 10 (n = 5) [3-23] | 19 (n = 6) [7-36] | |
| R Fludarabine-mitoxantrone + R | 2 (n = 1) [0-11] | 0 (0-11) | |
| Immunochemistry | |||
| % of CD8-positive cells | .001‡ | ||
| 1: < 10% | 0 (0-7) | 12 (n = 4) [4-29] | |
| 2: 10%-30% | 37 (n = 18) [24-53] | 66 (n = 21) [47-81] | |
| 3: 30%-40% | 44 (n = 21) [29-59] | 16 (n = 5) [5-33] | |
| 4: > 40% | 19 (n = 9) [9-33] | 6 (n = 2) [8-21] | |
| Score GrzB | |||
| % of GrzB-positive cells | NC | ||
| 1: < 10% | 8 (n = 4) [2-20] | 66 (n = 21) [47-81] | |
| 2: 10%-30% | 48 (n = 23) [33-63] | 34 (n = 11) [19-53] | |
| 3: > 30% | 44 (n = 21) [29-59] | 0 (0-11) | |
| Intensity of GrzB staining | NC | ||
| 1: + | 6 (n = 3) [1-17] | 75 (n = 24) [57-89] | |
| 2: ++ | 44 (n = 21) [29-59] | 25 (n = 8) [11-43] | |
| 3: +++ | 50 (n = 24) [35-65] | 0 (0-15) | |
| Variable . | Immunohistochemical analysis . | P . | |
|---|---|---|---|
| Group A . | Group B . | ||
| High GrzB . | Low GrzB . | ||
| % [95% CI], N = 48 . | % [95% CI], N = 32 . | ||
| Median age (range), y | 57.5 (26-83) | 58 (33-78) | .8742* |
| Sex | .121† | ||
| Male | 58 (n = 28) [43-72] | 41 (n = 13) (24-59] | |
| Female | 42 (n = 20) [28-57] | 59 (n = 19) [41-76] | |
| Stage | .628‡ | ||
| I | 4 (n = 2) [1-14] | 0 (0-11] | |
| II | 8 (n = 4) [1-20] | 9 (n = 3) [2-25] | |
| III | 17 (n = 8) [7-30] | 25 (n = 8) [11-43] | |
| IV | 71 (n = 34) [56-83] | 66 (n = 21) [47-81] | |
| FLIPI | .183† | ||
| Low risk (0-1) | 26 (n = 12) [14-40] | 9 (n = 3) [2-25] | |
| intermediate risk (2) | 32 (n = 15) [19-47] | 35 (n = 11) [19-53] | |
| High (≥ 3) | 42 (n = 20) [28-58] | 56 (n = 18) [38-74] | |
| GELF | .109* | ||
| GELF = 0 | 23 (n = 11) [12-38] | 9 (n = 3) [2-25] | |
| GELF > 0 | 77 (n = 36) [62-88] | 91 (n = 29) [75-98] | |
| BM involvement | 58 (n = 28) [43-72] | 63 (n = 20) [44-79] | .748* |
| Treatment | .592‡ | ||
| R-CHOP | 65 (n = 31) [49-78] | 50 (n = 16) [32-68] | |
| R-CVP | 17 (n = 8) [7-30] | 22 (n = 7) [9-40] | |
| R Fludarabine-mitoxantrone | 6 (n = 3) [13-17] | 9 (n = 3) [2-25] | |
| R-CHOP + R | 10 (n = 5) [3-23] | 19 (n = 6) [7-36] | |
| R Fludarabine-mitoxantrone + R | 2 (n = 1) [0-11] | 0 (0-11) | |
| Immunochemistry | |||
| % of CD8-positive cells | .001‡ | ||
| 1: < 10% | 0 (0-7) | 12 (n = 4) [4-29] | |
| 2: 10%-30% | 37 (n = 18) [24-53] | 66 (n = 21) [47-81] | |
| 3: 30%-40% | 44 (n = 21) [29-59] | 16 (n = 5) [5-33] | |
| 4: > 40% | 19 (n = 9) [9-33] | 6 (n = 2) [8-21] | |
| Score GrzB | |||
| % of GrzB-positive cells | NC | ||
| 1: < 10% | 8 (n = 4) [2-20] | 66 (n = 21) [47-81] | |
| 2: 10%-30% | 48 (n = 23) [33-63] | 34 (n = 11) [19-53] | |
| 3: > 30% | 44 (n = 21) [29-59] | 0 (0-11) | |
| Intensity of GrzB staining | NC | ||
| 1: + | 6 (n = 3) [1-17] | 75 (n = 24) [57-89] | |
| 2: ++ | 44 (n = 21) [29-59] | 25 (n = 8) [11-43] | |
| 3: +++ | 50 (n = 24) [35-65] | 0 (0-15) | |
On the basis of GrzB staining patients were classified in two groups: group A (high GrzB score) and group B (low GrzB score). Group A (high GrzB score) includes patients having score 4, 5, or 6. Group B (low GrzB score) includes patients having score 1, 2, or 3. Scores were calculated by adding values corresponding to: (i) the number of GrzB+ cells (ranging from 1 to 3: with 1 = < 10% GrzB+ cells; 2 = 10%-30% GrzB+ cells; 3 = > 30% GrzB+ cells out of the CD8+ cells) and (ii) values corresponding to the intensity of GrzB staining (ranging from 1 to 3: with 1 = +; 2 = ++; 3 = +++, as shown in supplemental Figure 1).
FL indicates follicular lymphoma; IHC, immunohistochemistry; CI, confidence interval; NC, not calculated; NR, not reached; FLIPI, Follicular Lymphoma International Prognostic Index; GELF, Groupe d'Etude des Lymphomes Folliculaires; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CVP, rituximab, cyclophosphamide, vincristine, and prednisone; R-CHOP + R, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone and rituximab maintenance; R-Fludarabine-mitoxantrone + R, rituximab, fludarabine, mitoxantrone, rituximab maintenance; and GrzB, granzyme B.
Student test.
χ2 test.
Fischer exact test.
Confocal microscopy
For confocal microscopy, samples from 10 FL lymph nodes and 5 reactive lymph nodes were fixed in 4% paraformaldehyde and frozen to perform 10 to 15-μm-thick cryostat cutting sections. Samples were pretreated by microwave incubation in pH 6.0, 0.1M sodium citrate. Samples were then permeabilized with 0.1% saponin (in PBS 3% BSA/HEPES, 10% goat serum, and 10% human serum), and stained overnight at 4°C with the following 3 primary Abs: CD3 (A452 Poly, dilution 1:25; DAKO), CD8 (clone C8/144b, dilution 1:10; DAKO) and Granzyme B (clone GrB-7, dilution 1:30; DAKO); CD79a (clone JCB117, dilution 1:25; DAKO); CD20 (clone L26, dilution 1:40; DAKO); active caspase 3 (a-CASP3; rabbit polyclonal, dilution 1:1000 Abcam); CD137 (clone BBK-2, dilution 1:30; Abcam) in PBS 3% BSA/HEPES, 0.1% saponin. Primary Abs were followed by goat anti–mouse isotype-specific Ab or goat anti–rabbit Abs labeled with Alexa 488, Alexa 633, and Alexa 555 (Molecular Probes) for 2 hours at room temperature.
In some experiments, CD8+ T cells were obtained from patient peripheral blood or from FL lymph nodes by negative selection using microbeads (Miltenyi Biotec). Freshly isolated CD8+ T-cell populations were cultured in RPMI 1640 medium, supplemented with 5% human serum and IL-2 (250 U/mL) in the presence of anti-CD3/CD28 mAb-coated Dynabeads (Invitrogen) at a ratio of 1 bead for 10 T cells. Expanded T cells were used between 15 and 20 days of culture. CTLs from FL peripheral blood or from lymph node tissue were cocultured with either EBV-transformed B cells (JY)23 or with autologous FL B cells, respectively, which were either unpulsed or pulsed with bacterial superantigen cocktail (SAg, 100 ng/mL TSST-1, SEC 1, SEB, SEE). After 1 hour of culture, cells were stained with Abs directed against a-CASP3, CD8 (clone UCH-T4, 1:40; Santa Cruz Biotechnology) and GrzB (clone GB11, 1:40; Santa Cruz Biotechnology).23 The samples were mounted in Fluorescence Mounting Medium (DAKO) and examined using a Zeiss LSM 510 or a Zeiss LSM 710 confocal microscope with a 63× Plan-Apochromat objective (1.4 oil). An argon laser at 488 nm was used to detect Alexa 488 fluorochrome. To detect Alexa 555 fluorescence, a helium laser was filtered at 543 nm. To detect Alexa 633 fluorescence, a helium laser was filtered at 633 nm. Under standard imaging conditions no signal from one fluorochrome could be detected with the other filter set. For each pair of Abs used, standardized conditions for pinhole size, and for gain and offset (brightness and contrast), were used for image capture. For 3-D images, a series of 15 to 25 z-sections taken at 0.5-μm distance was acquired for each T-cell–APC conjugate. Three-dimensional reconstruction of the images was performed using the Zeiss LSM software.
Confocal image quantification
All analyses were performed using Metamorph Imaging System software (Molecular Devices Corp). Ten FL versus 5 reactive lymph nodes were analyzed. To score CD3+CD8+GrzB+ cells, at least 5 fields were quantified for each sample. The number of CD3+CD8+GrzB+ cells was obtained as follows: CD3+CD8+ positive cells were identified by superposing for each field the CD3 (blue) staining with the CD8 (green) staining. The mask corresponding to CD3+CD8+ positive cells was superimposed to the GrzB (red) staining. CD3+CD8+GrzB+ cells were scored manually. The mean intensity of GrzB red fluorescence of individual CD3+CD8+GrzB+ cells, present in each field, was calculated by dividing the total GrzB+ red pixels by the number of CD3+CD8+GrzB+ cells scored. For the different samples standardized conditions for pinhole size, and for gain and offset (brightness and contrast), were used for image capture.
To score a-CASP3+ cells in contact with CD8+GrzB+ 13 to 19 confocal z-sections were acquired from 5 fields of 7 FL lymph nodes. The contact between cells was scored on 3-D reconstructed images by visual inspection.
Measurement of CTL-mediated cytoxicity
FL lymph nodes were gently teased in RPMI 1640 medium (Invitrogen) to obtain single-cell suspensions. The sheep RBCs (SRBCs) rosette method was used to separate T cells from B cells. SRBC rosette-based T-cell selection allowed the parallel isolation of FL B cells and CD8+ cells from relatively small fragments of FL lymph nodes and was instrumental to enhance the efficiency of CD8+ cell purification by negative selection. Briefly, FL lymph node cell suspensions were incubated with AET-SRBC during 30 minutes at 4°C and separated on a Ficoll gradient. FL B cells localized at the interface while T cells/AET-SRBC rosettes localized in the pellet. SRBC rosettes containing T cells were briefly incubated with sterile distilled water to destroy SRBC and then washed 2 times in PBS. CD8+ T cells were obtained by negative selection using microbeads (Miltenyi Biotec). T-cell and FL B cell purity was > 95%.
Freshly isolated CD8+ T-cell populations were cultured in RPMI 1640 medium, supplemented with 5% human serum and IL-2 (250 U/mL) in the presence of anti-CD3/CD28 mAb-coated Dynabeads (Invitrogen) at a ratio of 1 bead for 10 T cells. Expanded T cells were used between 15 and 20 days of culture.
In vitro–expanded CTLs were conjugated with target cells either unpulsed or pulsed with a bacterial superantigen cocktail (SAg, 100 ng/mL TSST-1, SEC 1, SEB, SEE) for 4 hours at a 2:1 E T ratio. To be excluded from the analysis, CTLs were labeled before conjugation with 1μM CMFDA (Molecular Probes) for 20 minutes at 37°C. Before FACS analysis, 0.125 μg/mL propidium iodide (PI; Molecular Probes) was added to each sample.
Statistical methods
The Student t test or the Wilcoxon-Mann-Whitney tests were used to compare FL lymph nodes with reactive lymph nodes for CTL infiltration as indicated in the Figure 2 legend. Analyses of T cells/a-CASP3+ cell contacts and killing assay measurement were performed using the paired t test (see Figure 3, supplemental Figure 4, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To compare clinical and histological characteristics between patients of group A and B, we performed a χ2 test or a Fischer exact test for qualitative variables and a Student t test for quantitative variables (Table 2).
For time-to-event analyses (see Figure 4, supplemental Figure 5), primary end point for PFS analysis was defined as time from the end of R-chemotherapy to progression. For univariate analysis, we performed Kaplan-Meier curves and log-rank test to assess association of granzyme B score with progression. We used the same tests to assess significance of GELF or FLIPI parameters, and number of CD8+ cells. Cox proportional-hazards model and Cox proportional-hazards regression curve wereperformed to test the simultaneous influence of all covariates on PFS with a P value < .30 in the univariate analysis. Using a backward stepwise removal method, only significant covariates were kept in the final Cox model.
All tests were 2-sided and statistical significance was set at a P value of .05. Analyses were performed using STATA Version 11.
Results
Infiltration of CD8+ granzyme B+ cells in the interfollicular spaces of FL lymph nodes
We investigated, on pretreatment lymph node biopsies of 80 FL patients (Table 1) CD8+GrzB+ cell infiltration by IHC. The majority of patients included in our study were referred to our institutions for treatment. For this reason, most of the patients displayed intermediate- or high-risk factors based on both FLIPI and GELF stratification as depicted in Table 1. The criterion of inclusion of a given patient in the study was treatment with R-chemotherapy. Three pathologists, ignoring patient clinical data, scored biopsies in a blinded fashion (see “IHC”).
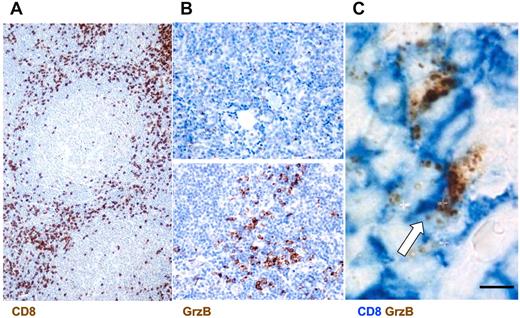
This analysis showed a rich infiltrate of CD8+ cells in the interfollicular spaces of FL lymph nodes (Figure 1A). CD8+ cells represented ∼ 30% of the total immune cell infiltrate ranging from 10% to 50% in the different patients (Table 2). In parallel, we scored the number and distribution of GrzB+ cells and the intensity of GrzB staining. This analysis showed that GrzB+ cells were enriched in the interfollicular spaces. They represented ∼ 15% of the total immune cells ranging from 1% to 30% in the different patients. The number of positive cells and the intensity of GrzB staining allowed us to calculate GrzB score for each patient as described in Table 2.
Infiltration of CD8+ GrzB+ cells in the interfollicular spaces of FL lymph nodes. (A) Representative section from FL lymph nodes of cells positive for CD8 as detected by IHC (original magnification ×200). (B) Examples of low (top panel) and high GrzB (bottom panel) staining (Original magnification, 400). (C) Double staining for CD8+GrzB+ cells. White arrow indicates double-positive cells (Original magnification, ×1000).
Infiltration of CD8+ GrzB+ cells in the interfollicular spaces of FL lymph nodes. (A) Representative section from FL lymph nodes of cells positive for CD8 as detected by IHC (original magnification ×200). (B) Examples of low (top panel) and high GrzB (bottom panel) staining (Original magnification, 400). (C) Double staining for CD8+GrzB+ cells. White arrow indicates double-positive cells (Original magnification, ×1000).
Scores were calculated by adding values corresponding to: (1) the number of GrzB+ cells (ranging from 1 to 3: with 1 = < 10% GrzB+ cells; 2 = 10%-30% GrzB+ cells; 3 = > 30% GrzB+ cells out of the CD8+ cells) and (2) values corresponding to the intensity of GrzB staining (ranging from 1 to 3: with 1 = +; 2 = ++; 3 = +++, as shown in supplemental Figure 1).
This GrzB score (based on percentage of GrzB+ cells out of CD8+ cells and on the intensity of GrzB staining) also allowed us to define 2 groups of patients: one exhibiting high GrzB staining in the interfollicular spaces (group A, corresponding to a GrzB score ≥ 4) and the other exhibiting a low GrzB staining (group B, corresponding to a GrzB score < 4, Table 2). Typical examples of low and high GrzB staining are depicted in Figure 1B.
Double staining for CD8 and GrzB confirmed the single staining data by showing that CD8+ cells, enriched in the FL interfollicular spaces, exhibited a granular intracellular GrzB staining (Figure 1C).
Taken together, the above results show a rich infiltrate of CD8+ and GrzB+ cells in the interfollicular spaces of FL lymph nodes and identify 2 groups of patients based on GrzB staining characteristics.
3-D distribution and activation status of CTL-infiltrating FL lymph nodes
The above-presented data, by showing that CD8+GrzB+ cells infiltrated FL interfollicular spaces, suggested that lytic granule-positive CTLs might be recruited to areas surrounding FL tumor nodules.
To better define the phenotype and the localization of these cells and to score their number using a nonsubjective method, we used confocal laser scanning microscopy allowing 3-color staining and 3-D reconstruction of acquired images.
Cryostat sections of new cohort of 10 FL and 5 control reactive lymph nodes were stained for CD3, CD8, and GrzB and inspected by acquiring high magnification fields in the interfollicular spaces. As shown in Figure 2A and supplemental Video 1, in FL lymph nodes a significant fraction of CD3+CD8+ T cells contained GrzB+ lytic granules, whereas GrzB+ cells were rare in reactive lymph nodes. Scoring of the total number of CD3+CD8+GrzB+ cells in at least 5 fields per each patient showed that in FL lymph nodes the number of armed CTLs was significantly increased (Figure 2B). To better characterize the microanatomy of CTL infiltration in FL lymph nodes, we performed 3-D reconstruction of confocal microscopy images taken at the follicular border. For this analysis, FL B cells were detected by CD79a staining and CTLs were detected by double staining for CD3 and GrzB. As shown in supplemental Video 2, this analysis showed that most of GrzB+ cells did not enter the FL nodules but were mostly located around the nodules, where they appeared to “attack” FL B cells. Interestingly, the establishment of contacts between CTLs and FL B cells, reminiscent of lytic synapses formed in vitro by human CTLs,23,24 was observed (supplemental Video 3).
Accumulation of activated CTLs expressing high levels of GrzB in FL nodes as quantified by confocal laser scanning microscopy. Tissue sections from FL or reactive lymph nodes were stained for CD8 (green), CD3 (blue), and GrzB (red). (A) Representative staining of CD3+CD8+GrzB+ cells in FL (top panels) or reactive (bottom panels) lymph nodes (bar = 10 μm in left panels and 5 μm in right panels). (B) Scoring of CD3+CD8+GrzB+ cells in the interfollicular area of 10 FL and 5 reactive lymph nodes. More than 5 fields from each of the 10 FL and of the 5 control lymph nodes were randomly scored. Statistical significance of difference between groups was evaluated by a Wilcoxon-Mann-Whitney test using STATA Version 11; P = .0024. (C) Measurement of GrzB expression in CD3+CD8+ cells in the interfollicular area of 10 FL and 5 reactive lymph nodes. Measurements for GrzB fluorescence intensity were performed as described in “Confocal Image Quantification.” More than 5 fields from each of the 10 FL and of the 5 control lymph nodes were randomly scored. Statistical significance of difference between groups was evaluated by a Student t test using STATA Version 11; P < .0001.
Accumulation of activated CTLs expressing high levels of GrzB in FL nodes as quantified by confocal laser scanning microscopy. Tissue sections from FL or reactive lymph nodes were stained for CD8 (green), CD3 (blue), and GrzB (red). (A) Representative staining of CD3+CD8+GrzB+ cells in FL (top panels) or reactive (bottom panels) lymph nodes (bar = 10 μm in left panels and 5 μm in right panels). (B) Scoring of CD3+CD8+GrzB+ cells in the interfollicular area of 10 FL and 5 reactive lymph nodes. More than 5 fields from each of the 10 FL and of the 5 control lymph nodes were randomly scored. Statistical significance of difference between groups was evaluated by a Wilcoxon-Mann-Whitney test using STATA Version 11; P = .0024. (C) Measurement of GrzB expression in CD3+CD8+ cells in the interfollicular area of 10 FL and 5 reactive lymph nodes. Measurements for GrzB fluorescence intensity were performed as described in “Confocal Image Quantification.” More than 5 fields from each of the 10 FL and of the 5 control lymph nodes were randomly scored. Statistical significance of difference between groups was evaluated by a Student t test using STATA Version 11; P < .0001.
We also investigated whether, at the individual cell level, CTLs present in FL interfollicular spaces might be “more armed” than CTLs in reactive lymph nodes. To address this question, we measured by confocal microscopy the expression of GrzB in individual CTL. This analysis was performed by measuring the mean fluorescence intensity of GrzB staining in the CD3+ and CD8+ cells present in > 5 fields (from each of the 10 FL and of the 5 control lymph nodes). As shown in Figure 2C, the mean fluorescence intensity of GrzB in FL cells was significantly higher.
Taken together, the above results extend the results of IHC by showing an enrichment of armed CTLs, exhibiting a strong content of GrzB, in the interfollicular spaces of FL lymph nodes.
FL-infiltrating CTLs exhibit a cytotoxic potential
We next investigated whether the CTLs enriched in the FL infiltrate were endowed of cytotoxicity potential. In a first step we used an in situ approach. We asked whether the presence of a rich infiltrate of GrzB+ CTLs could correlate with an increased induction of cell death in FL tissues. To address this question, lymph nodes from 7 FL patients were stained with an Ab directed against the activated form of caspase 3 (a-CASP3, a marker of apoptosis) in parallel with Abs directed against CD8+ and GrzB. Samples were visualized by 3-color confocal microscopy. As illustrated in Figure 3A and supplemental Video 4, a-CASP3+ cells, that exhibited cytosolic and/or nuclear staining, could be found at the follicular borders. Scoring of a-CASP3+ cells in 35 fields showed that the number of a-CASP3+ cells in contact with CD8+GrzB+ cells was significantly higher than the number of a-CASP3+ cells that were found not in contact with CD8+GrzB+ (Figure 3B). It should be noted that this analysis was performed by scoring the fields using 3-D reconstructed images to unambiguously define cellular contacts (see “Methods”). Nevertheless, this 3-color analysis did not allow the simultaneous visualization of CD8, GrzB, a-CASP3, and B-cell markers, raising the question of whether the a-CASP3+ cells were actually FL B cells. Two lines of evidence indicate that the scored a-CASP3+ cells are bona fide FL B cells. First, we performed this analysis at the border between the interfollicular space and the FL nodules were a “confrontation” between CTLs and FL B cells occurs (supplemental Videos 2-3). Second, parallel staining of FL samples for CD20, CD8, and a-CASP3 showed CD20+ a-CASP3+ cells in contact with CD8+ cells (supplemental Figure 2). We also investigated whether activation of CASP3 resembling to that observed in tissue staining could also be observed when the interaction of CTLs with target cells was studied in vitro. We initially addressed this question by using in vitro expanded CD8+ cells from patient peripheral blood with EBV-transformed B cells as target cells. CTLs were cocultured with target cells either unpulsed or pulsed with a cocktail of bacterial SAg. After 1 hour of culture, cells were stained with Abs directed against a-CASP3, CD8, and GrzB. Confocal laser-scanning visualization of cell-cell conjugates showed that CTL/target cell cognate interaction in vitro resulted in caspase 3 activation in target cells (supplemental Figure 3A-B). Similar results were obtained when in vitro–expanded CD8+ T cells from FL lymph nodes and autologous FL B cells were used (supplemental Figure 3C-D).
A significant fraction of a-CASP3+ cells is detected in contact with CD8+GrzB+ cells. Tissue sections from FL lymph nodes were stained for CD8 (green), a-CASP3 (blue) and GrzB (red). (A) Representative staining showing contact formation between CD8+GrzB+ cells and a-CASP3+ cells in a FL lymph node (bar = 5 μm). (B) Scoring of the total number of a-CASP3+ cells interacting with CD8+ or GrzB+ cells in 7 FL lymph nodes. The analysis was performed by scoring the fields using 3-D reconstructed images to unambiguously define cellular contacts (see “Confocal Image Quantification”). Statistical significance of difference between groups was evaluated by a paired Student t test using STATA Version 11; P = .0006.
A significant fraction of a-CASP3+ cells is detected in contact with CD8+GrzB+ cells. Tissue sections from FL lymph nodes were stained for CD8 (green), a-CASP3 (blue) and GrzB (red). (A) Representative staining showing contact formation between CD8+GrzB+ cells and a-CASP3+ cells in a FL lymph node (bar = 5 μm). (B) Scoring of the total number of a-CASP3+ cells interacting with CD8+ or GrzB+ cells in 7 FL lymph nodes. The analysis was performed by scoring the fields using 3-D reconstructed images to unambiguously define cellular contacts (see “Confocal Image Quantification”). Statistical significance of difference between groups was evaluated by a paired Student t test using STATA Version 11; P = .0006.
In a second step we used an in vitro approach. We investigated whether primary cell lines generated from CD8+ T cells infiltrating FL lymph nodes could exert cytotoxicity against autologous FL B cells pulsed with a cocktail of SAg. The rationale for this approach was to establish whether the balance between the in vitro efficiency of FL CTLs and intrinsic FL B cells resistance to cytotoxicity could be compatible with an in vivo cytotoxic activity. As shown in supplemental Figure 4, in vitro–expanded CD8+ T cells from FL lymph nodes exhibited cytotoxicity against SAg pulsed FL B cells.
Taken together, the above results show that FL-infiltrating CTLs establish cellular contacts with apoptotic cells in tissues and exhibit a cytotoxic activity against FL B cells in vitro. They suggest that FL CTLs play a role in the control of disease progression.
Infiltration of granzyme B+ cells in the interfollicular spaces of FL lymph nodes correlates with longer PFS
We finally asked whether GrzB staining in diagnostic biopsies might correlate with clinical outcome in our FL patient cohort. According to Cheson 1999 criteria,21 complete response (CR) or complete response unconfirmed (CRu) was observed in 76% whereas 23% of cases displayed partial response (PR). One patient died early. Mean and median follow-up were 32 and 26 months, respectively. The 2-year PFS and median PFS of this cohort was 78% (95% CI = 66-86) and 70 months, respectively (95% CI = 40-85).
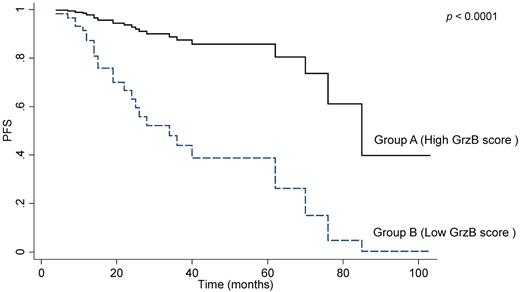
The comparison between group A (high GrzB staining score, as detected by IHC) and group B (low GrzB staining score, Figure 1) revealed no significant differences for BM infiltration, stage, FLIPI, or GELF profiles (Table 2), as well as for response rate. However, based on the entire cohort, group B patients displayed shorter PFS, compared with group A (Figure 4). Two-year PFS rates were also significantly different between group B and group A patients: 59% (95% CI = 40-74) versus 93% (95% CI = 70.5-97.9), (not adjusted P = .0008). Multivariate analysis revealed that only sex (HR = 4.3, 95% CI = 1.6-11.3, P = .003), R maintenance (HR = 0.15, 95% CI = 0.03-0.7, P = .015), response (PR versus CR or CRu; HR = 3.4, 95% CI = 1.5-8.1, P = .004) and GrzB score are independent predictors of PFS (HR = 6.8, 95% CI = 2.5-18.1, P < .0001). It should be noted that even though the analysis of GrzB score related to CD8+ cells correlated with clinical outcome, we did not find a significant correlation between the number of interfollicular CD8+ T cells and PFS of FL patients (Table 1).
Granzyme B is a prognostic marker of PFS in FL. The graph shows progression-free survival (PFS) in the 80 patients scored by IHC after R-combined chemotherapy. According to the GrzB scoring system, as described in “IHC,” association was found between a poor outcome of treatment and a low GrzB score.
Granzyme B is a prognostic marker of PFS in FL. The graph shows progression-free survival (PFS) in the 80 patients scored by IHC after R-combined chemotherapy. According to the GrzB scoring system, as described in “IHC,” association was found between a poor outcome of treatment and a low GrzB score.
This study was performed before the publication of the PRIMA study,20 which has shown PFS improvement in rituximab maintenance arm, compared with controls, in FL patients initially treated by R-chemotherapy whatever the design of chemotherapy (R-CHOP, R-CVP, R-fludarabine based). In our study, only 12 patients received rituximab in the context of the PRIMA study.20 Interestingly, in patients treated with R-chemotherapy without maintenance (n = 68), GrzB score also correlated with prolonged PFS (supplemental Figure 5).
Discussion
This work shows that CTLs are enriched in the interfollicular spaces of FL lymph nodes and that individual CTLs express high levels of GrzB. An IHC GrzB score was found to be highly predictive for PFS in FL patients treated with R-chemotherapy. It is interesting to note that, in the present study, while the score of GrzB related to CD8+ cells correlated with clinical outcome, the number of interfollicular CD8+ T cells did not correlate by itself with PFS (Table 1). These results suggest that the analysis of combined phenotypic and activation markers might be a suitable strategy to investigate CTL correlation with clinical outcome.
An innovative technical aspect of our study is that we investigated immune infiltration of FL lymphoid organs using both IHC and confocal microscopy, a technique rarely applied to the study of immunologic signatures in lymphomas. Using this approach, we show immunologic synapse-like CTL/B-cell contacts at the follicle border that have not been previously described.
The infiltration of FL lymph nodes by CD8+ cells, their preferential location in the interfollicular spaces, and the presence of CTLs in FL lymph nodes specimens have all been reported in earlier studies.8,13,14,25
However, our study characterizes these CD8+ cells as functional CTLs. First, a significant fraction of CD8+ cells displayed a high content of GrzB-containing cytotoxic granules, even though the percentage of CD3+CD8+GrzB+ cells, as well as the GrzB content per cell, varied among specimens. Second, primary cell lines of CD8+ T cells isolated from FL specimens exhibited cytotoxicity against autologous malignant B cells. Third, synapse-like contacts between CTLs and apoptotic cells were detected, in 3-D reconstructed confocal microscopy images, at the follicle border.
These results, taken together with the observation of a link between a strong CTL infiltration and a favorable disease course, suggest that activated CTLs could exhibit a “tonic control” over malignant FL B cells and therefore contribute to delay or limit disease development and/or relapses.
The sensitivity of FL cells to immune effectors is also suggested by the relative success of idiotype vaccination26 and the low rate of relapse after allogeneic stem cell transplantation performed in this context.27 This suggests that antiapoptosis equipment of FL cells, including Bcl-2 overexpression, deregulated Akt- or mTOR-dependent survival pathways,28 might not confer a high degree of protection against cellular cytotoxicity mediators such as the GrzB/perforin system. This idea is compatible with the observation that perforin deficient mice have a high incidence of malignancy in different lymphoid cell lineages, indicating a specific requirement for functional killer cells in the protection against lymphomagenesis.29
The correlation between CTL infiltration and favorable disease course does not exclude the possibility that FL B cells might exhibit a certain level of resistance to CTL attack. Indeed it has been described that both CD4+ and CD8+ human T cells from CLL and FL patients exhibit activation defects and defective immunologic synapses when conjugated with neoplastic B cells.30,31 We speculate that the observed strong enrichment of CTLs in interfollicular spaces could compensate for possible defects in CTL activation and/or effector function thus leading to a more favorable disease outcome.
Where does the “confrontation” between CTLs and FL take place? Our IHC and confocal microscopy analyses indicate that CTLs do not enter tumoral nodules but rather “intercept” FL B cells at the border of the nodules.
Our observations raise the question of whether the CTL-infiltrating FL lymph nodes are specific for Ags expressed in the tumor microenvironment. A first answer to this question came from our observation that a few CD3+GrzB+ cells are positive for the activation marker CD137 (data not shown). CD137 up-regulation has been recently proposed as an accurate marker for CTL activation via TCR.32 Thus a small, but significant, fraction of CTLs detected in FL lymph nodes might have been recently activated via TCR engagement with Ag displayed in the tumor microenvironment.
Previous studies described a correlation between the intensity of CD8+ cell infiltration and patient survival for solid tumors, including colon, breast, and ovarian carcinomas,15 as well as for FL.8,14,16 However, GrzB expression as a biomarker has received little attention since the study of Lee et al who showed using a TMA approach, no correlation between GrzB staining and FL survival in a 60 patient cohort.13 The latter study is in apparent contrast with our study because we show that CD8+-associated GrzB expression predicts a more favorable clinical outcome in FL patients, all treated with R-chemotherapy. This discrepancy can be explained by the different techniques used and, more importantly, by the type of patients (less advanced forms) as well as the longer period of recruitment (1974-1999) in the study by Lee et al,13 suggesting that most patients had not been treated with R-chemotherapy. However, we have to concede that most of our patients (85%) did not receive rituximab maintenance, a modality of treatment that is now part of FL therapy based on the PRIMA study.20 Whether granzyme B might serve as a biomarker beside other immunologic signatures33 at the era of R-chemotherapy plus rituximab maintenance remains to be investigated in prospective studies.
It is interesting to note that the granzyme B score was not found to correlate with initial FLIPI or GELF profile, suggesting that the activation status of CD8+ T cells influences more significantly the progression of the disease after R-chemotherapy than the natural history of the FL. This is an intriguing, but potentially important, observation because it suggests that R-chemotherapy could favor CD8+-mediated antitumor response. The role of chemotherapy as a stimulator of antitumor adoptive immunity has been recently proposed.34 It is also possible that rituximab renders more efficient CTL-mediated anti-FL response as suggested by a recent study in which rituximab attack of FL cells resulted in the induction of lymphoma idiotype-specific T-cell responses.35
In conclusion, we show that FL displays a potent CTL-mediated immune response and that a strong CTL infiltrate correlates with a positive FL prognosis after R-combined chemotherapy. Further research is required to define the cellular and molecular mechanisms by which activated CTLs might synergize with R-combined chemotherapy to influence FL outcome. Our results also provide a basis for future immune intervention strategies aiming at CTL stimulation in combination with rituximab.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank L. Dupré and L. Ysebaert for discussion, and M. March for IHC experiments. They also thank the Plateau technique de cytométrie Inserm U1063.
This work was supported by grants from Association pour la Recherche sur le Cancer, Fondation Banque Nationale de Paris Paribas, Institut National du Cancer (S.V.) and by a grant from the Société Française de Pathologie (C.L.).
Authorship
Contribution: C.L., S.M., T.A.-S., S.A., L.M.L., S.H., S.D., A.Q.-M., and G.L. collected and analyzed data; C.L., G.L., and S.V. designed the research and wrote the paper; C.L. and S.M. performed experiments; C.D. provided expert statistic analysis; S.H. and G.L. provided patient sample clinical information and analysis of outcome; and C.L., L.M.L., and P.B. provided expert histopathologist analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Salvatore Valitutti, Inserm U1043, CHU Purpan, 31059 Toulouse Cedex 3, France; e-mail: salvatore.valitutti@inserm.fr.
References
Supplemental data
For each video, you will need software that can open ZIP documents, such as WinZIP, Stuffit, or 7-Zip. A tissue section from a FL lymph node was stained for CD8 (green), a-CASP (blue) and GrzB (red). A detail of an a-CASP3+ cell in contact with CD8+GrzB+ cells is shown. 3-D reconstruction of z-sections was performed using the Zeiss LSM software.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal