Abstract
We evaluated 190 children with very high-risk leukemia, who underwent allogeneic hematopoietic cell transplantation in 2 sequential treatment eras, to determine whether those treated with contemporary protocols had a high risk of relapse or toxic death, and whether non–HLA-identical transplantations yielded poor outcomes. For the recent cohorts, the 5-year overall survival rates were 65% for the 37 patients with acute lymphoblastic leukemia and 74% for the 46 with acute myeloid leukemia; these rates compared favorably with those of earlier cohorts (28%, n = 57; and 34%, n = 50, respectively). Improvement in the recent cohorts was observed regardless of donor type (sibling, 70% vs 24%; unrelated, 61% vs 37%; and haploidentical, 88% vs 19%), attributable to less infection (hazard ratio [HR] = 0.12; P = .005), regimen-related toxicity (HR = 0.25; P = .002), and leukemia-related death (HR = 0.40; P = .01). Survival probability was dependent on leukemia status (first remission vs more advanced disease; HR = 0.63; P = .03) or minimal residual disease (positive vs negative; HR = 2.10; P = .01) at the time of transplantation. We concluded that transplantation has improved over time and should be considered for all children with very high-risk leukemia, regardless of matched donor availability.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is an established treatment for children with high-risk acute myeloid leukemia (AML) and very high-risk acute lymphoblastic leukemia (ALL).1,2 Some cooperative group studies have shown that the survival rates for AML patients treated with HLA-identical sibling HCT are superior to those of patients treated with chemotherapy alone,3,4 leading to a category B recommendation in favor of HCT by the American Society for Blood and Marrow Transplantation Evidence-Based Review Committee.2 Similarly, HCT produced higher cure rates in patients with ALL with t(9;22) (before the advent of tyrosine kinase inhibitors), t(4;11), or induction failure.5-9 In addition, patients with persistent minimal residual disease (MRD) or hematologic relapse while on therapy are also considered candidates for HCT in current protocols.10-13
As contemporary chemotherapy becomes more effective, the proportion of patients undergoing HCT is reduced. For example, patients with core-binding-factor AML or high-risk ALL are now typically treated with chemotherapy alone.8,14 Therefore, a key question now is whether HCT benefits patients whose leukemia is chemo-resistant to current intensive chemotherapy. One concern regarding the use of HCT in these patients is that its toxicity may be excessive because of preexisting organ dysfunction or infections caused by prior intensive chemotherapy. Another concern is that the ability of HCT to eradicate residual leukemia could be significantly reduced by the enhanced resilience of leukemic cells that survive highly intensive chemotherapy regimens. Finally, the role of HCT from alternative donor when matched sibling is not available is unclear in these patient populations.11,12 Besides their direct relevance to patient care, these questions are also important for the implementation of rational healthcare policies because HCT carries high costs and is associated with many chronic health conditions.15,16
We recently completed 2 leukemia treatment protocols that produced excellent overall treatment outcomes for pediatric patients with ALL and AML.17,18 In this context, we sought to evaluate the outcomes and causes of death of the 83 patients with very high-risk ALL or high-risk AML who underwent allogeneic HCT after receiving intensive multiagent chemotherapy in these protocols. We compared their results with those of 107 patients who were treated in previous protocols. The specific aims of this study were to determine whether patients who were treated with contemporary protocols had a higher risk of relapse or toxic death after HCT and whether non–HLA-identical HCT yielded poor outcomes.
Methods
Patients and donors
All of the 190 HCT patients were enrolled before transplantation in one of the following institutional leukemia treatment protocols: AML97 (1997-2002)19 or AML02 (2002-2008)17 for AML patients; Total Therapy 13 (1991-1998),20 Total Therapy 14 (1998-1999),20 or Total Therapy 15 (2000-2007)18 for ALL patients. The key differences between AML97 and AML02 were higher cumulative doses of daunorubicin and cytarabine and the addition of gemtuzumab ozogamicin in patients with persistent MRD in AML02. The 3-year event-free survival (EFS) rates of patients in these 2 protocols were 45% and 63%, respectively.17,19 Total Therapy 13 and 14 featured epipodophyllotoxin-based therapy, attaining 5-year EFS rates between 77% and 80%,20 and Total Therapy 15 incorporated intensive asparaginase, vincristine, and dexamethasone, achieving a 5-year EFS of 86%.18
The indications for HCT in initial remission for patients on AML97, included high-risk cytogenetics (−7 or 5q−), morphologic subtypes M6 or M7, myelodysplastic syndrome-derived or therapy-related AML, and persistent disease after 2 cycles of induction therapy. The indications in AML02 were similar, except that patients with t(6;9), FLT3-ITD, or persistent MRD (≥ 0.1% leukemic cells among bone marrow mononuclear cells) after 3 cycles of chemotherapy were also considered for HCT.17 HCTs were performed in first remission for ALL patients who had t(9;22) or had initial induction failure, and in Total Therapy 15 also for those with high levels of MRD (≥ 1%) after 6 weeks of remission induction.18 HCT was indicated for all relapsed AML and ALL, except B-lineage ALL that relapsed > 6 months after completion of chemotherapy or isolated extramedullary relapse.
The 190 HCTs were performed between June 1993 and December 2008. The order of preference for donors was consistent throughout this period: HLA-identical sibling donor, followed by 6 HLA-loci-matched unrelated donor, and then HLA-haplotype–matched (haploidentical) parental donor. Before 1995, HLA-A and HLA-B were typed serologically; thereafter, molecular typing was used.21 HLA-DRB1 alleles were identified by DNA techniques. HLA-C alleles were routinely typed since 2004. MRD was studied by flow cytometry and/or polymerase chain reaction as previously described.17,22 The sensitivity of MRD assay was 0.1% for AML and 0.01% for ALL.17,18
Transplantation regimen
The conditioning and GVHD prophylaxis regimens (including ex vivo T-cell depletion) have been previously described.21,23 Briefly, conditioning regimens were identical for AML and ALL and were adjusted according to donor type. For 145 of the 155 (94%) sibling and unrelated donor HCTs, a regimen based on total body irradiation (TBI) and cyclophosphamide was used (the remaining 10 patients did not receive TBI because of young age or comorbidity). Bone marrow from 60 of the 102 unrelated donors was T cell–depleted using either anti–T-cell antibodies plus complement or CD34+ cell selection, based on the availability of research protocols at the time of HCT. All patients received cyclosporine with methotrexate or mycophenolate mofetil as GVHD prophylaxis.
For the 35 HLA-haploidentical HCTs, 20 patients received a TBI-based regimen, and the other 15 received fludarabine and melphalan-based conditioning. Ex vivo T-cell depletion of the grafts in the early cohort was performed by anti–T-cell antibodies and complement (n = 5) or immunomagnetic selection using the Miltenyi CliniMACS system (n = 11), resulting in a T-cell dose of 0.44 ± 0.64 × 106/kg. For the 19 patients in recent cohort, all the grafts were T cell–depleted using the CliniMACS system (n = 19), and the T-cell dose was smaller (0.09 ± 0.07 × 106/kg; P = .025). Thirteen of the 30 immunomagnetic selections were CD34 cell enrichment, and the others were CD3 cell depletion. All patients received GVHD prophylaxis with either a calcineurin inhibitor (n = 18) or mycophenolate mofetil (n = 17).
All patients received prophylaxis for Pneumocystis pneumonia. Between 1993 and 2000, all patients who were seropositive for CMV or had a CMV-positive donor received ganciclovir until day 120 after HCT. Since 2001, all HCT recipients were monitored weekly by PCR assays for CMV, Epstein-Barr virus, and adenovirus, as well as galactomannan assay for aspergillus. Preemptive treatments, including ganciclovir, rituximab, cidofovir, and voriconazole, respectively, were used if the surveillance tests became positive. All patient studies were approved by the St Jude Institutional Review Board.
Statistics
We used exact test based on Pearson χ2 statistics to compare baseline variables among different types of HCT. The Kaplan-Meier method was used for survival estimates, and the log-rank test was used for comparisons of survival function across groups with different covariates, including the donor type, leukemia protocol, disease status, MRD, sex, race, TBI, T-cell depletion, and CMV status. Tests of heterogeneity were performed before results were combined across groups. Similar covariates were further investigated in a multivariable Cox proportional hazard model based on a stepwise selection strategy while the main effect variables for donor type and treatment era were held in all steps of model building. The assumption of proportional hazard was confirmed in all analyses.24 Cumulative incidences of various cause-specific deaths were estimated and compared by the Gray method, adjusting for competing events.25 The primary cause of death was used for analysis. Thus, in patients whose leukemia relapsed or progressed, leukemia was listed as cause of death regardless of other events. In patients with GVHD who died of infections, GVHD was listed as cause of death. Regimen toxicity referred to all nonrelapse complications, except GVHD and infection. All reported P values are 2-sided and are considered significant if < .05. Statistical analyses were performed with SAS software Version 9.2.
Results
The total number of patients enrolled in the leukemia protocols was 1199 (102 in AML97, 113 in AML02, 486 in Total Therapy 13/Total Therapy 14, and 498 in Total Therapy 15). Among the 190 patients with high-risk AML or very high-risk ALL who received HCT, 117 were in first complete remission at the time of HCT, including 32 in AML97, 37 in AML02, 21 in Total Therapy 13/Total Therapy 14, and 27 in Total Therapy 15. HCT was performed in 43 patients in second or third remission, and in 30 with leukemia not in remission.
Among the 190 HCT patients, 53 received transplants from an HLA-identical sibling donor, 102 from a matched unrelated donor, and 35 from a haploidentical family donor. Their demographics, stratified by donor source, are listed in Table 1. As expected, recipients of haploidentical HCT were more likely to be nonwhite (because a matched donor is often unavailable for these patients; P = .015) and from donors who were more often CMV-positive (P = .0038). A TBI-based regimen was more commonly used in sibling or unrelated donor transplantations (P < .001). All haploidentical grafts were T cell–depleted. There was no significant difference among the 3 donor sources with respect to sex, age, primary disease, leukemia protocol, or disease status at the time of HCT.
Patient characteristics stratified by donor type
| Characteristic . | All patients, N (%) . | Sibling donor, N (%) . | Unrelated donor, N (%) . | Haploidentical donor, N (%) . | P . |
|---|---|---|---|---|---|
| No. of patients | 190 (100) | 53 (28) | 102 (54) | 35 (18) | |
| Sex | .36 | ||||
| Male | 114 (60) | 36 (68) | 59 (58) | 19 (54) | |
| Female | 76 (40) | 17 (32) | 43 (42) | 16 (46) | |
| Race | .015 | ||||
| White | 139 (73) | 39 (74) | 81 (79) | 19 (54) | |
| Nonwhite | 51 (27) | 14 (26) | 21 (21) | 16 (46) | |
| Protocol | .74 | ||||
| AML97 | 50 (26) | 12 (23) | 29 (28) | 9 (26) | |
| AML02 | 46 (24) | 14 (26) | 21 (21) | 11 (31) | |
| Total Therapy 13/Total Therapy 14 | 57 (30) | 17 (32) | 33 (32) | 7 (20) | |
| Total Therapy 15 | 37 (19) | 10 (19) | 19 (19) | 8 (23) | |
| Disease status | .31 | ||||
| CR1 | 117 (62) | 34 (64) | 64 (63) | 19 (54) | |
| CR2 | 37 (19) | 7 (13) | 22 (22) | 8 (23) | |
| CR3 | 6 (3) | 2 (4) | 1 (1) | 3 (9) | |
| NR | 30 (16) | 10 (19) | 15 (15) | 5 (14) | |
| Indication for HCT in CR1 | .16 | ||||
| Persistent MRD | 61 (52) | 16 (47) | 31 (48) | 14 (74) | |
| HR cytogenetics | 31 (26) | 12 (35) | 19 (30) | 0 (0) | |
| M6/M7 | 12 (10) | 3 (9) | 6 (9) | 3 (16) | |
| Secondary AML | 13 (11) | 3 (9) | 8 (13) | 2 (10) | |
| MRD at HCT* | .94 | ||||
| Positive | 55 (45.1) | 15 (42.9) | 27 (45.0) | 13 (48.1) | |
| Negative | 67 (54.9) | 20 (57.1) | 33 (55.0) | 14 (51.9) | |
| Conditioning | < .001 | ||||
| TBI-based | 165 (87) | 47 (89) | 98 (96) | 20 (57) | |
| Non-TBI | 25 (13) | 6 (11) | 4 (4) | 15 (43) | |
| T-cell depletion | < .001 | ||||
| Yes | 96 (51) | 3 (6) | 60 (59) | 35 (100) | |
| No | 94 (49) | 50 (94) | 42 (41) | 0 (0) | |
| CMV status | .004 | ||||
| R+/D+ | 45 (24) | 15 (28) | 16 (16) | 14 (40) | |
| R+/D− | 36 (19) | 9 (17) | 24 (24) | 3 (9) | |
| R−/D+ | 44 (23) | 10 (19) | 21 (21) | 13 (37) | |
| R−/D− | 65 (34) | 19 (36) | 41 (40) | 5 (14) |
| Characteristic . | All patients, N (%) . | Sibling donor, N (%) . | Unrelated donor, N (%) . | Haploidentical donor, N (%) . | P . |
|---|---|---|---|---|---|
| No. of patients | 190 (100) | 53 (28) | 102 (54) | 35 (18) | |
| Sex | .36 | ||||
| Male | 114 (60) | 36 (68) | 59 (58) | 19 (54) | |
| Female | 76 (40) | 17 (32) | 43 (42) | 16 (46) | |
| Race | .015 | ||||
| White | 139 (73) | 39 (74) | 81 (79) | 19 (54) | |
| Nonwhite | 51 (27) | 14 (26) | 21 (21) | 16 (46) | |
| Protocol | .74 | ||||
| AML97 | 50 (26) | 12 (23) | 29 (28) | 9 (26) | |
| AML02 | 46 (24) | 14 (26) | 21 (21) | 11 (31) | |
| Total Therapy 13/Total Therapy 14 | 57 (30) | 17 (32) | 33 (32) | 7 (20) | |
| Total Therapy 15 | 37 (19) | 10 (19) | 19 (19) | 8 (23) | |
| Disease status | .31 | ||||
| CR1 | 117 (62) | 34 (64) | 64 (63) | 19 (54) | |
| CR2 | 37 (19) | 7 (13) | 22 (22) | 8 (23) | |
| CR3 | 6 (3) | 2 (4) | 1 (1) | 3 (9) | |
| NR | 30 (16) | 10 (19) | 15 (15) | 5 (14) | |
| Indication for HCT in CR1 | .16 | ||||
| Persistent MRD | 61 (52) | 16 (47) | 31 (48) | 14 (74) | |
| HR cytogenetics | 31 (26) | 12 (35) | 19 (30) | 0 (0) | |
| M6/M7 | 12 (10) | 3 (9) | 6 (9) | 3 (16) | |
| Secondary AML | 13 (11) | 3 (9) | 8 (13) | 2 (10) | |
| MRD at HCT* | .94 | ||||
| Positive | 55 (45.1) | 15 (42.9) | 27 (45.0) | 13 (48.1) | |
| Negative | 67 (54.9) | 20 (57.1) | 33 (55.0) | 14 (51.9) | |
| Conditioning | < .001 | ||||
| TBI-based | 165 (87) | 47 (89) | 98 (96) | 20 (57) | |
| Non-TBI | 25 (13) | 6 (11) | 4 (4) | 15 (43) | |
| T-cell depletion | < .001 | ||||
| Yes | 96 (51) | 3 (6) | 60 (59) | 35 (100) | |
| No | 94 (49) | 50 (94) | 42 (41) | 0 (0) | |
| CMV status | .004 | ||||
| R+/D+ | 45 (24) | 15 (28) | 16 (16) | 14 (40) | |
| R+/D− | 36 (19) | 9 (17) | 24 (24) | 3 (9) | |
| R−/D+ | 44 (23) | 10 (19) | 21 (21) | 13 (37) | |
| R−/D− | 65 (34) | 19 (36) | 41 (40) | 5 (14) |
P values are based on comparisons among the 3 donor groups.
CR indicates complete remission; NR, nonremission; HR, high-risk; R, recipient; and D, donor.
MRD was not measured in 68 patients because either MRD assay was not available or no leukemia-specific markers were identified.
Recent versus earlier cohorts
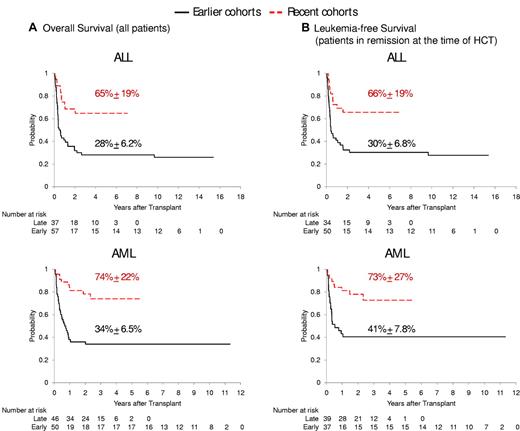
The 5-year overall survival estimate after HCT was 65% for the 37 ALL patients treated on our Total 15 protocol and 74% for the 46 AML patients enrolled in the AML02 protocol (Tables 2–3). These rates compared favorably with those of earlier cohorts on Total 13 or Total Therapy 14 (28%; n = 57; P = .0004) or AML97 (34%; n = 50; P < .0001), respectively (Figure 1A). In the 160 patients whose leukemia was in remission at the time of HCT, the leukemia-free survival rates were better in the recent cohorts than in the earlier cohorts (P = .0001; Figure 1B).
Overall survival estimates among earlier cohorts stratified by donor type
| Donor . | AML97 . | Total Therapy 13/Total Therapy 14 . | ||||
|---|---|---|---|---|---|---|
| N . | 1-year OS (95% CI), % . | 5-year OS (95% CI), % . | N . | 1-year OS (95% CI), % . | 5-year OS (95% CI), % . | |
| Sibling | 12 | 33 (10-59) | 33 (10-59) | 17 | 38 (16-60) | 15 (2.6-38) |
| Unrelated | 29 | 41 (24-58) | 38 (21-55) | 33 | 46 (28-61) | 36 (21-52) |
| Haploidentical | 9 | 33 (7.8-62) | 22 (3.4-51) | 7 | 29 (4.1-61) | 14 (0.7-47) |
| Combined | 50 | 38 (25-51) | 34 (21-47) | 57 | 41 (28-54) | 28 (17-40) |
| Donor . | AML97 . | Total Therapy 13/Total Therapy 14 . | ||||
|---|---|---|---|---|---|---|
| N . | 1-year OS (95% CI), % . | 5-year OS (95% CI), % . | N . | 1-year OS (95% CI), % . | 5-year OS (95% CI), % . | |
| Sibling | 12 | 33 (10-59) | 33 (10-59) | 17 | 38 (16-60) | 15 (2.6-38) |
| Unrelated | 29 | 41 (24-58) | 38 (21-55) | 33 | 46 (28-61) | 36 (21-52) |
| Haploidentical | 9 | 33 (7.8-62) | 22 (3.4-51) | 7 | 29 (4.1-61) | 14 (0.7-47) |
| Combined | 50 | 38 (25-51) | 34 (21-47) | 57 | 41 (28-54) | 28 (17-40) |
OS indicates overall survival.
Overall survival estimates among recent cohorts stratified by donor type
| Donor . | AML02 . | Total Therapy 15 . | ||||
|---|---|---|---|---|---|---|
| N . | 1-year OS (95% CI), % . | 5-year OS (95% CI), % . | N . | 1-year OS (95% CI), % . | 5-year OS (95% CI), % . | |
| Sibling | 14 | 93 (59-99) | 68 (25-90) | 10 | 80 (41-95) | 69 (31-89) |
| Unrelated | 21 | 74 (48-88) | 74 (48-88) | 19 | 60 (34-79) | 46 (21-68) |
| Haploidentical | 11 | 90 (47-99) | 77 (35-94) | 8 | 100 (100-100) | 100 (100-100) |
| Combined | 46 | 84 (69-92) | 74 (56-86) | 37 | 75 (57-86) | 65 (46-78) |
| Donor . | AML02 . | Total Therapy 15 . | ||||
|---|---|---|---|---|---|---|
| N . | 1-year OS (95% CI), % . | 5-year OS (95% CI), % . | N . | 1-year OS (95% CI), % . | 5-year OS (95% CI), % . | |
| Sibling | 14 | 93 (59-99) | 68 (25-90) | 10 | 80 (41-95) | 69 (31-89) |
| Unrelated | 21 | 74 (48-88) | 74 (48-88) | 19 | 60 (34-79) | 46 (21-68) |
| Haploidentical | 11 | 90 (47-99) | 77 (35-94) | 8 | 100 (100-100) | 100 (100-100) |
| Combined | 46 | 84 (69-92) | 74 (56-86) | 37 | 75 (57-86) | 65 (46-78) |
OS indicates overall survival.
Survival of patients after HCT stratified by disease category and treatment era. (A) Overall survival of ALL patients in earlier cohort Total Therapy 13/Total Therapy 14 versus recent cohort Total Therapy 15 (top panel), and of AML patients in earlier cohort AML97 versus recent cohort AML02 (bottom panel). (B) Leukemia-free survival of patients whose leukemia was in remission at the time of HCT.
Survival of patients after HCT stratified by disease category and treatment era. (A) Overall survival of ALL patients in earlier cohort Total Therapy 13/Total Therapy 14 versus recent cohort Total Therapy 15 (top panel), and of AML patients in earlier cohort AML97 versus recent cohort AML02 (bottom panel). (B) Leukemia-free survival of patients whose leukemia was in remission at the time of HCT.
The 5-year cumulative incidence of cause-specific death among HCT patients treated with various leukemia protocols ranged from 3.0% to 10.8% for GVHD, 2.9% to 22.0% for infection, 6.6% to 32.2% for regimen-related mortality, and 12.0% to 26.0% for leukemia relapse (Table 4). Compared with the earlier cohorts treated for AML or ALL, recent cohorts had a significantly lower hazard of death from infection (hazard ratio [HR] = 0.12; P = .005), regimen-related toxicity (HR = 0.25; P = .002), or leukemia relapse (HR = 0.4; P = .013). The difference in GVHD was not statistically significant (HR = 0.38; P = .16).
Cumulative incidence of cause-specific death at 5 years stratified by protocol
| Cause . | Earlier cohorts . | Recent cohorts . | Recent vs earlier . | |||
|---|---|---|---|---|---|---|
| AML97 (N = 50), cumulative incidence (95% CI), % . | Total Therapy 13/Total Therapy 14 (N = 57), cumulative incidence (95% CI), % . | AML02 (N = 46), cumulative incidence (95% CI), % . | Total Therapy 15 (N = 37), cumulative incidence (95% CI), % . | Hazard ratio (CI) . | P . | |
| GVHD | 4.0 (0.7-12.2) | 10.8 (4.35-20.6) | 3.0 (0.2-13.5) | 5.5 (0.98-16.2) | 0.38 (0.1-1.4) | .16 |
| Infection | 22.0 (11.7-34.3) | 10.8 (4.24-20.8) | 4.4 (0.28-18.6) | 2.9 (0.21-13.1) | 0.12 (0.03-0.5) | .005 |
| Regimen toxicity | 12.0 (4.7-22.9) | 32.2 (20.4-44.6) | 6.6 (1.7-16.3) | 9.4 (2.2-23) | 0.25 (0.1-0.6) | .002 |
| Relapse | 26.0 (14.7-38.8) | 18.1 (9.1-29.3) | 12.0 (4.3-23.9) | 17.5 (7.0-32) | 0.40 (0.2-0.8) | .013 |
| Cause . | Earlier cohorts . | Recent cohorts . | Recent vs earlier . | |||
|---|---|---|---|---|---|---|
| AML97 (N = 50), cumulative incidence (95% CI), % . | Total Therapy 13/Total Therapy 14 (N = 57), cumulative incidence (95% CI), % . | AML02 (N = 46), cumulative incidence (95% CI), % . | Total Therapy 15 (N = 37), cumulative incidence (95% CI), % . | Hazard ratio (CI) . | P . | |
| GVHD | 4.0 (0.7-12.2) | 10.8 (4.35-20.6) | 3.0 (0.2-13.5) | 5.5 (0.98-16.2) | 0.38 (0.1-1.4) | .16 |
| Infection | 22.0 (11.7-34.3) | 10.8 (4.24-20.8) | 4.4 (0.28-18.6) | 2.9 (0.21-13.1) | 0.12 (0.03-0.5) | .005 |
| Regimen toxicity | 12.0 (4.7-22.9) | 32.2 (20.4-44.6) | 6.6 (1.7-16.3) | 9.4 (2.2-23) | 0.25 (0.1-0.6) | .002 |
| Relapse | 26.0 (14.7-38.8) | 18.1 (9.1-29.3) | 12.0 (4.3-23.9) | 17.5 (7.0-32) | 0.40 (0.2-0.8) | .013 |
Impact of donor type
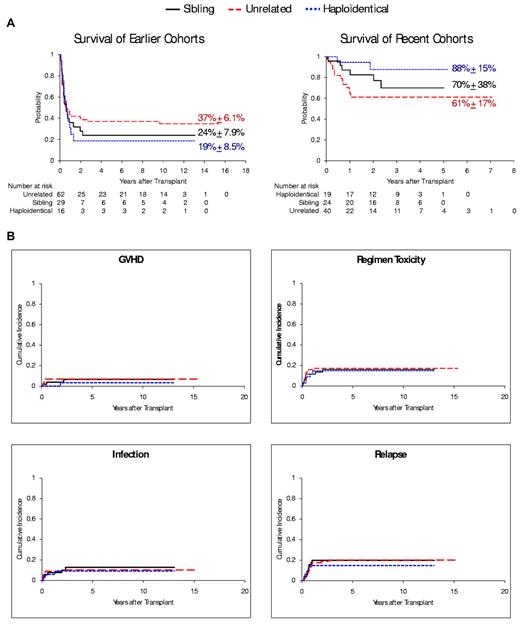
The improvement in 5-year overall survival rates observed in recent cohorts occurred regardless of donor type: sibling (70% [n = 24] vs 24% [n = 29] of earlier cohorts, P < .001); unrelated (61% [n = 40] vs 37% [n = 62], P = .014); and haploidentical (88% [n = 19] vs 19% [n = 16], P < .001; Figure 2A). There was no significant difference in the risk of any cause-specific deaths (GVHD, infection, regimen-related toxicity, and leukemia relapse) between patients who received HCT from sibling donors and those from unrelated donors (P > .69) or haploidentical donors (P > .49) in either cohort of patients (Table 5; Figure 2B), even though haploidentical HCTs had more unfavorable factors (including more nonwhite recipients, T-cell depletion, CMV positivity, and reduced use of TBI). In risk-factor analyses, none of these variables was statistically significant (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Outcomes after HCT stratified by donor type. (A) Overall survival of patients in the earlier cohorts (left panel) and the recent cohorts (right panel) after receiving HCT from a sibling donor, unrelated donor, or HLA haploidentical donor. (B) Cumulative incidence of cause-specific death among patients stratified by the 3 donor types, including death related to GVHD, infection, transplantation-regimen toxicity, and leukemia relapse.
Outcomes after HCT stratified by donor type. (A) Overall survival of patients in the earlier cohorts (left panel) and the recent cohorts (right panel) after receiving HCT from a sibling donor, unrelated donor, or HLA haploidentical donor. (B) Cumulative incidence of cause-specific death among patients stratified by the 3 donor types, including death related to GVHD, infection, transplantation-regimen toxicity, and leukemia relapse.
Cumulative incidence of cause-specific death at 5 years stratified by donor type
| Cause . | Sibling donor (N = 53), CI (95%) . | Unrelated donor (N = 102), CI (95%) . | Haploidentical donor (N = 35), CI (95%) . | Unrelated vs sibling HR (CI), P . | Haplo vs sibling HR (CI), P . |
|---|---|---|---|---|---|
| GVHD | 6.6 (1.6-16.6) | 7.0 (3.03-13.2) | 3.4 (0.22-15.5) | 1.28 (0.3-4.9), .72 | 0.48 (0.1-4.6), .52 |
| Infection | 12.7 (4.9-24.2) | 10.2 (5.2-17.2) | 9.1 (2.25-22) | 0.90 (0.3-2.5), .85 | 0.71 (0.2-2.8), .63 |
| Regimen toxicity | 16.0 (7.3-27.6) | 17.1 (10.4-25.2) | 14.8 (5.3-28.8) | 1.19 (0.5-2.7), .69 | 0.88 (0.3-2.7), .83 |
| Relapse | 19.7 (9.9-31.8) | 20.1 (12.6-28.9) | 14.7 (3.7-27.3) | 1.09 (0.5-2.3), .83 | 0.68 (0.2-2.0), .49 |
| Cause . | Sibling donor (N = 53), CI (95%) . | Unrelated donor (N = 102), CI (95%) . | Haploidentical donor (N = 35), CI (95%) . | Unrelated vs sibling HR (CI), P . | Haplo vs sibling HR (CI), P . |
|---|---|---|---|---|---|
| GVHD | 6.6 (1.6-16.6) | 7.0 (3.03-13.2) | 3.4 (0.22-15.5) | 1.28 (0.3-4.9), .72 | 0.48 (0.1-4.6), .52 |
| Infection | 12.7 (4.9-24.2) | 10.2 (5.2-17.2) | 9.1 (2.25-22) | 0.90 (0.3-2.5), .85 | 0.71 (0.2-2.8), .63 |
| Regimen toxicity | 16.0 (7.3-27.6) | 17.1 (10.4-25.2) | 14.8 (5.3-28.8) | 1.19 (0.5-2.7), .69 | 0.88 (0.3-2.7), .83 |
| Relapse | 19.7 (9.9-31.8) | 20.1 (12.6-28.9) | 14.7 (3.7-27.3) | 1.09 (0.5-2.3), .83 | 0.68 (0.2-2.0), .49 |
Grades 2-4 GVHD was observed in 13.2% of the recipients with sibling donor, 26.4% with unrelated donor, and 25.7% with haploidentical donor. The corresponding rates for grades 3-4 GVHD were 7.5%, 11.7%, and 11.4%, respectively.
Factors affecting outcome
Only 2 factors were associated with survival outcomes after HCT in multivariable analysis: treatment era (early vs recent cohort; HR = 3.01; 95% confidence interval [CI], 1.82-4.98; P < .0001) and type of remission (first remission vs more advanced disease; HR = 0.63; 95% CI, 0.41-0.96; P = .03). When stratified by remission status at the time of HCT, the early cohort had worse progression-free survival than the recent cohort in patients who were in first morphologic remission (HR = 3.25; 95% CI, 1.81-5.87; P < .0001) or not in remission (HR = 2.67; 95% CI, 1.00-7.17; P = .05), but not in those who were in second or third remission (HR = 1.72; 95% CI, 0.59-5.04; P = .32). In the subset of patients with available MRD data at the time of HCT, positive MRD was associated with poorer survival and higher rate of relapse in both ALL and AML patients (Table 6).
Overall survival and cumulative incidence of relapse at 5 years stratified by MRD level immediately before HCT
| Outcome . | ALL . | AML . | ||||
|---|---|---|---|---|---|---|
| MRD− (N = 34) . | MRD+ (N = 30) . | P . | MRD− (N = 33) . | MRD+ (N = 25) . | P . | |
| Survival (95% CI), % | 68.0 (48.2-81.3) | 29.8 (14.0-47.0) | .008 | 67.8 (46.8-82.3) | 46.6 (26.3-64.6) | .05 |
| Relapse (95% CI), % | 6.3 (1.1-17.1) | 27.8 (12.4-46) | .03 | 6.6 (1.1-20.8) | 36.9 (18.3-55.8) | .005 |
| Outcome . | ALL . | AML . | ||||
|---|---|---|---|---|---|---|
| MRD− (N = 34) . | MRD+ (N = 30) . | P . | MRD− (N = 33) . | MRD+ (N = 25) . | P . | |
| Survival (95% CI), % | 68.0 (48.2-81.3) | 29.8 (14.0-47.0) | .008 | 67.8 (46.8-82.3) | 46.6 (26.3-64.6) | .05 |
| Relapse (95% CI), % | 6.3 (1.1-17.1) | 27.8 (12.4-46) | .03 | 6.6 (1.1-20.8) | 36.9 (18.3-55.8) | .005 |
Discussion
We found that patients who had very high-risk leukemia in our recent AML and ALL protocols had a favorable outcome after HCT. The risk of transplantation regimen–related death for these patients was much lower than for patients treated on earlier protocols. Furthermore, because of the reduced risk of infection- and relapse-related death, the overall survival rate of HCT patients in recent cohorts was more than double that of earlier cohorts for both ALL and AML.
Some of the cooperative-group studies have shown modest change in the outcome of recent HCT trials in patients with poor-prognosis leukemia. For instance, in a study by the European Group for Blood and Marrow Transplantation on 18 803 consecutive pediatric HCTs, the 2-year HCT-related mortality was 21% for transplantations performed between 1970 and 1995 and 22% for those between 1996 and 2002 (P = .78).26 In a study combining data from 5 cooperative group clinical trials and including more than 3000 pediatric patients with AML in first complete remission, improvement in survival with HCT was restricted to the intermediate-risk disease group and did not extend to the poor-risk group (33% with HCT vs 35% with chemotherapy).27 Similarly, in the BFM-AML98 study, intent-to-treat analysis revealed no difference in 5-year disease-free survival among 275 high-risk patients with or without an HLA-identical sibling donor (47% vs 41%, P = .4).14 The CCG1941 study found no advantage of HCT for ALL patients with early relapse (3-year disease-free survival 29% with sibling HCT vs 27% with chemotherapy alone), and the CCG1952 study found no advantage in either early relapse (2-year EFS, 43% for HCT and 38% for chemotherapy) or late relapse (56% for HCT and 62% for chemotherapy).28,29
An HLA-identical sibling donor has been considered the gold standard as stem cell source.21,30 If a matched sibling donor is not available and the disease carries a sufficiently poor prognosis, then the order of alternative donor selection for most institutions and cooperative group studies is an unrelated adult donor or a cord blood unit, followed by an HLA-haploidentical family donor.31 There have been significant advances in alternative donor HCT using unrelated adult,32-34 cord blood,35 and haploidentical graft.36 However, even 8 of 8 allelic matched unrelated donor HCT was associated with a 2- to 4-fold higher risk of GVHD and mortality compared with sibling-donor transplantations.37 Recently, a large study on 4099 patients by the Center for International Blood and Marrow Transplant Research confirmed that unrelated-donor HCT carried a higher risk of HCT-related mortality (relative risk = 2.76, P < .001), without a superior GVL effect, than sibling donor HCT.38 The results of our hapoidentical HCT were particularly intriguing because these transplantations were more often performed in nonwhite patients,39 grafts were extensively T cell– depleted,40 donors were more often CMV-positive,41 and a TBI-based conditioning regimen was used less often.42,43 All these features would predict a higher risk of GVHD, infection, or leukemia relapse. Whereas the improvement in our unrelated donor HCT could be related to the inclusion of HLA-C matching30 (thereby 36 of the 40 unrelated donors in recent cohorts were HLA-C matched) and better supportive care (eg, molecular microbiology monitoring and newer agents, including cidofovir for adenovirus and rituximab for Epstein-Barr virus lymphoproliferative disorder),44,45 the favorable results of haploidentical donor HCTs may be related to less toxic non-TBI regimen and better leukemia and infection control by NK cells because of efficient T-cell depletion46 and the comprehensive KIR typing available recently in our center for donor selection (thereby 15 of the 19 donors in recent cohorts were KIR mismatched).47 T-cell depletion is important to optimize NK-cell function, as T-cell alloreactivity dominates NK-cell alloreactivity,46 and the prophylaxis and treatment of T cell–mediated GVHD may compromise NK-cell activity. Although the beneficial effects of KIR mismatch were only observed in adult AML but not in ALL, recent studies by our group and others have shown that pediatric ALL is also susceptible to KIR mismatch recognition.48,49 The role of NK cells, however, could not be established definitely in this study as KIR typing was not performed in earlier cohorts.
The prognostic value of MRD level during chemotherapy for childhood leukemia has been well recognized,10 and risk-directed therapy based on this measure has become the mainstay of many contemporary chemotherapy regimens.17,18 The prognostic value of MRD level before HCT is less well established. Two recent studies showed a high relapse rate (57%-76%) and poor 5-year survival rate (18%-27%) in ALL patients who had MRD > 10−4 before HCT or in AML patients with WT1 expression level > 0.5 relative to K562 cells.50,51 In our patients with very high-risk leukemia, there was a relatively low risk of relapse (6% for ALL and 7% for AML) and a relatively high likelihood of survival (68% for both ALL and AML) for patients with negative MRD. Even patients with positive MRD (resistant to intensive reinduction attempt) had higher than expected survival rates with HCT (30% for ALL and 47% for AML). Eventually, these patients should benefit from new strategies to induce deeper remission before HCT, such as NK-cell therapy.52
Our study has several limitations. First, although a variety of cell sources were examined in detail, cord blood grafts were not included because parental grafts were used in our center for patients without a matched donor. In 2007 and 2008, 24% of the allogeneic HCTs in patients younger than 20 years worldwide were performed using cord blood grafts.53 Second, although our patients received well-defined chemotherapy regimens with criteria for HCT determined a priori, thus resulting in high internal validity in statistical comparisons, the number of events was small in subgroup analyses. For example, our analyses on donor sources were susceptible to type II statistical error and should not be considered definitively as equivalency, even though the favorable results in all donor sources would support the consideration of alternative donor HCT in all patients without sibling donor. Third, the exact causes of improvement in HCTs over time cannot be established in this retrospective study, as many transplant approaches evolved simultaneously.44 Fourth, as matching in HLA-C was not required for unrelated donor HCTs before 2008 in our institution, outcomes after contemporary 8 of 8 loci-matched transplantations may even be better than those favorable rates reported herein.
In conclusion, our results indicate that all patients with very high-risk leukemia should be considered as candidates for HCT early in the course of diagnosis or relapse treatment, regardless of the availability of a matched donor or the intensity of prior chemotherapy. HLA typing, donor search, and transplant center referral should be performed as soon as possible. Unfortunately, insurance approval is frequently denied in the United States for HCT because of the notion that these patients are not salvageable by this procedure, especially if matched sibling donor is not available. Our findings contradict this belief and show that HCT should be pursued with a curative intent for these patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank David Galloway, ELS, for assistance with manuscript editing; our clinical, laboratory, and research office colleagues for data collection; and the many patients and parents who participated in the transplantation and cellular therapy research program.
This study was supported in part by the National Institutes of Health Cancer Center Support (grants P30 CA021765-30, U01 GM092666, and RO1 CA115422), the State of Tennessee (Center of Excellence Grant), the Assisi Foundation of Memphis, and the American Lebanese Syrian Associated Charities. C.-H.P. is an American Cancer Society Professor.
National Institutes of Health
Authorship
Contribution: W.L. designed the study, analyzed and interpreted data, and wrote the paper; W.L., D.C., J.E.R., J.T.S., R.C.R., A.S., C.H., B.M.T., M.D., A.P., R.H., J.H.L., and C.-H.P. provided study material and patient information and contributed to the interpretation of data; W.L., E.C.-S., and K.G. collected and assembled laboratory data; J.Y. and D.P. performed statistical analyses; and all authors contributed to the revisions of the draft and approval of the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for R.H. is University Children's Hospital, Tübingen, Germany.
Correspondence: Wing Leung, St Jude Children's Research Hospital, 262 Danny Thomas Pl, Memphis, TN 38105-2794; e-mail: wing.leung@stjude.org.