Abstract
We genotyped 370 subjects with primary myelofibrosis (PMF) and 148 with postpolycythemia vera/postessential thrombocythemia (PPV/PET) MF for mutations of EZH2. Mutational status at diagnosis was correlated with hematologic parameters, clinical manifestations, and outcome. A total of 25 different EZH2 mutations were detected in 5.9% of PMF, 1.2% of PPV-MF, and 9.4% of PET-MF patients; most were exonic heterozygous missense changes. EZH2 mutation coexisted with JAK2V617F or ASXL1 mutation in 12 of 29 (41.4%) and 6 of 27 (22.2%) evaluated patients; TET2 and CBL mutations were found in 2 and 1 patients, respectively. EZH2-mutated PMF patients had significantly higher leukocyte counts, blast-cell counts, and larger spleens at diagnosis, and most of them (52.6%) were in the high-risk International Prognostic Score System (IPSS) category. After a median follow-up of 39 months, 128 patients (25.9%) died, 81 (63.3%) because of leukemia. Leukemia-free survival (LFS) and overall survival (OS) were significantly reduced in EZH2-mutated PMF patients (P = .028 and P < .001, respectively); no such impact was seen for PPV/PET-MF patients, possibly due to the low number of mutated cases. In multivariate analysis, survival of PMF patients was predicted by IPSS high-risk category, a < 25% JAK2V617F allele burden, and EZH2 mutation status. We conclude that EZH2 mutations are independently associated with shorter survival in patients with PMF.
Introduction
The identification of the JAK2V617F mutation1-4 represented a seminal discovery in the field of Philadelphia-chromosome–negative chronic myeloproliferative neoplasms (MPNs),5 providing clues to the pathogenesis,6 prompting a revision of the diagnostic criteria,7 and culminating in the development of clinical trials with JAK2 (and JAK1) inhibitors.8,9 The JAK2V617F mutation occurs in almost all patients with polycythemia vera (PV) and in 50%-70% of those with essential thrombocythemia (ET) and primary myelofibrosis (PMF). Soon after the identification of the JAK2V617F mutation, mutations in JAK2 exon 12 were described in rare patients with JAK2V617F-negative PV and mutations in MPL were reported in 5%-10% of ET or PMF subjects. The complexity of the molecular pathogenesis of MPNs is reinforced by discovery of additional mutations in TET2,10 ASXL1,11 CBL,12 IDH1/IDH2,13 and IKZF1.14 These mutations are detected in a minority of patients at different phases of the disorder, including leukemic transformation, and are variably associated each other and with JAK2 or MPL mutations.
We recently identified novel loss-of-function mutations in EZH2 in 1 of 30 (3%) PV and in 4 of 30 PMF patients (13%), as well as in 11%-25% of patients with myelodysplastic syndromes (MDS) and in 10% of patients with MDS/MPN.15 Mutations were spread throughout the gene and included missense, nonsense, and premature stop codons; both monoallelic and biallelic mutations were described. Among patients with MDS/MPN, survival was significantly worse in those with EZH2 mutation. Furthermore, subjects with homozygous mutations had a trend toward inferior survival compared with heterozygous patients.15 Several different EZH2 mutations were also reported in an independent series of 102 patients with MDS,16 whereas a functionally distinct heterozygous missense point mutation at codon Y641 of EZH2 has been described in patients with follicular lymphoma (7%) and diffuse, germinal center-origin, large B-cell lymphomas (22%).17,18
The aim of current study was to determine the frequency and characteristics of EZH2 mutations in a large series of patients with PMF and PPV/PET MF and to analyze the prognostic relevance of a mutated EZH2 status.
Methods
Patients and samples
Patients diagnosed with PMF or PPV/PET-MF according to World Health Organization (WHO)7 and International Working Group for Myelofibrosis Research and Treatment (IWG-MRT) criteria,19 respectively, were recruited for this study from the database at 6 hematology units: Florence, Italy; Pavia, Italy; Southampton, United Kingdom; Barcelona, Spain; Athens, Greece; and Mannheim, Germany. For PET-MF patients, only those who had a previous confirmed diagnosis of “true” ET as opposed to “prefibrotic” MF were considered for this study. Patients provided informed consent for the use of archival material for mutational analysis, and the study was performed under a Florence University Institutional Review Board–approved protocol in referring institutions. The study was conducted in accordance with the Declaration of Helsinki.
Genotyping for EZH2 mutations
DNA was purified from peripheral blood (PB) whole leukocytes (n = 71) or gradient-purified granulocytes (n = 447) that had been collected at diagnosis of PMF or PPV/PET-MF, or no later than 1 year afterward provided the patient had remained free of cytotoxic treatment. DNA was purified using conventional methods and subjected to whole-genome amplification with the Illustra GenomiPhi V2 DNA Amplification Kit (GE Healthcare). EZH2 mutation analysis was performed using high-resolution melting (HRM) in a Rotor-Gene 6000 instrument (Corbett Life Science), using primer sets as described previously.15 Products showing abnormal melt pattern were subjected to bidirectional direct sequencing. Sequence analysis was performed using Mutation Surveyor (SoftGenetics). All mutations were further validated by repeating PCR and direct sequencing on genomic (ie, not subjected to whole-genome amplification) DNA from the archival sample. Details of the techniques used are provided in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Genotyping for JAK2, MPL, IDH1/IDH2, CBL, TET2, and ASXL1 mutations
Patients were also genotyped for additional mutations, including JAK2, MPL, IDH1, IDH2, and ASXL1. The presence of the JAK2V617F mutation and the mutated allele burden were determined by quantitative real-time PCR (QRT-PCR)20 or pyrosequencing assays.21 MPLW515L/K mutations were determined using QRT-PCR, as described previously.22 Mutational analysis for IDH exon 4, ASXL1 exon 12, (primers are listed in supplemental Table 1) and CBL exons 8 and 912 was performed by direct sequencing of the PCR products amplified from genomic DNA. The complete coding sequence of TET2 was analyzed by HRM.15
Statistical analysis
Our primary aim was to determine the correlation between EZH2 mutational status and major outcome events, which included overall survival (OS) and transformation to acute leukemia. We also investigated whether EZH2 status was correlated with specific laboratory parameters or clinical features, including RBC indexes, leukocyte or platelet count, percentage of PB blasts, splenomegaly, constitutional symptoms, and the ranking of patients according to the International Prognostic Score System (IPSS) developed by the IWG-MRT.23 Constitutional symptoms included loss of 10% or more of body weight in the last 6-months, drenching night sweats, or unexplained fever. Splenomegaly was measured in centimeters from the left costal margin (LCM); we considered 2 groups of patients who presented a spleen enlargement smaller than or greater than 10 cm from the LCM, respectively.
The χ2 or Fisher exact test (2 × 2 table) or the χ2 test for trend (larger contingency table) were used as appropriate to compare the variables among the different patient groups that had been categorized according to mutational status. The analysis of continuous variables among the groups was performed using the Mann-Whitney U test (2 groups) or the Kruskal-Wallis test with the use of the Dunn method for multiple comparison. Kaplan-Meier analysis and the log-rank test were used to estimate OS. Cox regression models were used to perform multivariate analysis. P < .05 was considered to indicate statistical significance; all tests were 2-tailed. Data were processed using SPSS Version 17.0 software (StatSoft).
Results
Patient characteristics
Hematologic and clinical features of the 518 patients included in the study are listed in supplemental Table 2; they comprised 370 subjects with PMF, 84 with PPV-MF, and 64 with PET-MF. A total of 321 subjects were JAK2V617F mutated (62%): 213 with PMF (58%), 78 with PPV-MF (93%), and 30 with PET-MF (47%). The median V617F allele burden was 42% (range 3%-100%) in PMF, 69% (range 11%-100%) in PPV-MF, and 48% (range 2%-100%) in PET-MF. Considering only patients with PMF, 31 (14.5%) had a V617F allele burden < 25%. The MPLW515L/K mutation was found in 18 patients (3.8%): 13 with PMF (3.7%) and 5 (10.6%) with PET-MF. Results of cytogenetic analysis at diagnosis were available in 188 patients; of these, 21 with PMF (15.3% of evaluated), 7 with PPV-MF (21.2%), and 6 with PET-MF (33.3%) had unfavorable cytogenetic abnormalities (ie, complex karyotype or single or 2 abnormalities including −8, −7/7q−, i(17q), −5/5q−, 12p−, inv(3), or 11q23 rearrangement).24
The stratification of PMF patients according to the IPSS reflected that reported in large patients series, with 30.7%, 25.5%, 22.4%, and 21.4% in the low-risk, intermediate 1–risk, intermediate 2–risk, and high-risk categories, respectively, indicating that our patient population was well representative of all of the different IPSS risk categories.
Results of EZH2 genotyping
We screened all of the 20 coding exons of EZH2 by HRM followed by direct-sequencing confirmation. A total of 29 patients (5.6% of total) were EZH2 mutated; of these, 22 had PMF (5.9% of all PMF), 1 had PPV-MF (1.2%), and 6 had PET-MF (9.4%; supplemental Tables 2 and 3). Because only 1 of 84 PPV-MF patients was EZH2 mutated, we carefully reviewed his records and confirmed the original diagnosis of PV using the 2008 WHO criteria7 and the diagnosis of progression to PPV-MF according to the IWG-MRT criteria.19 This was a 62-year-old man who had a 14-year-long history of heavily phlebotomized PV treated with hydroxyurea at the time he was diagnosed as PPV-MF.
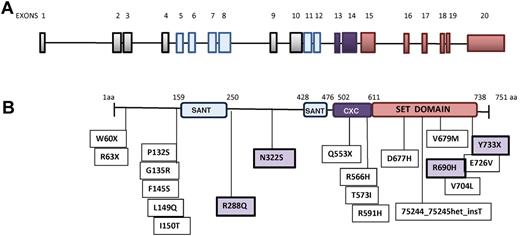
We identified a total of 25 different mutations, of which 20 were exonic and 5 intronic. Seven mutations (in 9 patients) were located in the suppressor of variegation3-9, enhancer of zeste and trithorax (SET) domain and 4 (in 5 patients) in the CXC domain; other mutations were located in exons 3, 5, 8, and 9 (Figure 1). Most exonic mutations were heterozygous missense changes caused by single-nucleotide substitution; in 5 patients (17%; patient numbers 2, 15, 17, 26, and 28 in supplemental Table 3), a homozygous mutation was detected, including one intronic change (patient number 26 in supplemental Table 3), and 1 patient presented with 2 different mutations (patient number 19 in supplemental Table 3). Mutations were confirmed as somatic for 5 of 5 patients with available germline DNA extracted from buccal epithelial cells: 74938G > GA/G > A (patient numbers 1 and 2), 54557T > TA (patient number 7), 73990G > C (patient number 10), and 70231C > CT (patient numbers 11 and 12) abnormalities. Other patients were denoted as having mutations based on the exclusion of known single nucleotide polymorphisms in published databases (Ensembl and National Center for Biotechnology Information). Five intronic mutations were also identified (supplemental Figure 1); of these, 3 (patient numbers 25, 26, and 28) affected the absolutely conserved AG/GT exon flanking splice sites and are therefore very likely to be causative. The changes in patients 27 and 29 are of uncertain significance, but were considered as causative for the analysis below. Twelve EZH2-mutated subjects (41.4%) harbored the JAK2V617F mutation; of these, 9 had PMF, 1 PPV-MF, and 2 PET/MF. No EZH2-mutated patient had mutations in IDH1/IDH2, whereas 6 of 27 evaluated subjects (22.2%) concurrently harbored an ASXL1 exon 12 mutation. The simultaneous occurrence of the EZH2, JAK2V617F, and ASXL1 mutations was not documented. Two patients had mutations in TET2, one of whom was also ASXL1 mutated (patient number 6 in supplemental Table 3). One additional patient (patient number 26) was EZH2, CBL, and ASXL1 mutated. Finally, no concurrent EZH2 and MPLW515L/K mutation was detected.
EZH2 schematic structure and localization of mutations. (A) Representation of EZH2 exons. Blue and purple bars correspond to exons encoding the SANT_DNA–binding domain and the SET domain, respectively. (B) Domain structure of EZH2 and positions of mutations carried by subjects. Missense mutations highlighted in the violet boxes (R288Q, N322S, R690H, and Y733X) have been described previously.15,42
EZH2 schematic structure and localization of mutations. (A) Representation of EZH2 exons. Blue and purple bars correspond to exons encoding the SANT_DNA–binding domain and the SET domain, respectively. (B) Domain structure of EZH2 and positions of mutations carried by subjects. Missense mutations highlighted in the violet boxes (R288Q, N322S, R690H, and Y733X) have been described previously.15,42
We also analyzed a prospective cohort of 118 PMF patients who had EZH2 wild-type genotype at diagnosis and for whom we had stored at least 1 additional blood sample collected after a minimum of 1 year from diagnosis (median 38; range 12-84 months). Acquisition of the EZH2 mutation was demonstrated in 1 patient (patient number 13 in supplemental Table 3) at 32 months after diagnosis; she has been followed for an additional 24 months without obvious changes in her illness. Finally, in 1 EZH2/ASXL1–mutated PMF patient who evolved to leukemia after 17.6 months from diagnosis (patient number 1 in supplemental Table 3), the blast cells showed maintenance of both mutations. In this patient, granulocytes collected at diagnosis and blast cells at leukemic transformation tested negative for JAK2V617F, MPLW515L/K, TET2, IDH1/IDH2, and CBL mutations. Conversely, none of 7 EZH2 wild-type PMF patients at diagnosis who later evolved to leukemia acquired an EZH2 mutation. Of these, 5 were JAK2V617F mutated at chronic phase and maintained this mutation in leukemic blasts.
Association of EZH2-mutated genotype with hematologic and clinical characteristics
To establish the correlation, if any, of the EZH2 mutation with clinical characteristics, we compared EZH2-mutated PMF patients with their wild-type counterparts (Table 1). PMF patients harboring an EZH2 mutation (including the 5 intronic variants) displayed at diagnosis a higher leukocyte count (median 17.8; range 3.5-47.9 vs 8.5 × 109/L; range 1.4-106, P = .001), more frequently had a blast count > 1% (52.6% vs 20.7%, P = .002), and presented with a larger spleen (the proportion of those with palpable spleen at > 10 cm from the LCM was 54.5% vs 29.7%, P = .016). Variables associated with leukocytosis in multivariate analysis were: age > 65 years (P = .030), presence of constitutional symptoms (P = .018), and EZH2 mutation (P = .023). Factors associated with > 1% blast cells in univariate analysis were ASXL1 mutation (P = .001), hemoglobin < 100 g/L (P = .001), leukocytosis (> 25 × 109/L) (P = .001), and EZH2 mutation (P = .003); however, in multivariate analysis, EZH2 lost its significant association in favor of the others. Finally, variables associated with splenomegaly > 10 cm in univariate analysis were: the presence of constitutional symptoms (P = .027), a > 25% V617F allele burden (P = .014), and EZH2 mutation (P = .019); however, all of these variables lost their significant association in multivariate analysis. Finally, there was no difference in age, sex, hemoglobin level, platelet count, or occurrence of constitutional symptoms between EZH2 mutated and wild-type patients. The proportion of the JAK2V617F mutation in the 2 groups was also similar (40.9 vs 58.8%).
Hematologic and clinical characteristics of patients stratified according to EZH2 mutational status
| . | PMF . | P . | PPV/PET-MF . | P . | ||
|---|---|---|---|---|---|---|
| EZH2 . | EZH2 . | |||||
| Wild-type . | Mutated . | Wild-type . | Mutated . | |||
| N | 348 | 22 | 141 | 7 | ||
| Median follow-up, mo, (range) (n = 500)* | 39.567 (1-340) | 28.183 (8-183) | .365 | 29.7 (1-234) | 16.8 (1-70) | .256 |
| Median age, y (range) | 60.0 (14-90) | 66.0 (38-90) | .135 | 62.0 (32-84) | 62.0 (52-78) | .731 |
| Male sex, no. (%) (n = 303)* | 221 (60.6) | 15 (68.2) | .481 | 73 (51.8) | 4 (57.1) | .781 |
| Leukocytes, × 106/L, mean ± SD (n = 500)* | 12.3 ± 13.3 | 18.7 ± 11.5 | .001 | 14.3 ± 14.1 | 13.8 ± 7.0 | .360 |
| Hb, g/L, mean ± SD (n = 497)* | 114 ± 27 | 112 ± 19 | .700 | 114 ± 26 | 100 ± 18 | .153 |
| Platelets, × 106/L, mean ± SD, (n = 501)* | 341.0 ± 345.6 | 405.0 ± 258.7 | .831 | 404.1 ± 317.6 | 232.1 ± 198.0 | .097 |
| Peripheral blast cells, %, mean ± SD (n = 329)* | 0.7 ± 2.1 | 1.6 ± 2.1 | .002 | 0.5 ± 2.0 | 0.4 ± 0.9 | .959 |
| Constitutional symptoms, no. (%) (n = 333)* | 67 (28.2) | 8 (42.1) | .198 | 34 (47.9) | 2 (40.0) | .733 |
| Splenomegaly, no. (%) (n = 494)*† | .016 | .059 | ||||
| 0 | 91 (27.6) | 1 (4.5) | 17 (12.6) | 3 (42.9) | ||
| 1 | 141 (42.7) | 9 (40.9) | 58 (43.0) | 1 (14.3) | ||
| 2 | 98 (29.7) | 12 (54.5) | 60 (44.4) | 3 (42.9) | ||
| Abnormal karyotype, no. (%) (n = 195)* | 26 (20.2) | 4 (36.4) | .209 | 17 (32.7) | 2 (66.7) | .229 |
| Unfavorable karyotype, no. (%) (n = 188)* | 18 (14.3) | 3 (27.3) | .252 | 11 (22.9) | 2 (66.7) | .092 |
| JAK2V617F, no. (%) (n = 518)* | 204 (58.8) | 9 (40.9) | .100 | 105 (74.5) | 3 (42.9) | .066 |
| JAK2V617F allele burden, %, mean ± SD | 45.7 ± 22.9 | 42.2 ± 22.1 | .603 | 64.1 ± 23.4 | 61.1 ± 52.6 | .747 |
| MPL W515L/K, no. (%) (n = 466)* | 13 (3.9) | 0 (0.0) | .344 | 5 (4.7) | 0 (0.0) | .586 |
| IWG-MRT score, no. (%) (n = 192)* | .002 | - | ||||
| Low | 55 (31.8) | 4 (21.1) | ||||
| Int-1 | 44 (25.4) | 5 (26.3) | ||||
| Int-2 | 43 (24.9) | 0 (0.0) | ||||
| High | 31 (17.9) | 10 (52.6) | ||||
| Progression to acute leukemia, no. (%) (n = 443)* | 57 (17.6) | 7 (31.8) | .098 | 16 (17.2) | 1 (20.0) | .872 |
| Death, no. (%) (n = 494)* | 86 (26.0) | 13 (61.9) | < .001 | 28 (20.6) | 1 (16.7) | .816 |
| . | PMF . | P . | PPV/PET-MF . | P . | ||
|---|---|---|---|---|---|---|
| EZH2 . | EZH2 . | |||||
| Wild-type . | Mutated . | Wild-type . | Mutated . | |||
| N | 348 | 22 | 141 | 7 | ||
| Median follow-up, mo, (range) (n = 500)* | 39.567 (1-340) | 28.183 (8-183) | .365 | 29.7 (1-234) | 16.8 (1-70) | .256 |
| Median age, y (range) | 60.0 (14-90) | 66.0 (38-90) | .135 | 62.0 (32-84) | 62.0 (52-78) | .731 |
| Male sex, no. (%) (n = 303)* | 221 (60.6) | 15 (68.2) | .481 | 73 (51.8) | 4 (57.1) | .781 |
| Leukocytes, × 106/L, mean ± SD (n = 500)* | 12.3 ± 13.3 | 18.7 ± 11.5 | .001 | 14.3 ± 14.1 | 13.8 ± 7.0 | .360 |
| Hb, g/L, mean ± SD (n = 497)* | 114 ± 27 | 112 ± 19 | .700 | 114 ± 26 | 100 ± 18 | .153 |
| Platelets, × 106/L, mean ± SD, (n = 501)* | 341.0 ± 345.6 | 405.0 ± 258.7 | .831 | 404.1 ± 317.6 | 232.1 ± 198.0 | .097 |
| Peripheral blast cells, %, mean ± SD (n = 329)* | 0.7 ± 2.1 | 1.6 ± 2.1 | .002 | 0.5 ± 2.0 | 0.4 ± 0.9 | .959 |
| Constitutional symptoms, no. (%) (n = 333)* | 67 (28.2) | 8 (42.1) | .198 | 34 (47.9) | 2 (40.0) | .733 |
| Splenomegaly, no. (%) (n = 494)*† | .016 | .059 | ||||
| 0 | 91 (27.6) | 1 (4.5) | 17 (12.6) | 3 (42.9) | ||
| 1 | 141 (42.7) | 9 (40.9) | 58 (43.0) | 1 (14.3) | ||
| 2 | 98 (29.7) | 12 (54.5) | 60 (44.4) | 3 (42.9) | ||
| Abnormal karyotype, no. (%) (n = 195)* | 26 (20.2) | 4 (36.4) | .209 | 17 (32.7) | 2 (66.7) | .229 |
| Unfavorable karyotype, no. (%) (n = 188)* | 18 (14.3) | 3 (27.3) | .252 | 11 (22.9) | 2 (66.7) | .092 |
| JAK2V617F, no. (%) (n = 518)* | 204 (58.8) | 9 (40.9) | .100 | 105 (74.5) | 3 (42.9) | .066 |
| JAK2V617F allele burden, %, mean ± SD | 45.7 ± 22.9 | 42.2 ± 22.1 | .603 | 64.1 ± 23.4 | 61.1 ± 52.6 | .747 |
| MPL W515L/K, no. (%) (n = 466)* | 13 (3.9) | 0 (0.0) | .344 | 5 (4.7) | 0 (0.0) | .586 |
| IWG-MRT score, no. (%) (n = 192)* | .002 | - | ||||
| Low | 55 (31.8) | 4 (21.1) | ||||
| Int-1 | 44 (25.4) | 5 (26.3) | ||||
| Int-2 | 43 (24.9) | 0 (0.0) | ||||
| High | 31 (17.9) | 10 (52.6) | ||||
| Progression to acute leukemia, no. (%) (n = 443)* | 57 (17.6) | 7 (31.8) | .098 | 16 (17.2) | 1 (20.0) | .872 |
| Death, no. (%) (n = 494)* | 86 (26.0) | 13 (61.9) | < .001 | 28 (20.6) | 1 (16.7) | .816 |
P values in bold indicate statistical significance.
Number of patients for whom information was available.
Splenomegaly: 0 = not palpable; 1 = palpable at < 10 cm from left costal margin; and 2 = palpable at > 10 cm from left costal margin.
The analysis of the PPV/PET-MF patients did not reveal any significant differences in terms of hematologic and clinical parameters that could be meaningfully associated with their EZH2 mutational status (Table 1). Similar results were obtained when PET-MF patients (n = 6) were considered separately from the single PPV-MF–mutated patient (data not shown).
To assess whether EZH2 status was correlated with IPSS prognostic score, we evaluated the distribution of PMF patients in the different IPSS risk categories.23 We observed that most EZH2-mutated patients (52.6%) clustered in the high-risk category compared with the low-risk group (21.1%; P = .002). The low number of EZH2-mutated patients who had cytogenetic analysis available (n = 11) did not allow us to ascertain the correlation of EZH2 mutational status with the Dynamic IPSS Plus (DIPPS Plus) score.24 However, the proportion of patients with unfavorable karyotype was double among EZH2-mutated versus wild-type subjects (27.3% vs 14.3% for PMF and 66.7% vs 22.9% in PPV/PET-MF patients, respectively), although the difference was not statistically significant possibly because of the low number of patients.
We also stratified PMF patients according to 4 different categories defined by their EZH2 and JAK2V617F mutational status (Table 2). Among EZH2 wild-type subjects, the presence of the JAK2V617F mutation was associated with significantly older age, higher leukocyte and hemoglobin levels, and a lower frequency of the MPLW515 mutation; conversely, there was no difference with regard to sex, platelet count, PB blast cell count, constitutional symptoms, splenomegaly, IPSS score, or proportion of patients progressing to leukemia. In EZH2-mutated patients, the concurrent presence of the JAK2V617F mutation did not affect the hematologic or clinical phenotype at all (Table 2).
Hematologic and clinical characteristics of EZH2-mutated subjects with PMF stratified according to JAK2V617F mutational status
| . | EZH2 wild-type . | P . | EZH2 mutated . | P . | ||
|---|---|---|---|---|---|---|
| JAK2 . | JAK2 . | |||||
| Wild-type . | V617F . | Wild-type . | V617F . | |||
| N | 143 | 204 | 13 | 9 | ||
| Follow-up, mo (range) n = 354)* | 49.4 (1-340) | 37.9 (1-282) | .020 | 25.7 (8-182) | 30.7 (11-84) | .616 |
| Median age, y, (range) | 55.0 (14-88) | 63.0 (18-90) | .001 | 57.0 (41-81) | 70 (38-90) | .324 |
| Male sex, no. (%) (n = 369)* | 90 (62.9) | 120 (58.8) | .440 | 8 (61.5) | 7 (77.8) | .421 |
| Leukocytes, × 106/L, mean ± SD (n = 354)* | 11.3 ± 13.0 | 13.1 ± 13.5 | .005 | 16.2 ± 11.5 | 22.4 ± 11.1 | .117 |
| Hemoglobin, g/L, mean ± SD (n = 352)* | 109 ± 24 | 118 ± 28 | .002 | 107 ± 22 | 118 ± 14 | .171 |
| Platelet, × 106/L, mean ± SD (n = 355)* | 447.0 ± 403.2 | 408.7 ± 308.0 | .840 | 392.8 ± 255.0 | 422.5 ± 278.6 | .764 |
| Peripheral blast cells, %, mean ± SD (n = 240)* | 1.0 ± 2.6 | 0.5 ± 1.7 | .090 | 1.4 ± 2.4 | 1.8 ± 1.8 | .299 |
| Constitutional symptoms, no. (%) (n = 257)* | 26 (28.3) | 41 (28.1) | .976 | 3 (27.3) | 5 (62.5) | .125 |
| Splenomegaly, no. (%) (n = 352)*† | .747 | .518 | ||||
| 0 | 41 (29.7) | 50 (26.0) | 1 (7.7) | 0 (0.0) | ||
| 1 | 58 (42.0) | 83 (43.2) | 6 (46.2) | 3 (33.3) | ||
| 2 | 39 (28.3) | 59 (30.7) | 6 (46.2) | 6 (66.7) | ||
| Unfavorable karyotype, no. (%) (n = 137) | 8 (12.1) | 10 (16.7) | .466 | 2 (28.6) | 1 (25.0) | .898 |
| JAK2V617F allele burden, %, mean ± SD | 45.9 ± 23.0 | 42.2 ± 22.1 | ||||
| MPL W515L/K, no. (%) (n = 354)* | 12 (8.8) | 1 (0.5) | < .0001 | 0 (0.0) | 0 (0.0) | |
| IWG-MRT score, no. (%) (n = 192)* | .484 | .247 | ||||
| Low | 26 (36.6) | 29 (28.4) | 3 (27.3) | 1 (12.5) | ||
| Int-1 | 14 (19.7) | 30 (29.4) | 4 (36.4) | 1 (12.5) | ||
| Int-2 | 18 (25.4) | 25 (24.5) | 0 (0.0) | 0 (0.0) | ||
| High | 13 (18.3) | 18 (17.6) | 4 (36.4) | 6 (75.0) | ||
| Progression to acute leukemia, no. (%) (n = 345)* | 26 (19.3) | 31 (16.5) | .520 | 6 (46.2) | 1 (11.1) | .083 |
| Death, no. (%) (n = 352)* | 38 (27.7) | 48 (24.7) | .541 | 7 (53.8) | 6 (75.0) | .332 |
| . | EZH2 wild-type . | P . | EZH2 mutated . | P . | ||
|---|---|---|---|---|---|---|
| JAK2 . | JAK2 . | |||||
| Wild-type . | V617F . | Wild-type . | V617F . | |||
| N | 143 | 204 | 13 | 9 | ||
| Follow-up, mo (range) n = 354)* | 49.4 (1-340) | 37.9 (1-282) | .020 | 25.7 (8-182) | 30.7 (11-84) | .616 |
| Median age, y, (range) | 55.0 (14-88) | 63.0 (18-90) | .001 | 57.0 (41-81) | 70 (38-90) | .324 |
| Male sex, no. (%) (n = 369)* | 90 (62.9) | 120 (58.8) | .440 | 8 (61.5) | 7 (77.8) | .421 |
| Leukocytes, × 106/L, mean ± SD (n = 354)* | 11.3 ± 13.0 | 13.1 ± 13.5 | .005 | 16.2 ± 11.5 | 22.4 ± 11.1 | .117 |
| Hemoglobin, g/L, mean ± SD (n = 352)* | 109 ± 24 | 118 ± 28 | .002 | 107 ± 22 | 118 ± 14 | .171 |
| Platelet, × 106/L, mean ± SD (n = 355)* | 447.0 ± 403.2 | 408.7 ± 308.0 | .840 | 392.8 ± 255.0 | 422.5 ± 278.6 | .764 |
| Peripheral blast cells, %, mean ± SD (n = 240)* | 1.0 ± 2.6 | 0.5 ± 1.7 | .090 | 1.4 ± 2.4 | 1.8 ± 1.8 | .299 |
| Constitutional symptoms, no. (%) (n = 257)* | 26 (28.3) | 41 (28.1) | .976 | 3 (27.3) | 5 (62.5) | .125 |
| Splenomegaly, no. (%) (n = 352)*† | .747 | .518 | ||||
| 0 | 41 (29.7) | 50 (26.0) | 1 (7.7) | 0 (0.0) | ||
| 1 | 58 (42.0) | 83 (43.2) | 6 (46.2) | 3 (33.3) | ||
| 2 | 39 (28.3) | 59 (30.7) | 6 (46.2) | 6 (66.7) | ||
| Unfavorable karyotype, no. (%) (n = 137) | 8 (12.1) | 10 (16.7) | .466 | 2 (28.6) | 1 (25.0) | .898 |
| JAK2V617F allele burden, %, mean ± SD | 45.9 ± 23.0 | 42.2 ± 22.1 | ||||
| MPL W515L/K, no. (%) (n = 354)* | 12 (8.8) | 1 (0.5) | < .0001 | 0 (0.0) | 0 (0.0) | |
| IWG-MRT score, no. (%) (n = 192)* | .484 | .247 | ||||
| Low | 26 (36.6) | 29 (28.4) | 3 (27.3) | 1 (12.5) | ||
| Int-1 | 14 (19.7) | 30 (29.4) | 4 (36.4) | 1 (12.5) | ||
| Int-2 | 18 (25.4) | 25 (24.5) | 0 (0.0) | 0 (0.0) | ||
| High | 13 (18.3) | 18 (17.6) | 4 (36.4) | 6 (75.0) | ||
| Progression to acute leukemia, no. (%) (n = 345)* | 26 (19.3) | 31 (16.5) | .520 | 6 (46.2) | 1 (11.1) | .083 |
| Death, no. (%) (n = 352)* | 38 (27.7) | 48 (24.7) | .541 | 7 (53.8) | 6 (75.0) | .332 |
P values in bold indicate statistical significance.
Number of patients for whom information was available.
Splenomegaly: 0 = not palpable; 1 = palpable at < 10 cm from left costal margin; and 2 = palpable at > 10 cm from left costal margin.
Association of EZH2-mutated genotype with disease outcome
Information about progression to acute leukemia and death were available in 443 and 494 patients, respectively. After a median follow-up of 39 months (range 1-340), 128 patients (25.9%) died; of these, 99 had PMF (28.1% of all PMF), 18 had PPV-MF (23.1%), and 11 had PET-MF (17.2%; supplemental Table 2). The median survival was 128 months in PMF patients (95% confidence interval [95% CI], 92-163) and 103.3 months (95% CI, 79-128) in PPV/PET-MF (supplemental Figure 2A-B). Considering PMF patients only, survival varied according to the 4 IPSS risk categories (supplemental Figure 2C); the median survival was 264 months (95% CI, 50-478) in the low-risk category, was not reached in the intermediate 1–risk category, 80 months (95% CI, 71-90) in the intermediate 2–risk category, and 32 months (95% CI, 18-46) in the high-risk category patients (P < .001). As reported previously,20,25 survival was also significantly reduced in JAK2V617F-mutated PMF patients, who presented an allelic burden < 25% compared with higher allelic burden quartiles (supplemental Figure 2D). JAK2V617F allele burden had no impact on survival in PPV/PET-MF patients (not shown), confirming previous findings.26
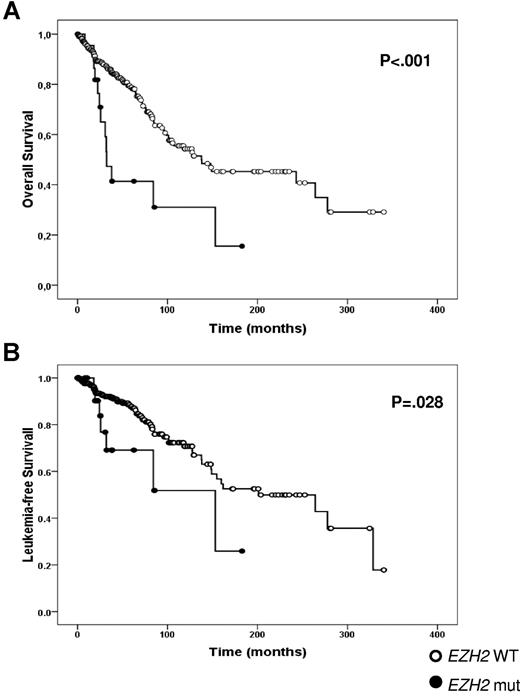
Among patients who died, 14 (13 with PMF and 1 with PET-MF) and 114 were EZH2 mutated or wild-type, respectively, corresponding to 51.9% and 24.4% of their respective categories (P < .001). The median OS was significantly shortened in EZH2-mutated PMF patients (31.6 months; 95% CI, 23-43) compared with wild-type (137 months; 95% CI, 53-222; P < .001; Figure 2A). Variables associated with reduced survival among PMF patients in univariate analysis were sex, IPSS score, low platelet count, low (< 25%) JAK2V617F allele burden, and EZH2-mutated status (Table 3). On multivariate analysis, OS was predicted by IPSS score (P < .0001), a < 25% JAK2V617F allele burden (P = .046), and EZH2-mutated status (P = .016; Table 3). The significant association between EZH2 mutation and OS was maintained even if the 3 intronic mutations in PMF subjects (supplemental Table 3) were not considered as causative; in this case, OS was 32.3 months (95% CI, 21.7-43.0) in EZH2-mutated patients (P < .0001 vs EZH2 wild-type). Finally, the fact that only one event was recorded in the PPV/PET-MF group prevented statistical analysis of survival between EZH2-mutated and wild-type patients.
OS (A) and LFS (B) measured from disease diagnosis to leukemia transformation in EZH2-mutated and wild-type patients with PMF.
OS (A) and LFS (B) measured from disease diagnosis to leukemia transformation in EZH2-mutated and wild-type patients with PMF.
Univariate and multivariate analysis for OS in PMF patients
| . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Univariate | ||
| Male sex | 0.547 (0.358-0.835) | .005 |
| IPSS score | 4.807 (2.807-8.232) | < .0001 |
| Platelet count < 100 × 109/L | 0.999 (0.998-0.999) | < .0001 |
| EZH2 mutation | 2.817 (1.565-5.072) | .001 |
| V617F burden < 25% | 2.256 (1.034-4.921) | .040 |
| Multivariate | ||
| IPSS score | 6.087 (2.723-13.606) | < .0001 |
| EZH2 mutation | 3.585 (1.274-10.091) | .016 |
| V617F burden < 25% | 1.082 (1.014-1.101) | .046 |
| . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Univariate | ||
| Male sex | 0.547 (0.358-0.835) | .005 |
| IPSS score | 4.807 (2.807-8.232) | < .0001 |
| Platelet count < 100 × 109/L | 0.999 (0.998-0.999) | < .0001 |
| EZH2 mutation | 2.817 (1.565-5.072) | .001 |
| V617F burden < 25% | 2.256 (1.034-4.921) | .040 |
| Multivariate | ||
| IPSS score | 6.087 (2.723-13.606) | < .0001 |
| EZH2 mutation | 3.585 (1.274-10.091) | .016 |
| V617F burden < 25% | 1.082 (1.014-1.101) | .046 |
Acute leukemia occurred in 81 patients (18.3%), corresponding to 19%, 14%, and 21% of PMF, PPV-MF, and PET-MF patients, respectively. Among these, 73 were EZH2 wild-type (17.5%) and 8 EZH2 mutated (26.9%). The leukemia-free survival (LFS), measured from diagnosis to the time of leukemia transformation, was significantly shorter in EZH2-mutated PMF patients (n = 7; 153.1 months, 95% CI, 42-264) compared with EZH2 wild-type patients (n = 57; 201.07 months, 95% CI, 103-299; P = .028) (Figure 2B). Again, the significant association between EZH2 mutation and LFS was maintained without the 3 intronic mutations; in this case, LFS was 84.2 months (95% CI, 12.9-155.5) in EZH2-mutated patients (P = .014 vs EZH2 wild-type). The low number of cases harboring the EZH2 mutation prevented any multivariate analysis of variables associated with reduced LFS. Due to low number of events, we were unable to determine the statistical significance of EZH2 mutational status for LFS in the PPV/PET-MF group.
Discussion
PMF is associated with poorer survival compared with other classic BCR-ABL–negative chronic MPNs.27 The identification of variables associated with prognosis is of considerable importance for driving therapeutic decisions, particularly concerning the choice between drug therapy and hematopoietic stem cell transplantation,28 which remains the only potentially curative therapeutic approach even in the era of JAK2 inhibitors. The IPSS, which includes older age, the presence of constitutional symptoms, anemia, leukocytosis, and blood blasts > 1% as risk variables, reliably discriminated 4 categories of patients with significantly different median survival times of 135, 95, 48, and 27 months.23 Further refinement of the IPSS are the DIPSS 29 and the DIPSS Plus score, which includes cytogenetic abnormalities, transfusion dependency, and thrombocytopenia as additional variables.24 Several studies have focused on the newly discovered somatic mutations in MPNs to ascertain their relationships with disease phenotype and, eventually, their prognostic value. Although with conflicting results, the presence of JAK2V617F in patients with PMF has been associated with older age, higher leukocyte and RBC indexes, and splenomegaly.25,30-32 However, most studies concluded that the OS of JAK2V617F-mutated patients was no different from their wild-type counterparts.20,25,31 Conversely, quantitative analysis of the V617F allele burden revealed a prognostically negative impact of a low allele burden (ie, the lowest quartile) in 2 independent series from the GIMEMA group20 and the Mayo Clinic.25 In patients with PPV-MF or PET-MF, no meaningful differences in survival were found depending on the mutated status and allelic burden of the JAK2V617F mutation.26 Finally, there is conflicting information about the prognostic relevance of a nullizygous status for the JAK2 predisposition haplotype 46/1 (“GGCC”).33,34 Other mutated genotypes, including MPLW515L/K,35,36 CBL,12 TET2,10 ASXL1,11 LNK,37,38 and IDH1/IDH2,39 have not been shown to be prognostically informative, although most patient series analyzed to date were too small to allow reliable statistical analyses.40 The aim of the present study was to clarify the prognostic relevance, if any, of newly discovered mutations in EZH2 in a large series of patients with PMF.
EZH2, the PcG Enhancer of Zeste Homolog 2, is the catalytic component of the polycomb repressive complex 2 (PRC2), which serves to trimethylate histone H3 lysine 27 (H3K27me3). It contains 3 main functional domains: the SANT1 and SANT2 domains, which are involved in DNA binding; a cysteine-rich (CXC) domain; and the catalytically active SET domain. H3K27 methylation by EZH2 requires the presence of 2 additional proteins: embryonic ectoderm development (EED) and suppressor of zeste 12 (SUZ12). PRC2 complexes contain other proteins, including PHD finger protein 1 (PHF1), which specifically promotes H3K27 trimethylation rather than dimethylation; sirtuin 1 (SIRT1); and jumonji, AT rich interactive domain 2 (Jarid2). Trimethylation at H3K27 results in transcriptional repression, as opposed to H3K4 trimethylation, catalyzed by the trithorax homolog myeloid/lymphoid leukemia (MLL), which is associated with transcriptional activation.
The gene encoding EZH2 is located at 7q36.1, comprises 20 exons, and extends for > 40 kb. Macro- and microdeletions specifically involving this region have been found in about 10% of MDS patients,41 and a few patients had loss-of-heterozygosity because of acquired uniparental disomy.15,16 Mutations of EZH2 have been reported in patients with PMF, MDS, MDS/MPN,15,16,42 and lymphoma.17,18 Unlike the heterozygous, gain-of-function missense mutation of Tyr641 in the SET domain that occurs in lymphoma43 and the overexpression of EZH2 seen in epithelial malignancies, mutations in MPN and MDS are scattered throughout the gene and result in loss of function. These data indicate that EZH2 may behave as a tumor suppressor or an oncogene depending on the cellular context, presumably by controlling chromatin structure and gene accessibility.44
In this large cohort of 518 patients with MF, EZH2 mutations were detected in approximately 6% of PMF patients, 9% of PET-MF patients, and only in only 1 PPV-MF patient (1.2%). Such a frequency is lower than originally reported in a smaller group of 30 subjects (13%),15 but is similar to a recent study that included 46 PMF cases (7%).45 EZH2-mutated patients concurrently harbored JAK2V617F, CBL, TET2, and ASXL1 mutations in 41.4%, 5.9%, 11.8%, and 22.2%% of cases, respectively. No EZH2-mutated patients also harbored a MPL mutation; however, these 2 rare molecular abnormalities are not necessarily mutually exclusive, because at least one such case has been described recently.45 IDH1/IDH2 mutations, reported preferentially in patients in leukemic transformation,39 were not detected in association with EZH2 mutations. Finally, in a prospective cohort of 118 PMF patients followed for a median of 39 months who tested negative for EZH2 mutation at diagnosis, only one became EZH2 mutation positive. Whereas we cannot exclude that this mutation was already present at very low level at diagnosis and went undetected, these data suggest that EZH2 mutations are usually already present at the time of diagnosis. In addition, we found that EZH2 mutations can be maintained in leukemic blasts at the time of leukemia transformation, as described for JAK2V617F.46 On the contrary, we found no evidence for EZH2 mutations being acquired at the time of leukemia transformation in any of the 8 patients who were wild-type at diagnosis.
The analysis of hematologic-clinical correlates highlighted only subtle differences associated with the EZH2 mutation in PMF patients, such as more pronounced leukocytosis, larger spleens, and higher circulating blast cells; therefore, we conclude that the EZH2 mutation does not contribute a specific phenotypic signature in patients with primary and PPV/PET PMF. Conversely, we found that EZH2 mutational status had a significant negative impact on disease outcome among PMF patients. This is supported by the following findings: (1) EZH2-mutated patients preferentially clustered in the IPSS high-risk category; (2) both OS and LFS were shortened in EZH2-mutated subjects compared with their wild-type counterparts; and (3) in a multivariate analysis, EZH2 mutational status maintained a negative prognostic significance together with the IPPS score and a low JAK2V617F allele burden. We believe that it is very unlikely that the adverse impact of the EZH2 mutation could be attributable to mutations in another gene known to be mutated in PMF, because there is no consistent evidence of an association between changes in CBL, TET2, and LNK and a poor prognosis in myeloid disorders,12,47,48 and because we found that only 6 of 27 (22.2%) evaluable EZH2-mutated cases were also mutated for ASXL1. Furthermore, it is noteworthy that a negative prognostic impact of the EZH2 mutation on survival has also been reported for patients with MDS/MPN, chronic myelomonocytic leukemia, and MDS.15,16 Conversely, the low number of events (death and leukemia) recorded in the PPV/PET-MF group prevented statistical testing of a possible impact of EZH2 mutational status on OS and LFS; data from a larger series of patients are needed before any firm conclusion can be drawn.
Our series was representative of the usual distribution of patients in the 4 IPSS-defined risk categories; however, the median survival of the entire cohort, and in particular that of 13 patients falling in the low- and intermediate 1–risk group, was longer than reported in the original IPSS cohort.23 This is probably a consequence of the relatively short follow-up time for our patients compared with the IPSS study; indeed, disease-related deaths in the low- and intermediate 1–risk categories occurred after 5 and 3 years, respectively, unlike the higher-risk groups, in which disease-related deaths typically occurred much earlier. Therefore, the prognostic impact of the EZH2 mutation could be even greater than reported herein once a greater number of events are accrued with longer follow-up times.
In summary, the results of this study indicate that an EZH2-mutated genotype represents a novel variable independently associated with adverse outcome in patients with PMF. The pathogenetic mechanisms underlying this correlation remain to be established, but nonetheless there is increasing evidence that disruption of the epigenetic machinery by mutations in genes such as EZH2, TET2, and ASXL1 makes an important contribution to the pathogenesis of MPN, and might therefore represent novel therapeutic targets.49
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the Associazione Italiana per la Ricerca sul Cancro, Milan, Italy (to A.M.V.; IG 9034); by a Leukemia & Lymphoma Research Specialist program grant (to N.C.P.C.); by the Associazione Italiana per la Ricerca sul Cancro (AIRC, Milan, Italy) “Special Program Molecular Clinical Oncology 5 × 1000” (grant 1005) to the AIRC-Gruppo Italiano Malattie Mieloproliferative (AGIMM). A detailed description of the AGIMM project is available at: http://www.progettoagimm.it.
Authorship
Contribution: P.G. collected patient samples, performed the research, and contributed to data analysis and manuscript writing; F.B., J.S., T.F., C.H-C, N.W. T.E., and A.V.J. performed the research and contributed to data analysis and manuscript writing; F.C., M.M., K.Z., A.R. A.D., L.V., A.B., and G.B. provided patient samples and clinical information and contributed to manuscript writing; and N.C.P.C. and A.M.V. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alessandro M. Vannucchi, MD, Department of Hematology, University of Florence, Viale Morgagni 85, 50134 Florence, Italy; e-mail: amvannucchi@unifi.it or Nicholas C. P. Cross, PhD, Wessex Regional Genetics Laboratory, Salisbury District Hospital, Salisbury SP2 8BJ, United Kingdom; e-mail: ncpc@soton.ac.uk.
References
Author notes
P.G., F.B., J.S., N.C.P.C., and A.M.V. contributed equally to this work.