Abstract
Human CD4+FoxP3+ T cells are functionally and phenotypically heterogeneous providing plasticity to immune activation and regulation. To better understand the functional dynamics within this subset, we first used a combined strategy of subcellular fractionation and proteomics to describe differences at the protein level between highly purified human CD4+CD25+ and CD4+CD25− T-cell populations. This identified a set of membrane proteins highly expressed on the cell surface of human regulatory T cells (Tregs), including CD71, CD95, CD147, and CD148. CD147 (Basigin or Emmprin) divided CD4+CD25+ cells into distinct subsets. Furthermore, CD147, CD25, FoxP3, and in particular CTLA-4 expression correlated. Phenotypical and functional analyses suggested that CD147 marks the switch between resting (CD45RA+) and activated (CD45RO+) subsets within the FoxP3+ T-cell population. Sorting of regulatory T cells into CD147− and CD147+ populations demonstrated that CD147 identifies an activated and highly suppressive CD45RO+ Treg subset. When analyzing CD4+ T cells for their cytokine producing potential, CD147 levels grouped the FoxP3+ subset into 3 categories with different ability to produce IL-2, TNF-α, IFN-γ, and IL-17. Together, this suggests that CD147 is a direct marker for activated Tregs within the CD4+FoxP3+ subset and may provide means to manipulate cells important for immune homeostasis.
Introduction
Human regulatory T cells (Tregs) are critical in maintaining self-tolerance and immune homeostasis and their development and function depends on the expression of the transcription factor FoxP3.1-3 Deletion or mutations in the FOXP3 gene leads to severe autoimmune disease and immunopathology in humans and mice4,5 and alteration or disruption of Treg function has been linked to the development of a broad array of human diseases including autoimmunity and cancer. An essential and sufficient role of FoxP3 in maintaining self-tolerance is supported by spontaneous immune activation and disruption of immune homeostasis in normal mice where the FoxP3 gene is conditionally deleted.6 Extensive characterization of Foxp3-dependent gene expression profiles combined with genome-wide screening of FoxP3 target sequences have identified a transcriptional signature for FoxP3+ Tregs7-10 that include the classic Treg markers CD25, CTLA-4 and GITR. Combined with the absence of CD127 expression, these are still the most used markers for identification and purification of Tregs11 in mice and humans. However, the global profiling of FoxP3+ cells has not yet led to clarification of whether a central molecular mechanism of suppression exists or whether the immune modulating activity of Tregs is composed of a repertoire of several mechanisms.12-14 This may partly be because of the fact that FoxP3 acts in concert with a set of other transcription factors, including NFAT, NF-kB, Eos, and Runx1 to integrate a transcriptional program necessary for Treg function.15-18 In humans, Treg biology is further complicated by functional heterogeneity between different FoxP3+ subsets and more dynamic expression of FoxP3 itself. In addition, gene profiling studies do not take into account regulation at the translational level and differences in protein expression that can be significant in the functional regulation of Tregs. As part of a comprehensive approach to characterize human Tregs, we performed a qualitative proteome analysis between highly purified CD4+CD25− and CD4+CD25+ T cells. To increase the resolution and hence the number of proteins identified in our experiments, we performed subcellular fractionation of each T-cell subset before analysis of in-gel tryptic digests by nano-LC-ESI-LTQ Orbitrap mass spectrometry. From this we identified several candidates with a preferential expression on CD4+CD25+ T cells including galectin-3, TNF receptor superfamily member 6 (CD95), transferrin receptor protein 1 (CD71) and receptor-type tyrosine-protein phosphatase η (CD148), proteins that previously have been reported to be either up-regulated in Tregs19,20 or regulated in a FoxP3-dependent manner.21 We were also able to identify the specific expression of FoxP3 itself in our nucleic/insoluble cytoskeleton fractions. Furthermore, tryptic peptides from membrane fractions of CD4+CD25+ cells identified the immunoglobulin family member CD147 or extracellular matrix metalloproteinase inducer (Emmprin) or Basigin. CD147 is expressed at different levels on several cell types and contains a single highly conserved transmembrane domain containing a glutamic acid22,23 that mediates interactions with other transmembrane proteins.24 This may also explain why CD147 has been implicated in a wide range of physiologic and pathologic functions. It has been reported to associate with the monocarboxylate transporters MCT1 and MCT4,24 integrins25,26 and caveolin-127 and act as a receptor for extracellular cyclophilins.28,29 However, it was originally identified and has been mostly studied as an inducer of matrix metalloproteinases (MMPs)30 in adjacent fibroblasts or in tumor cells.
CD147 has also been linked to the regulation of T-cell development and function. Thymocytes at the DN4 stage demonstrate high expression of CD147 and addition of blocking antibody arrests further development, suggesting a role for CD147 in thymocyte expansion.31 In the periphery, CD147 is expressed on activated lymphocytes and has been reported to be correlated with CD25 and HLA-DR on human CD4+ T cells and regulate their migration response to extracellular cyclophilin A.32 Certain antibodies toward CD147 block the proliferative response to anti-CD3 stimulation in a concentration dependent manner and lymphocytes from CD147 deficient mice display more robust proliferation in mixed lymphocyte assays.33 Interestingly, the expression of CD147 is increased on total CD3+ cells from Systemic Lupus Erythematosus (SLE) patients34 and targeting of CD147 seems to improve the treatment of graft versus host disease (GVHD).35 Our results, describing CD147 as a subset marker for activated human FoxP3+ Tregs, suggest a role for CD147 in the regulation of immune homeostasis.
Methods
Cells, reagents, and flow cytometry
Unless otherwise stated, cells were cultured in RPMI 1640 (Gibco) supplemented with 10% FCS, 100 U/mL penicillin/streptomycin, 1mM sodium pyruvate, and 1:100 nonessential amino acids.
See supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) for source of antibodies. Staining for intracellular FoxP3 protein was performed using the cell-fixation/cell-permeabilization buffers included in the FoxP3 staining kit (e-Bioscience). For analysis of cell surface markers and intracellular proteins, cells were first stained for cell-surface markers followed by fixing, permeabilization and intracellular staining procedures according to the manufacturer's instructions (e-Bioscience). Cells were washed once in PBS containing 2% FCS before data acquisition on a flow cytometer (FACSCalibur, FACSCanto II or FACSAria, BD Biosciences) and analyzed using FlowJo 8.8.2 software (TreeStar), ModFit LT 3.3 software (Topsham), or Cytobank (http://cytobank.stanford.edu).
Isolation of primary human CD4+CD25− and CD4+CD25+ T cells
Human peripheral blood was obtained from normal healthy donors (Oslo University Hospital, Ullevaal Blood Center, Oslo, Norway with approval from the Regional Ethics Review Board), and CD4+ T cells were enriched by incubating buffy coats with RosetteStep CD4+ enrichment cocktail (StemCell Technologies) for 20 minutes at RT before Isopaque-Ficoll (Lymphoprep; Axis-Shield) density gradient centrifugation. For proteomic analysis, CD4+CD25+ and CD4+CD25− T cells were isolated using a CD4+CD25+ Treg cell isolation kit according to the manufacturer's manual (Miltenyi Biotec). Briefly, CD4+ T cells were enriched from buffy coats as described in this section and separated into a CD25+ and a CD25− fraction by CD25 MicroBeads and magnetic separation on a MACS column. The cells were routinely analyzed by flow cytometry and the purity of the CD4+CD25+ and CD4+CD25− populations were > 98%. For Western blot analysis, CD4+CD25+CD127low and CD4+CD25−CD127high T cells were isolated by FACS sorting (FACSAria; BD Biosciences) to reach > 99% purity.
Cellular subfractionation and gel electrophoresis
Approximately 2.0 × 106 CD4+CD25+ and CD4+CD25− T cells were fractionated into cytosolic, membrane and nucleic/insoluble cytoskeleton fractions by the Qproteome Cell Compartment kit (QIAGEN). Protein content from each fraction was precipitated with cold acetone and dried under nitrogen. The resulting pellet was redissolved in 1× LDS NuPAGE Sample buffer containing 1× reducing agent (Invitrogen) and heated for 5 minutes at 95°C before gel electrophoresis on a 4%-12% NuPAGE gel (200V, 30 minutes; Invitrogen). Proteins were visualized by SimplyBlue safestain (Invitrogen).
Protein identification by LC-MS/MS
Stained gel lanes were cut into 14 slices and each in-gel digested with 0.1 μg trypsin (Promega) in 20 μL 25mM ammonium bicarbonate, pH 7.8 at 37°C for 16 hours. For each slice the tryptic peptides were purified with μ-C18 ZipTips (Millipore) and dried using a Speed Vac concentrator (Savant), then dissolved in 10 μL 1% formic acid in water and 5 μL were injected onto an LC/MS system consisting of a Dionex Ultimate 3000 nano-LC system connected to an LTQ OrbitrapXL mass spectrometer (ThermoElectron) equipped with a nanoelectrospray ion source. For liquid chromatography, an Acclaim PepMap 100 column (C18, 3 μm, 100 Å; Dionex) was used with a flow rate of 300 nL/min and the solvent gradient 7% B to 50% B in 40 minutes (Solvent A, 0.1% formic acid; solvent B 90% acetonitrile in 0.1% formic acid).
The mass spectrometer was operated in the data-dependent mode to automatically switch between Orbitrap-MS and LTQ-MS/MS acquisition. Other instrument parameters were as previously described.36 Raw data were processed using DTA Version 1.33 supercharge software to generate mgf files. A database search was performed by MS/MS ion search algorithms using Mascot 2.2.1 by database comparisons37 with human entries (18 117 sequences) from the UniProt database (http://www.uniprot.org). Trypsin was selected as enzyme with at maximum one missed cleavage site and tolerance, 5 ppm for the precursor ion and 0.5 Da for the MS/MS fragments. Methionine oxidation, acetylation at protein N-terminus, deamidation of asparagines and glutamines and propionamide formation of cysteines were allowed as variable modifications. Proteins were considered to be identified if at least 1 peptide revealed a Mascot score of above 30.
Western blotting
Total cell lysates of CD4+CD25+CD127low and CD4+CD25−CD127high T cells were prepared and proteins separated by gel electrophoresis as described in “Isolation of primary human CD4+CD25− and CD4+CD25− T cells” and “Cellular subfraction and gel electrophoresis” and transferred to nitrocellulose membranes using the iBlot unit (Invitrogen). Membranes were blocked with 5% nonfat dry milk followed by incubation with primary antibodies and visualized using a HRP-conjugated secondary antibody and the SuperSignal West Pico kit (Pierce).
In vitro activation of human T cells
Purified cells were diluted to 1 × 106 cells/mL in complete medium and activated by the addition of anti-CD3/anti-CD2/anti-CD28–coated beads (T-cell Activation/Expansion kit; Miltenyi Biotec) at a bead to cell ratio of 1:2. Cells were then cultured for 48 h at 37°C and 5% CO2.
Coculture and in vitro suppression assay
Human naive responder T cells (CD4+ CD25−CD147− or CD4+CD25−CD147+) were sorted on FACSAria flow cytometer (BD Biosciences) and stained with 2mM CFSE before stimulation and coculture with sorted suppressor cells (CD4+CD25+CD147high or CD4+CD25+CD147low) at indicated cell:cell ratios. Cells were stimulated for 4 days with anti-CD3/anti-CD2/anti-CD28–coated beads at a bead-to-cell ratio of 1:5 before analysis by flow cytometry (FACSCalibur: BD Biosciences).
Intracellular cytokine staining
For detection of intracellular cytokine production, CD4+ T cells were stimulated with 50 ng/mL of phorbol myristate acetate (PMA) plus 2 μg/mL of ionomycin for 6 hours in the presence of 10 μg/mL brefeldin A. Cells were washed twice in 2% FCS, fixed and permeabilized before staining with anti-FoxP3, anti-CD147, anti-CD4 and either anti–IFN-γ, anti–TNF-α, anti–IL-10, anti-CD152 (CTLA-4), anti–IL-2, or anti–IL-17 according to manufacturer's instructions (e-Bioscience and BD Biosciences) and analyzed by flow cytometry (FACSAria; BD Biosciences).
Results
Identification of potential Treg biomarkers using a proteomic strategy
The work flow applied in the presented work is summarized in Figure 1A. To investigate the protein expression profiles in Tregs versus effector T cells, CD4+CD25+ and CD4+CD25− T cells were isolated from human blood. Each cell population was then subfractionated into cytosolic, membrane and nucleic/insoluble cytoskeleton compartments before SDS-PAGE. Each lane of a SimplyBlue stained gel was cut into 14 pieces and proteins in-gel digested with trypsin. The peptides were analyzed by nanoLC-ESI-LTQ-Orbitrap mass spectrometry. MS/MS spectra were searched against the Uniprot database using MASCOT as search engine and an ion score > 30 for at least 1 peptide was set as a threshold for identification. By comparing protein expression profiles of CD4+CD25+ and CD4+CD25− T cells from 3 independent experiments, a total of 28 proteins were uniquely identified in CD4+CD25+ cells, including the transcription factor FoxP3 (Figure 1B). In addition, 14 proteins were only observed in the CD4+CD25− T cells (supplemental Table 1). Of the proteins highly expressed in the CD25+ cells, 12 proteins were found in the cytosolic compartment, 11 proteins in the membrane compartment and 5 proteins in the nucleic/insoluble cytoskeleton fraction. Furthermore, while all except 1 protein (MCM4) in the CD4+CD25+ membrane fraction were transmembrane proteins, 4 of these were also known to have extracellular domains according to Uniprot, that is, Basigin/Emmprin (CD147), receptor-type tyrosine-protein phosphatase-η (CD148), transferrin receptor protein 1 (CD71) and tumor necrosis factor receptor superfamily member 6 (CD95).
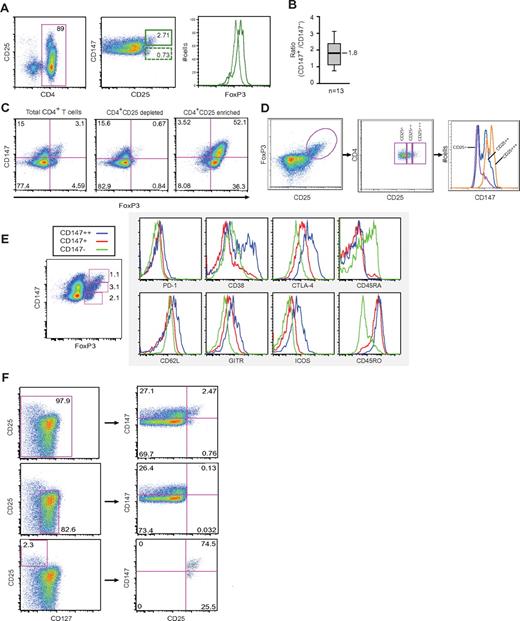
Identification of possible Treg biomarkers. (A) Work flow of the strategy applied to identify and validate potential Treg biomarkers. CD4+CD25+ and CD4+CD25− T cells were isolated by antibody coupled magnetic beads. Isolated cells were subfractionated into their respective cytosolic, membrane and nucleic/insoluble cytoskeleton fractions. Each protein fraction was precipitated by ice-cold acetone followed by SDS-PAGE. SimplyBlue SafeStain stained protein bands were excised, in-gel trypsinated and the resulting peptides enriched on a C18 gel-loader tip column before nano-LC-MS/MS analysis. Resulting peptide fragmentation spectra were searched against the Uniprot database using Mascot as a search engine. A selected set of proteins were taken further for validation by complementary methods and finally functional analysis. (B) Proteins observed exclusively in CD4+CD25+ cells, number of observations and observed peptides, total Mascot score and the proteins respective cellular compartments. To fulfill our criteria, proteins had to be observed in CD4+CD25 enriched cells in at least 2 of 3 experiments and not in the CD4+CD25 depleted cells. A Mascot cut-off ion score of 30 was applied on the peptide level to ensure high confidence. (C) Among the identified membrane proteins, 4 were already designated with a number in the CD nomenclature and were known to contain an extracellular domain, CD95, CD147, CD71, and CD148. (C) CD4+ cells were either depleted or enriched for CD25 and cell surface stained with an antibody cocktail containing conjugated anti-CD4, anti-CD3, anti-CD25, and either anti-CD95, anti-CD147, anti-CD71, or anti-CD148 before flow cytometry.
Identification of possible Treg biomarkers. (A) Work flow of the strategy applied to identify and validate potential Treg biomarkers. CD4+CD25+ and CD4+CD25− T cells were isolated by antibody coupled magnetic beads. Isolated cells were subfractionated into their respective cytosolic, membrane and nucleic/insoluble cytoskeleton fractions. Each protein fraction was precipitated by ice-cold acetone followed by SDS-PAGE. SimplyBlue SafeStain stained protein bands were excised, in-gel trypsinated and the resulting peptides enriched on a C18 gel-loader tip column before nano-LC-MS/MS analysis. Resulting peptide fragmentation spectra were searched against the Uniprot database using Mascot as a search engine. A selected set of proteins were taken further for validation by complementary methods and finally functional analysis. (B) Proteins observed exclusively in CD4+CD25+ cells, number of observations and observed peptides, total Mascot score and the proteins respective cellular compartments. To fulfill our criteria, proteins had to be observed in CD4+CD25 enriched cells in at least 2 of 3 experiments and not in the CD4+CD25 depleted cells. A Mascot cut-off ion score of 30 was applied on the peptide level to ensure high confidence. (C) Among the identified membrane proteins, 4 were already designated with a number in the CD nomenclature and were known to contain an extracellular domain, CD95, CD147, CD71, and CD148. (C) CD4+ cells were either depleted or enriched for CD25 and cell surface stained with an antibody cocktail containing conjugated anti-CD4, anti-CD3, anti-CD25, and either anti-CD95, anti-CD147, anti-CD71, or anti-CD148 before flow cytometry.
To validate our proteomic data we analyzed CD4+ T cells either enriched or depleted for CD25 by flow cytometry using a cocktail of conjugated anti-CD3, anti-CD4, anti-CD25, and either anti-CD71, anti-CD95, anti-CD148, or anti-CD147 (Figure 1C). All 4 cell surface proteins revealed a clear correlation with CD25 expression. Analysis of the data showed that 82.2% of the CD25+ cells stained positive for CD95, 59.7% for CD147, 10.1% for CD71, and 19.6% of the CD25+ cells were positive for CD148, while only 30.9% of the CD25− cells stained positive for CD95, 13.9% for CD147, 0.48% for CD71 and 0.95% for CD148. The correlation of the presence of these transmembrane proteins with CD25 expression and the positive identification of FoxP3 in the nuclear fraction (supplemental Figure 1) support the validity of our proteomic approach. We were also able to confirm the preferential expression of Mitofusin2 (MFN2) and CAAX prenyl protease 1 homolog (FACE1) identified in the proteomic screen in CD4+CD25+ T cells by Western blot analysis (supplemental Figure 1).
CD147 identifies a subset of FoxP3 positive human T cells
Results in Figure 1C suggested that subsets of CD4+CD25+ cells can be defined based on CD147 expression. Analysis of CD4+ T cells obtained from 13 healthy individuals demonstrated that the average ratio of CD4+CD25+CD147+ to CD4+CD25+CD147− T cells was 1.8 (Figure 2A-B) and comparison of the CD4+CD25+CD147+ and CD4+CD25+CD147− populations revealed that the CD147+ cells were more abundant (P < .01, n = 13). To further dissect the relationship between CD147 and Tregs, we looked at the FoxP3 expression in CD4+CD25+CD147+ versus CD4+CD25+CD147− T cells which revealed that the CD147+ population expressed significantly higher levels of FoxP3 than the CD147− population (Figure 2A). Staining of CD25 enriched and CD25 depleted CD4+ cells confirmed a distribution of FoxP3+ cells with CD147 and suggested a positive correlation between FoxP3 and CD147 expression (Figure 2C). This was further supported by analyzing 3 subsets of CD4+FoxP3+ T cells as defined by CD25 for their CD147 expression. Indeed, an increased expression of CD147 was observed with increasing levels of CD25 expression in FoxP3 positive T cells (Figure 2D). To characterize human Tregs in greater detail on the basis of CD147 expression, CD4+FoxP3+ cells were gated into CD147−, CD147+, and CD147++ as indicated and analyzed for the expression of phenotypical markers (Figure 2E). While the expression of PD-1 and CD62L were comparable between the subsets, the expression of the Treg related markers GITR, ICOS, and CTLA-4 increased with the level of CD147 expression. Furthermore, CD147++ cells were positive for CD38, previously demonstrated to be expressed on Treg subsets.38 Importantly, both the CD147+ and CD147++ subsets were uniformly CD45RO+ in contrast to CD147− Tregs that expressed CD45RA, suggesting that up-regulation of CD147 marks the switch between naive and activated Tregs (Figure 2E).
High expression of CD147 correlates with high levels of FoxP3 and CD45RO and low levels of CD127 expression. (A-C) CD4+ T cells from human buffy coats were analyzed for their FoxP3 expression with respect to CD147. Total CD4 (A-B), CD4+CD25 enriched or CD4+CD25 depleted (C) T-cell fractions were analyzed by FACS using anti-CD4, anti-CD25, anti-CD147, and anti-FoxP3 under nonstimulating conditions. The ratio of CD4+CD25+CD147+ versus CD4+CD25+CD147− T cells in CD4 enriched cell fractions from 13 healthy individual blood donors is shown (B). Distribution of the obtained ratios of CD4+CD25+CD147+ versus CD4+CD25+CD147− T cells in n = 13 donors with median (line), 25-75 (box), and 2.5-97.5 (bar) percentiles shown. (D) CD147 expression in 3 subsets of CD4+ FoxP3+ T cells as defined by CD25 expression. (E) Phenotypical analysis of CD4+CD147−FoxP3+ and CD4+CD147+FoxP3+ T cells. Cell surface marker staining was determined by flow cytometry. Similar results were observed in at least 3 independent experiments for each marker, and the results of a representative experiment are shown. (F) Flow cytometric analysis using anti-CD4, anti-CD25, anti-CD147, and anti-CD127 demonstrates the negative correlation between CD147 and CD127.
High expression of CD147 correlates with high levels of FoxP3 and CD45RO and low levels of CD127 expression. (A-C) CD4+ T cells from human buffy coats were analyzed for their FoxP3 expression with respect to CD147. Total CD4 (A-B), CD4+CD25 enriched or CD4+CD25 depleted (C) T-cell fractions were analyzed by FACS using anti-CD4, anti-CD25, anti-CD147, and anti-FoxP3 under nonstimulating conditions. The ratio of CD4+CD25+CD147+ versus CD4+CD25+CD147− T cells in CD4 enriched cell fractions from 13 healthy individual blood donors is shown (B). Distribution of the obtained ratios of CD4+CD25+CD147+ versus CD4+CD25+CD147− T cells in n = 13 donors with median (line), 25-75 (box), and 2.5-97.5 (bar) percentiles shown. (D) CD147 expression in 3 subsets of CD4+ FoxP3+ T cells as defined by CD25 expression. (E) Phenotypical analysis of CD4+CD147−FoxP3+ and CD4+CD147+FoxP3+ T cells. Cell surface marker staining was determined by flow cytometry. Similar results were observed in at least 3 independent experiments for each marker, and the results of a representative experiment are shown. (F) Flow cytometric analysis using anti-CD4, anti-CD25, anti-CD147, and anti-CD127 demonstrates the negative correlation between CD147 and CD127.
The combination of CD127 and CD25 cell surface expression is commonly used to distinguish between regulatory and conventional CD4+ cells.11 To determine the relationship between different cell populations gated on CD25 and CD127 and their level of CD147 expression, we stained human CD4+ T cells with a combination of anti-CD25, anti-CD127, and anti-CD147 antibodies. As illustrated in Figure 2F, the CD25highCD127− gated population directly corresponds to the subsets defined in Figure 2A and D as CD25highCD147+ and demonstrates that CD147 identifies a unique Treg profile that is not identified by CD25 and CD127 expression levels alone.
Stability of CD147 expression under activating conditions
We next tested the stability and expression profile of CD147 in CD25 depleted and CD25 enriched populations of CD4+ T cells under both resting and activated conditions (Figure 3A). The number of CD147+CD25+ or CD147+ FoxP3+ doubly positive cells appeared to be independent of T-cell activation in the CD25 enriched population but not in the CD25 depleted cells where doubly positive cells increased as analyzed by cell numbers or MFI. Cells doubly positive for CD147 and CD45RO were observed to increase significantly in both CD25 enriched and CD25 depleted fractions, but at a higher level in the CD25 depleted population (Figure 3A cell numbers and MFI). To follow the development within each cell subset in more detail, we decided to isolate CD4+CD147+CD25high (Fraction II) and CD4+CD147−CD25high (Fraction III) by FACS sorting before activation by CD2/CD3/CD28-coated beads for 48 hours (Figure 3B). As expected both subsets significantly up-regulated CD25 on stimulation and Fraction III cells increased their CD147 expression to a level comparable with Fraction II cells. Fraction III cells also clearly increased their expression of Foxp3, while Fraction II cells displayed a small reduction. This suggest that the Fraction III cells obtain a phenotype similar to Fraction II cells on TCR activation and hence provide a source for the CD147+FoxP3+ cells.
Stability of CD147 expression. (A) CD4+ T cells, either enriched or depleted for CD25, were stimulated for 48 hours by anti-CD3/anti-CD2/anti-CD28–coated beads at a 1:2 ratio of beads to cells. Resting and activated T cells were then analyzed for their expression of CD25, FoxP3, and CD45RO versus CD147. Mean fluorescence intensities (MFI) in doubly positive cells are indicated. (B) CD4+CD25+CD147+ and CD4+CD25+CD147− T cells were FACS sorted to > 96% purity as determined by their CD147 expression and activated by CD23/CD3/CD28-coated beads for 48 hours. Expression profiles of CD25, CD147, and FoxP3 under resting and stimulating conditions were analyzed by flow cytometry and compared in histogram-overlay analysis.
Stability of CD147 expression. (A) CD4+ T cells, either enriched or depleted for CD25, were stimulated for 48 hours by anti-CD3/anti-CD2/anti-CD28–coated beads at a 1:2 ratio of beads to cells. Resting and activated T cells were then analyzed for their expression of CD25, FoxP3, and CD45RO versus CD147. Mean fluorescence intensities (MFI) in doubly positive cells are indicated. (B) CD4+CD25+CD147+ and CD4+CD25+CD147− T cells were FACS sorted to > 96% purity as determined by their CD147 expression and activated by CD23/CD3/CD28-coated beads for 48 hours. Expression profiles of CD25, CD147, and FoxP3 under resting and stimulating conditions were analyzed by flow cytometry and compared in histogram-overlay analysis.
CD147 identifies a highly suppressive subset of human Tregs
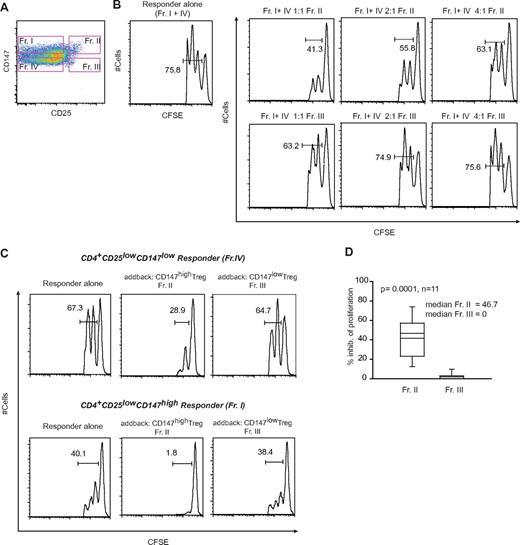
To analyze the functional difference between the CD147+ and CD147− Tregs, we examined the ability of these two subsets to suppress the in vitro proliferative responses of CD4+CD25low T cells. Since CD147 not only splits the Treg population but also the effector T-cell population, we divided the total CD4+ population into 4 different fractions by cell sorting. Whereas Fraction II and III are identical with the Treg Fractions CD25highCD147+ and CD25highCD14; Fraction I and IV describe the two effector T-cell fractions staining positive or negative for CD147, respectively (Figure 4A). As shown in Figure 4B, CFSE dye dilution assay analyzed by flow cytometry of CFSE-labeled responder CD4+CD25lowCD147+/− T cells (Fraction I + IV) demonstrated that CD147+CD25high Tregs (Fraction II) are able to suppress T-cell proliferation more efficiently than CD147−CD25high Tregs (Fraction III). Furthermore, to test the proliferative response of Fraction I and IV alone, as well as the suppressive activity of the CD147+ and CD147− Tregs on each of these responder T-cell fractions, we performed suppression assays where we used Fraction I and IV as separate responder T-cell subsets. As shown in Figure 4C, the suppressive activity of CD147+ (Fraction II) and CD147− (Fraction III) Tregs on the proliferation of Fraction I or IV did not differ significantly from the results obtained in Figure 4B. The proliferative response, however, did vary quite dramatic between the CD147+ and the CD147− responder T cells. As CD147−CD25− responders proliferated strongly, the CD147+CD25− displayed a very modest proliferation on activation using anti-CD3/anti-CD2/anti-CD28–coated beads. When comparing the suppressive activity of CD147− (Fraction III) and CD147+ (Fraction II) Tregs isolated from 11 different donors a clear and reproducible distinction was seen (Figure 4D), supporting that CD147 provides a useful marker to dissect the function of human regulatory T cells. Analyses of the same data in Figure 4B through D with the cell-cycle analysis software ModFit Lt for nonproliferating cells yielded similar results.
Suppressive activity of CD147+ and CD147− Treg cells. (A) CD4+ T cells were FACS sorted into 4 major different subsets (Fraction I-IV) based on their CD147 and CD25 expression levels. (B) Responder cells, Fraction I and IV, were sorted as one fraction and labeled with CFSE. FACS sorted CD147+ Treg (Fraction II) or CD147− Treg (Fraction III) were added as indicated at ratios versus responder cells that varied from 1:1 to 1:4. Cultures were activated using anti-CD3/anti-CD2/anti-CD28-coated beads at a 1:5 ratio of beads to cells and number of proliferating cells identified in FlowJo Version 8.8.2. (C) Responder cells, Fraction I and IV were FACS sorted, CFSE labeled and cultured separately. Treg suppression assay was performed as in panel B with a CD147+ Treg (Fraction II) or CD147− Treg (Fraction III) cell (addback cells) versus responder cell ratio of 1:2. (D) Proliferation data from Treg suppression assays were normalized and the suppressive activity of CD147+ Tregs and CD147− Tregs were compared from 11 healthy individual blood donors. Statistical significance was determined with Student t test with P < .001. Median (line), 25-75 (box) and 2.5-97.5 (bar) percentiles are shown.
Suppressive activity of CD147+ and CD147− Treg cells. (A) CD4+ T cells were FACS sorted into 4 major different subsets (Fraction I-IV) based on their CD147 and CD25 expression levels. (B) Responder cells, Fraction I and IV, were sorted as one fraction and labeled with CFSE. FACS sorted CD147+ Treg (Fraction II) or CD147− Treg (Fraction III) were added as indicated at ratios versus responder cells that varied from 1:1 to 1:4. Cultures were activated using anti-CD3/anti-CD2/anti-CD28-coated beads at a 1:5 ratio of beads to cells and number of proliferating cells identified in FlowJo Version 8.8.2. (C) Responder cells, Fraction I and IV were FACS sorted, CFSE labeled and cultured separately. Treg suppression assay was performed as in panel B with a CD147+ Treg (Fraction II) or CD147− Treg (Fraction III) cell (addback cells) versus responder cell ratio of 1:2. (D) Proliferation data from Treg suppression assays were normalized and the suppressive activity of CD147+ Tregs and CD147− Tregs were compared from 11 healthy individual blood donors. Statistical significance was determined with Student t test with P < .001. Median (line), 25-75 (box) and 2.5-97.5 (bar) percentiles are shown.
CD147 expression correlates with CTLA-4 and defines CD4+ subsets with different cytokine potential
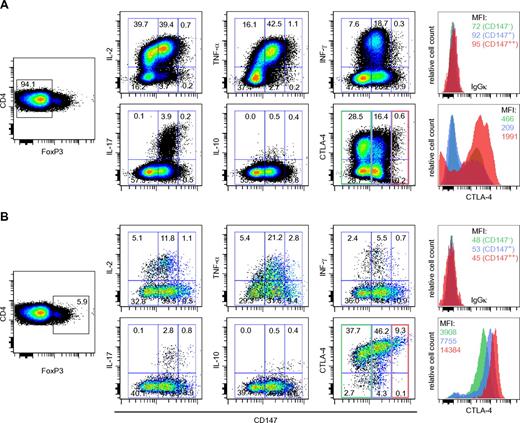
Having demonstrated that CD4+CD25highCD147+ cells have a much higher suppressive activity compared with CD4+CD25highCD147− T cells, we assessed the cytokine producing potential of the CD4+FoxP3− and CD4+FoxP3+ cells based on their CD147 expression. Intracellular cytokine staining was used to analyze the production of IL-2, IL-10, IFN-γ, TNF-α, and IL-17 on phorbol myristate acetate (PMA) and ionomycin treatment in the presence of brefeldin A. As illustrated in Figure 5, we were able to split the CD4+FoxP3− and the CD4+FoxP3+ populations into 3 different subsets with cytokine producing potential based on their CD147 expression (CD147−, CD147+, and CD147++). Irrespective of the FoxP3 expression, the CD147+ subset demonstrated the most pronounced production of IL-2, IFN-γ, and TNF-α after PMA and ionomycin treatment (Figure 5A-B) and interestingly, IL-17 production was also mainly restricted to this subset. The CD147++ cells demonstrated limited ability to produce any of the cytokines tested (Figure 5A-B). In the same experimental setup, we also tested the correlation between CTLA-4 and CD147 expression. CTLA-4 is described to play a key role in regulating Treg suppressive activity39 and high expression correlates with activated highly suppressive human Tregs.13 As demonstrated in Figure 5B, there is a strong positive correlation between increasing CD147 expression and CTLA-4 within the FoxP3+ subset. This positive correlation is not observed in the FoxP3− population where the majority of CTLA-4+ cells are in the CD147− population. Together this further supports that CD147 can be a useful marker to identify and study functional subsets of human Tregs.
CD147 expression levels divide CD4+ FoxP3− and FoxP3+ T cells into 3 functionally different subsets with different levels of cytokine production. CD4+FoxP3− (A) or CD4+FoxP3+ (B) T cells were analyzed for their capacity to produce cytokines (IL-2, TNF-α, IFN-γ, IL-17, and IL-10) and the expression of CTLA-4 after stimulation with PMA and ionomycin and stratified by their CD147 expression. Percent cytokine producing cells are shown from one representative of 4 independent experiments. Histograms are shown for CTLA-4 expression at different levels of CD147 expression.
CD147 expression levels divide CD4+ FoxP3− and FoxP3+ T cells into 3 functionally different subsets with different levels of cytokine production. CD4+FoxP3− (A) or CD4+FoxP3+ (B) T cells were analyzed for their capacity to produce cytokines (IL-2, TNF-α, IFN-γ, IL-17, and IL-10) and the expression of CTLA-4 after stimulation with PMA and ionomycin and stratified by their CD147 expression. Percent cytokine producing cells are shown from one representative of 4 independent experiments. Histograms are shown for CTLA-4 expression at different levels of CD147 expression.
CD147 expression level directly relates to CD45RA and FoxP3 defined human T-cell subsets
Recently, human FoxP3+ T cells have been classified into 3 subsets as defined by their expression profile of FoxP3 and CD45RA.13 Activated, highly suppressive cells are CD45RA−FoxP3++ while a cytokine producing nonsuppressive population is CD45RA−FoxP3+. Naive Tregs are CD45RA+FoxP3+ before becoming CD45RA−FoxP3++ and highly suppressive on activation, hence the switch from CD45RA+ to CD45RA− (or CD45RO+) seems to mark a functional shift within the Treg population. To test how CD147 expression relates to the described CD45RA/FoxP3 subsets, we gated CD4+ T cells based on their CD147 expression (as defined in Figure 5) and analyzed each subset with respect to CD45RA and FoxP3 expression. As demonstrated in Figure 6A, the level of CD147 correlated closely with the CD45RA/FoxP3 defined Treg subsets. Whereas almost all of the naive Tregs and some of the cytokine producing cells were CD147−, most of the cytokine producing CD45RA−FoxP3+ cells (not suppressive) were found in the CD147+ population which is in line with the results in Figure 5. In contrast, activated FoxP3++ cells were almost exclusively identified among the CD147++ gated cells. A similar relationship was seen when performing the opposite gating based on CD45RA and FoxP3 expression (Figure 6B), supporting the notion that the level of CD147 expression is linked to the functional status of Treg subsets. Distribution of these cell populations in 6 healthy blood donors, is shown in Figure 6C. Lastly, analysis of the methylation status of 10 methylation sites in the Treg-specfic demethylated region (TSDR) of the FoxP3 gene13 demonstrated the TSDR to be > 90% demethylated in CD147++CD25++ activated Tregs as well as in CD147++CD25− activated effector T cells and 100% demethylated in CD147−CD25++ resting Tregs (supplemental Figure 2). In contrast, the TSDR was 75% methylated in CD147−CD25− naive T cells. The status of this epigenetic mark would indicate stable expression of FoxP3 in the CD147++CD25++ activated Tregs.
CD147 expression directly correlates with CD45RA/FoxP3-defined subsets. (A) CD4+ T cells were stained with an antibody cocktail consisting of conjugated anti-CD4, anti-CD147, anti-CD45RA, and anti-FoxP3 before flow cytometric analysis. The total CD4+ population was split into 3 different subsets depending on their CD147 expression levels, that is, CD147−/CD147+/CD147++ and analyzed with respect to their CD45RA and FoxP3 staining. The presented figure was obtained from one representative of 6 healthy blood donors. (B) A histogram overlay analysis revealed the positive correlation of CD147 expression and the activation status of Tregs. (C) A graphic presentation of the variations observed in the 6 individuals analyzed in panel A.
CD147 expression directly correlates with CD45RA/FoxP3-defined subsets. (A) CD4+ T cells were stained with an antibody cocktail consisting of conjugated anti-CD4, anti-CD147, anti-CD45RA, and anti-FoxP3 before flow cytometric analysis. The total CD4+ population was split into 3 different subsets depending on their CD147 expression levels, that is, CD147−/CD147+/CD147++ and analyzed with respect to their CD45RA and FoxP3 staining. The presented figure was obtained from one representative of 6 healthy blood donors. (B) A histogram overlay analysis revealed the positive correlation of CD147 expression and the activation status of Tregs. (C) A graphic presentation of the variations observed in the 6 individuals analyzed in panel A.
Discussion
The molecular mechanisms underlying Treg mediated suppression of other immune cells will provide a fundamental understanding of physiology of immune regulation and are expected to unravel important new targets for immune therapy. Although great efforts have been made to characterize FoxP3+ cells, the molecular nature of the suppressive mechanism remains obscure. This has probably been complicated by the fact that in humans CD4+ FoxP3+ T cells are heterogeneous in nature with potential to dynamically change their functional properties based on the coexpression of other regulatory factors. First, Treg suppressive function probably involves integrated activity of FoxP3 with other transcription factors like NFAT, NFκB, Runx1, and Eos.15-18 Furthermore, it is becoming more evident that there is greater heterogeneity and dynamic distribution of human T-cell subsets, including FoxP3 positive subsets, and a context-dependent and overlapping expression of the lineage factors GATA-3, T-bet, RORγt, and FoxP3.40 This again directs their cytokine potential and functional role in each case and together provides an explanation on the observed functional differences between FoxP3 positive cells in human and mice. In addition, this provides a model that depending on the context and immune activation, FoxP3 expressing T cells can adapt and adjust their functions to balance and guide immune responses as needed. Therefore, markers that make it possible to dissect out the individual properties of FoxP3 positive subsets may be important in linking function to molecular characteristics. In this study, we aimed to identify proteins preferentially expressed in CD4+CD25+ enriched T cells, specifically focusing on membrane proteins containing extracellular domains. The identification of membrane proteins by a general proteomic approach is, however, not always straightforward. Despite constituting 30% of the total genome, membrane proteins are under-represented in many proteome profiles.41 Their under-representation is attributed mainly because of the heterogeneous, hydrophobic and low abundance nature. To overcome some of these limitations, we performed a subcellular fractionation of highly purified CD4+CD25+ and CD4+CD25− T cells before proteomic analysis. The 2 techniques form an ideal partnership when it comes to enrichment and analysis of intracellular organelles, membranes and low abundant multiprotein complexes. Subcellular fractionation results in reduced sample complexity, higher numbers of protein identifications and can efficiently be combined with gel electrophoresis/mass spectrometry. By this approach, we identified CD147 as a subset marker of human CD4+FoxP3+ T cells and demonstrated by flow cytometry that differential expression of CD147 can be used to define FoxP3+ subsets with different phenotypical properties. In addition, we also identified several other proteins that seemed to be expressed at higher levels in CD25 enriched T cells of which some already have been shown to be specifically enriched in Tregs.19-21 CD147 has previously been described to be expressed on activated lymphocyte populations and positively correlate with CD25 expression.32 Here, we show that CD147 splits the FoxP3 population and directly identifies the CD45RO+ subset with a positive correlation with CTLA-4 and FoxP3 itself. As mentioned in “Introduction,” CD147 is expressed on activated lymphocyte populations. By comparing the CD147 expression profile in CD25-depleted and CD25-enriched cell fractions under resting and activating conditions with respect to CD25, FoxP3, and CD45RO, we observed that the CD147 profile was rather stable for the CD25-enriched cell fraction as opposed to the CD25-depleted cells. We were further able to show that FACS sorted CD25highCD147− cells up-regulated CD147 and FoxP3 after crosslinking of CD3, CD2, and CD28, suggesting that CD147 correlates with activation and functional maturation of naive Tregs. Functional characterization based on cell sorting of CD4+CD25highCD147+ or CD4+CD25highCD147− cells suggested that FoxP3+ cells expressing CD147 are more suppressive in vitro than cells from CD147 negative subset both against CD147 negative and positive responder cells. Furthermore, when analyzing the cytokine producing potential of FoxP3+/− cells based on CD147 expression, a separation into 3 functional groups was observed, ie CD147−, CD147+ and CD147++, with CD147+ cells demonstrating the highest potential to produce IL-2, IL-17, TNF-α, and IFN-γ−. In contrast, FoxP3+ cells expressing high levels of CD147 (CD147++) displayed little or no ability to produce any of the cytokines tested.
The recent classification of human FoxP3+ T cells into 3 subsets defined by their expression profile of FoxP3 and CD45RA provides a framework to better understand human Treg biology.13 While CD45RA+FoxP3+ and CD45RA−FoxP3++ subsets identify cells with potent suppressive capacity, the CD45RA−FoxP3+ cells were nonsuppressive with a significant potential to produce IL-2, IFN-γ and IL-17. The CD147++ Treg subset appears to belong to the CD45RA−FoxP3++ activated Tregs, while the CD147+ cell subset appears to be CD45RA−FoxP3+ cells as they are also cytokine producing. The distribution within these subsets changes depending on the immune status and is significantly skewed in cases of autoimmunity. Together these results further suggest that the distribution and functionality within each subset are relevant when assessing immune status in health and disease. Although these subsets expressed different amounts of FoxP3 protein, displayed distinct cytokine producing potential and suppressive ability, they expressed similar levels of FoxP3 transcripts. This points to the relevance of defining and delineating Treg subset differences at the protein level and the need to better characterize their functions. By direct comparison we demonstrate that the level of CD147 staining directly relates to the CD45RA/FoxP3 defined subsets and that the level of CD147 expression correlates with the activation status and function of FoxP3+ human cells. This was further substantiated by the demethylated status of the Foxp3 gene TSDR in CD147++ cells. In conclusion, the CD147high population selectively identifies CD45RA−FoxP3++ cells and demonstrate them to be highly suppressive activated Tregs, while the cytokine producing CD45RA−FoxP3+ cells were mainly found in the CD147 intermediate population as described in Figure 6A. Almost all naive Tregs and the remaining cytokine producing FoxP3+ cells were FoxP3+CD147−, again clearly suggesting that CD147 is an activation marker on FoxP3+ cells.
Our results pointed to a strong correlation between CD147 and CTLA-4 within the FoxP3 subset and the coexpression of these proteins may have implications for the behavior and function of Tregs in different tissues. In humans, a constitutively high expression of CTLA-4 was only seen on activated FoxP3+ cells and the highest levels were observed within the CD45RA−FoxP3++ subset. Although antibody blockade of CTLA-4 in mice leads to organ specific autoimmunity42,43 and conditional elimination of CTLA-4 in FoxP3 positive cells leads to lymphoproliferative disease,39 it is not clear whether CTLA-4 can be part of a direct suppressive mechanism between Tregs and effector T cells. One interesting perspective is the possibility that the high expression of CTLA-4 down regulates the level of CD80 and CD86 on dendritic cells, thereby reducing their ability to stimulate effector cells via CD28.39 This indirect suppression by altering the function of surrounding cells may partly explain why a relatively small population can control peripheral tolerance. Along the same line of thought, high expression of CD147 on activated Tregs could induce expression of MMPs from surrounding cells and regulate barrier function and activity of inflammatory cytokines and chemokines.44 It could potentially also directly affect the ability of FoxP3+ cells to invade tissues during immune activation and further regulate recruitment of leukocytes which may be related to disease status as seen with cancers.45,46 Similarly, the production of MMPs and profound infiltration of inflammatory cells into the synovium during rheumatoid arthritis (RA) could be associated with expression of CD147 on lymphoid cells. In general, there appears to be a direct correlation between increased levels of MMPs in RA and increased levels of CD147 in rheumatoid synovium.47 It is also interesting to note that synovial fluid from RA patients has elevated levels of cyclophilin A (CypA)48 which has been described to have a chemotactic effect on leukocytes49 including activated human CD4+ cells.32 The migratory effect of CypA on activated CD4 T cells seems to be dependent on binding to CD147 as antibody blocking reduced migration in vitro and dampened collagen-induced arthritis in a mouse model.50 Whether CypA has the same effect on CD147 positive Tregs as on CD4+ cells remain to be seen, but it clearly suggests an important functional role of CD147 in the regulation of FoxP3+ cells.
In conclusion, our results suggest that CD147 identifies all human FoxP3 cells with an activated phenotype and could be a useful marker both to isolate and to better understand delineation and functional properties of human Tregs in health and disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The present study was supported by the Norwegian Functional Genomics Program (FUGE), the Norwegian Research Council, the Norwegian Cancer Society, and Novo Nordic Foundation.
Authorship
Contribution: T.S., S.J.B, and J.L. did experiments and analyzed data; K.M.T. and K.T. supervised the project; B.T. and E.M.A. provided essential advice and analyzed data; and T.S., S.J.B., K.T., and K.M.T. wrote the paper. All authors read and commented on the draft versions of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests. The University of Oslo Technology Transfer Office, Inven2 AS, has filed a pending patent application on methods to identify activated Tregs.
The current affiliation for T.S. is Norwegian Medicines Agency, Oslo, Norway. The current affiliation for K.M.T. is Pfizer AS, Oslo, Norway.
Correspondence: Kjetil Taskén, Centre for Molecular Medicine Norway, Nordic European Molecular Biology Laboratory Partnership, University of Oslo, PO Box 1137, Blindern, N-0318 Oslo, Norway; e-mail: kjetil.tasken@ncmm.uio.no.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal