Abstract
The microenviroment of acute myelogenous leukemia (AML) is suppressive for immune effector cells. Regulatory T cells (Tregs) have been recognized as a contributor factor and may be recruited and exploited by leukemic cells to evade immunesurveillance. Studies have shown that the frequencies of marrow and blood Tregs are greater in patients with AML than in control patients. Although increased Tregs have been associated with a decreased risk of GVHD after allogeneic HCT and hence may impede the graft-versus-tumor effect, recent findings indicate that that this may not be the case. Because there is a need to improve outcomes of standard treatment (chemotherapy with or without allogeneic HCT) in AML, targeting Tregs present an outstanding opportunity in AML because discoveries may apply throughout its treatment. Here, we review data on the roles of Tregs in mediating immune system-AML interactions. We focused on in vitro, animal, and observational human studies of Tregs in AML biology, development, prognosis, and therapy in different settings (eg, vaccination and HCT). Manipulation of Tregs or other types of immunomodulation may become a part of AML treatment in the future.
Introduction
Current treatments for acute myelogenous leukemia (AML) have not changed for several decades and have not resulted in satisfactory outcomes. Modulating the immune system may improve survival in patients with AML because the immune system is highly active against leukemic cells. The most compelling evidence for an antileukemic immune effect is seen in recipients of allogeneic hematopoietic stem cell transplantation (alloHCT).1,2 Donor natural killer (NK) cells, γδ T cells, and cytotoxic T lymphocytes (CTLs) kill leukemic cells after alloHCT.2,3 Donor lymphocyte infusions (DLIs) can induce modest and often transient responses in patients with AML who relapse after transplantation.1,4
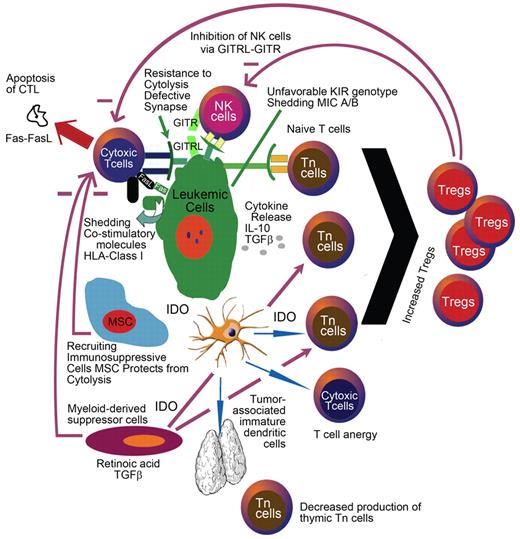
Conversely, patients with AML have dysfunctional T cells and NK cells at diagnosis5,6 as well as a greater frequency of immature NK cells during first complete remission (CR).7 An emerging body of evidence demonstrates that these functional abnormalities are at least in part induced by the tumor itself. For example, direct contact between leukemic cells and NK cells induces a loss or decrease in natural cytotoxicity receptors on NK cells (NCRdull),8 a phenotype associated with poor overall survival (OS). Another example is seen with defective or immature dendritic cells (DCs). Defective DCs are found in the peripheral blood (PB) of patients with AML and can induce tolerance toward leukemic cells.9 NK cells, which are deficient in AML, are at least partly responsible for removing some of these DCs.10 Taken together, these studies indicate that defects in antileukemic effector cells in patients with AML can contribute to the development and persistence of the disease (Figure 1). In addition to tolerogenic DCs, the authors of recent studies in mice and humans have implicated that immune suppressive regulatory T cells (Tregs) contribute to a defective antileukemic immune response.11,12
AML leukemic cells can inhibit immune effector cells by contact-dependent or -independent means. Shedding of costimulatory molecules, increased levels of suppressive cytokines, and increased IDO expression are some of the mechanisms by which leukemia cells evade from immune surveillance.
AML leukemic cells can inhibit immune effector cells by contact-dependent or -independent means. Shedding of costimulatory molecules, increased levels of suppressive cytokines, and increased IDO expression are some of the mechanisms by which leukemia cells evade from immune surveillance.
Tregs in the immunosuppressive microenviroment of AML
The AML microenviroment is immunosuppressive and antiapoptotic, favoring the survival of malignant hematopoietic cells.13 The authors of in vitro studies have shown that AML cells secrete factors, which inhibit T-cell activation and proliferation and limit proinflammatory T helper-1 cytokine production.13,14 This suppressive effect is reversed, however, when Tregs and other T lymphocytes are removed from the microenvironment in vitro, leading to augmented immune responses to AML.14 In mice, Tregs accumulate in leukemic sites and impede the proliferative and cytolytic capacity of adoptively transferred anti-AML reactive CTLs.15 Depletion of CD25 (IL-2 receptor α-chain)–expressing Tregs by the administration of IL-2 diphtheria toxin results in temporary tumor regression associated with increased CTLs at tumor sites. Combination therapy with IL-2 diphtheria toxin and anti-AML adoptive CTL transfer not only reduces tumor mass but also improves OS in mice. Moreover, mice display resistance to AML cells on rechallenge, implying the development of effective adaptive immunity.15 AML-induced DCs also have a marked chemotactic effect on Tregs compared with other lymphocyte subtypes in vitro, which may contribute to the accumulation of Tregs around leukemic sites.16 Collectively, these data indicate that the AML microenvironment contains a variety of immune suppressive elements, including secreted factors and immune cells, namely Tregs, which appear to be mediators of immune suppression.
Treg development and function
Tregs are a diverse population of CD4+ T cells with suppressive functions crucial in self-tolerance with different origins, phenotypes, and subtypes (Table 1). Tregs can develop naturally in the thymus (nTregs) or be induced by other T cells in the periphery (inducible/adaptive Tregs, iTregs). nTregs develop directly from CD4+ T-cell precursors by a process of positive selection through their interaction with thymus epithelium and DCs in the presence of MHC II and self-peptide. nTregs account for 5%-10% of all peripheral CD4+ lymphocytes and are detectable in the PB, lymph nodes, and spleen. They are relative anergic to stimulation via the T-cell receptor, compared with T effector cells (Teffs), and unable to produce IL-2 in vitro, even though IL-2 is essential for the generation, survival, and activation of nTregs.
T cells with suppressive properties are summarized
| Regulatory/suppressor T cells . | ||
|---|---|---|
| Cell type . | Phenotype . | Suggested immunosuppressive mechanisms . |
| CD4+ | ||
| nTreg | CD25 + FOXP3 + 45RO+ CTLA-4+ GITR+CD134+CD62L+CD103+ lymphocyte activation gene-3+ CD127lo CD26+ | Cell-to-cell contact-dependent in vitro (CTLA-4) Cell-to-cell contact- and cytokine-dependent in vivo (IL-10 and TGF-β) |
| iTreg | ||
| Th3 | CD25±FOXP3±45RO+ CTLA-4+ | Cytokine-mediated (TGF-β production > > IL-10) |
| Tr1 | CD25±FOXP3±45RO+ CTLA-4− | Cell-to-cell contact Cytokine-mediated (IL-10 > > TGF-β, IL-5, IFN-γ production) |
| TGF-β/IL-10 double-positive Treg | CD25− FOXP3− | Cytokine-mediated (IL-10 and TGF-β production) |
| CD8+ | ||
| T suppressor cells (Ts) | ||
| Naturally occurring | FOXP3+ 45RO+CD25+CTLA-4+GITR+ | Cell-to-cell contact-dependent (CTLA-4) Cytokine-mediated (TGF-β production) |
| Non–antigen specific | CD28−Foxp3−CD56− | Cytokine-mediated (IL-10 production) |
| Inducible | CD25+Foxp3+CD28+GITR+CTLA-4+ | Cell-to-cell contact |
| γ/δ T cells | Cell-to-cell contact Cytokine-mediated (IL-2, IL-10, IL-17, IFN-γ, TGF-β, TNF-α production) | |
| CD4−CD8− αβ T cells | CD25+30+45+69+LFA-1+CTLA-4+ | Cell-to-cell contact Produce IFN-γ and TNF-α |
| NKT cells | CD161 | Cytokine-mediated, produce both Th-1 (IFN-γ and TNF-α) and Th-2 cytokines (IL-4, -10,-13) |
| Regulatory/suppressor T cells . | ||
|---|---|---|
| Cell type . | Phenotype . | Suggested immunosuppressive mechanisms . |
| CD4+ | ||
| nTreg | CD25 + FOXP3 + 45RO+ CTLA-4+ GITR+CD134+CD62L+CD103+ lymphocyte activation gene-3+ CD127lo CD26+ | Cell-to-cell contact-dependent in vitro (CTLA-4) Cell-to-cell contact- and cytokine-dependent in vivo (IL-10 and TGF-β) |
| iTreg | ||
| Th3 | CD25±FOXP3±45RO+ CTLA-4+ | Cytokine-mediated (TGF-β production > > IL-10) |
| Tr1 | CD25±FOXP3±45RO+ CTLA-4− | Cell-to-cell contact Cytokine-mediated (IL-10 > > TGF-β, IL-5, IFN-γ production) |
| TGF-β/IL-10 double-positive Treg | CD25− FOXP3− | Cytokine-mediated (IL-10 and TGF-β production) |
| CD8+ | ||
| T suppressor cells (Ts) | ||
| Naturally occurring | FOXP3+ 45RO+CD25+CTLA-4+GITR+ | Cell-to-cell contact-dependent (CTLA-4) Cytokine-mediated (TGF-β production) |
| Non–antigen specific | CD28−Foxp3−CD56− | Cytokine-mediated (IL-10 production) |
| Inducible | CD25+Foxp3+CD28+GITR+CTLA-4+ | Cell-to-cell contact |
| γ/δ T cells | Cell-to-cell contact Cytokine-mediated (IL-2, IL-10, IL-17, IFN-γ, TGF-β, TNF-α production) | |
| CD4−CD8− αβ T cells | CD25+30+45+69+LFA-1+CTLA-4+ | Cell-to-cell contact Produce IFN-γ and TNF-α |
| NKT cells | CD161 | Cytokine-mediated, produce both Th-1 (IFN-γ and TNF-α) and Th-2 cytokines (IL-4, -10,-13) |
Suppressive T cells are mostly present in CD4+ cell population. However, some CD8+, double-negative, and NKT cells also have suppressive functions. Some of these T cells demonstrate their suppressive activity by cell-cell contact manners, whereas others release cytokines and inhibit effector immune system cells.
CTLA-4 indicates cytotoxic T-lymphocyte antigen-4; GITR, glucocorticoid-induced TNFR-related protein; iTreg, inducible regulatory T cells, NKT cells, batural killer T cells; nTreg, natural regulatory T cells; Tr1, regulatory T cells type 1; and Th3, T helper 3.
Various subgroups of iTregs are generated from naive conventional CD4+25− T cells in the presence of APCs, such as DCs, and cytokines, including TGF-β, IL-10, or IL-2 (Table 1).17,18 Although the majority of Tregs are present in the CD4+CD25+ T-cell population, other suppressive T cells have been found in the CD4+CD25−, CD8+, and CD4−CD8− subgroups (Table 1).19,20 There are differences between mice and humans Tregs. In humans, Tregs may be CD25hi, CD25int, or CD25lo; CD25hi, which comprise 2%-4% of CD4+ T cells, are the most highly suppressive subset. In mice, ∼ 10% of CD4+ T cells are CD4+CD25+ Tregs and are clearly distinguishable from CD4+25− T cells. Two and one FOXP3 gene isotopes are present in humans and mice, respectively. Phenotypically mice Tregs are neuropilin-1+CD62L (L-selectin)+ granzyme B+ (on activation), whereas Tregs of humans are neuropilin-1−CD62L (L-selectin)−/+ granzyme A+ (on activation).
The concept of T cells with a suppressive function was first introduced in the early 1970s.21 Supporting evidence for Tregs in self-tolerance came much later. In 1995, Sakaguchi et al identified the CD25 molecule as a bona fide cell-surface marker for immunosuppressive CD4+ T cells in mice.22 In this study, CD4+CD25+ cells were found to be critical in maintaining self-tolerance by down-regulating immune responses to self- and non-self antigens.22 In 2001, mutations in the FOXP3 gene were found to cause various autoimmune and inflammatory disorders in scurfy mice and humans.23,24 FOXP3, a member of the forkhead/winged-helix family of transcriptional regulators, is a master regulator of key biologic processes in Tregs. FOXP3 regulates the development and activation of CD4+CD25+ Tregs and stabilizes and amplifies their regulatory functions.25,26 Ectopic expression of a transgene encoding FOXP3 in mice or retroviral transduction of FOXP3 converts CD4+ CD25− T cells to CD25+CD4+ Treg-like cells, and these genetically modified cells have suppressive effects on other T cells and autoimmune disorders.25,26
In addition to nTregs, iTregs also can express FOXP3 on activation.18 To accommodate the diverse outcomes (eg, autoimmunity vs tolerance to infections or tumors) mediated by Tregs, a response to the differential expression of various factors (eg, cytokines, hypoxia, retinoic acid) at the site of the immune response is needed to achieve an outcome most beneficial to the host. For example, the anti-inflammatory protein TGF-β induces FOXP3 expression and generation of iTregs,27 whereas the proinflammatory protein IL-6 suppresses TGF-β–mediated FOXP3 induction and generation of iTregs, favoring the generation of proinflammatory Th17 cells.28 Conversely, retinoic acid suppresses inflammation by enhancing TGF-β–driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression, thereby increasing FOXP3+ Tregs and inhibiting development of Th-17 cells.29
Immunosuppressive mechanisms of Tregs
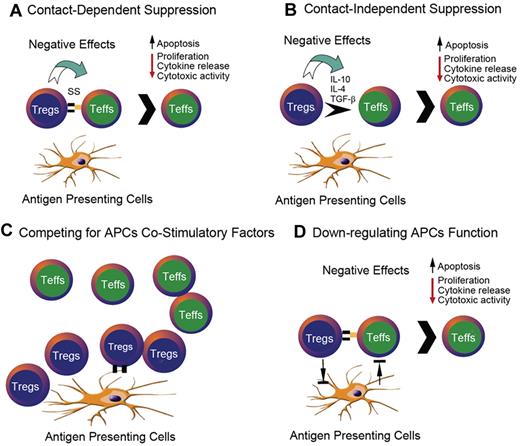
Tregs suppress effector cells and APCs either in a contact-dependent or -independent manner (Figure 2; Table 1). Whereas nTregs can use both mechanisms, iTregs induce immunosuppression through cytokines, including IL-4, IL-10, or TGF-β. Cell-to-cell interactions can occur between Tregs and Teffs with or without APCs.30-32 Treg-to-Teff contact results in the suppression or apoptosis of Teffs. On contact, formation of gap junctions occurs between these 2 T cells.31 cAMP transferred through the gap from Tregs to Teffs suppresses the proliferation of Teffs by decreasing IL-2 production31 and phosphodiesterase 3 inhibitors, which increase cAMP, also increase Tregs.33 In patients with AML, Tregs were found to more efficiently hydrolyze adenosine triphosphate to adenosine compared with Tregs in healthy control patients.34 It can be speculated that this might increase Treg cAMP levels in patients with AML and might explain the finding that Tregs in AML patients are more suppressive than Tregs in control patients.35 Tregs can also suppress IL-2 mRNA in responding T cells.36 Depleting IL-2 from the microenviroment through its highly expressed CD25 is another mechanism to competitively decrease IL-2 available for Teffs.37 Tregs also induce apoptosis in CD4+CD25− effector cells through the granzyme B-dependent, perforin-independent mechanism in mice.38
Some of the mechanisms by which CD25+CD4+ Tregs suppress the activation and proliferation of other T cells. The inhibition between these 2 T-cell subtypes occurs as either contact-dependent (suppressor synapse) or -independent manners. APCs play an important role in this interaction. (A) Tregs can suppress the functions of Teffs via direct contact. (B) Tregs can suppress the functions of Teffs via releasing inhibitory cytokines. (C) Tregs can complete with Teffs for the costimulatory signals of APCs. (D) Tregs can suppress APCs and thus prevent the stimulation of Teffs by APCs.
Some of the mechanisms by which CD25+CD4+ Tregs suppress the activation and proliferation of other T cells. The inhibition between these 2 T-cell subtypes occurs as either contact-dependent (suppressor synapse) or -independent manners. APCs play an important role in this interaction. (A) Tregs can suppress the functions of Teffs via direct contact. (B) Tregs can suppress the functions of Teffs via releasing inhibitory cytokines. (C) Tregs can complete with Teffs for the costimulatory signals of APCs. (D) Tregs can suppress APCs and thus prevent the stimulation of Teffs by APCs.
Tregs have been shown to inhibit NK-cell functions as well.39 In an allogeneic transplant murine model, the prior removal of host Tregs, but not CD8+ T cells, significantly enhanced NK cell–mediated BM graft rejection.39 In addition, treatment with anti-TGF-β mAb increased graft rejection, suggesting that iTreg development or maintenance were compromised because of elimination of TGF-β. NK cell–mediated hybrid resistance to a BM graft was successfully abrogated when CD4+CD25+ Tregs were transferred to the microenviroment. Similarly, in leukemia-bearing mice, a combination of IL-2 (to activate and expand NK cells) and anti-CD25 (to deplete Tregs) led to superior in vitro and in vivo antitumor effects compared with either treatment alone.40
The role of indoleamine 2,3-dioxygenase in Treg generation and function
In addition to the aforementioned mechanisms, Tregs suppress Teffs through APCs via various mechanisms, including competing with Teffs or naive T cells for the same APC-derived costimulatory signals (eg, CD80/86) and preventing DCs from forming stable contacts with conventional T cells in vivo (Figure 2).41 Tregs also alter the function and restrain the maturity of DCs; thus, DCs remain immature and more tolerogenic.42 In turn, these DCs promote Tregs. Immature DCs express indoleamine 2,3-dioxygenase (IDO), causing immunosuppression both by depleting tryptophan, which inhibits T-cell proliferation, and increasing tryptophan metabolites, kynurenines, thereby promoting T-cell apoptosis.43 IDO has also been shown to promote the generation of Tregs from CD4+CD25− T cells through tryptophan starvation and tryptophan catabolites in murine models.44 Human plasmacytoid DCs can drive naive allogeneic CD4+CD25− T cells to differentiate into CD4+CD25+FOXP3+Tregs via the IDO pathway.45 IDO-expressing plasmacytoid DCs in tumor-draining lymph nodes promote de novo differentiation and activation of Tregs,46 resulting in tumoral immune resistance.47 IDO is also used by human BM stromal cells (mesenchymal stem cells) to suppress allogeneic T cells.48 Human mesenchymal stem cells favor the differentiation of CD4+ T-cell subsets coexpressing CD25 and/or CTLA4 in vitro.49
Another potent T-cell suppressive (antigen-specific or -nonspecific) group of cells, myeloid-derived suppressor cells (MDSCs), are found to be increased in PB of patients with various malignancies. This heterogeneous population of myeloid cells includes hematopoietic progenitor cells and precursors of macrophages, DCs, and granulocytes. The phenotype of MDSCs is Lin−HLA-DR−CD33+ or CD11b+CD14−CD33+. In steady state, MDSCs are present in the BM; however, in the active state they pick up and carry antigens to peripheral lymphoid organs and process and present these antigens to T cells.50 This interaction induces tolerance in Teffs toward the antigen presented.51 MDSCs also promote the de novo development of Tregs in vivo in tumor-bearing mice and ex vivo.52,53 Hoechst et al demonstrated that MDSCs obtained from human PBs induced FOXP3+ Tregs ex vivo.54 In addition, tumor-infiltrating MDSCs can express IDO in mice,55 although some MDSCs express arginase-I, which catabolizes L-arginine, and not IDO. The relative potency of Tregs and MDSCs in the tumor models has not been systemically analyzed in most tumor models. These data show that Tregs can be generated after contact with various suppressive cells and in interaction with them, and in some cases, IDO within the tumor microenvironment can play a critical role in Treg induction.
The immunosuppressive mechanisms of AML
IDO and Tregs in AML
Especially relevant to this review, AML cells express an active IDO protein.12,56 IDO activity has been found to be greater in the serum of patients with AML than healthy control patients.57 Curti et al found primary myeloblasts were positive for IDO expression in 40 of 76 patients (52%) at the time of diagnosis.12 AML patients with IDO-positive myeloblasts had more CD4+25+ T cells compared with IDO-negative patients. In vitro, IDO-positive myeloblasts could increase the number of Tregs, and this effect was completely abrogated by addition of an IDO inhibitor. These Tregs inhibited the proliferation of naive T cells. Likewise, IDO-positive leukemic DCs generated from AML cells suppressed T cells in vitro because of increased Tregs and tryptophan catabolism.58 Increased Tregs directly inhibited naive T-cell proliferation and impaired maturation of normal DCs.58 This result could potentially contribute to the limited efficacy of cellular vaccinations that use leukemic DCs to induce cytotoxic effector cells.58 IDO-positive leukemia or leukemia/lymphoma cells in humans and mice were able to convert CD4+CD25− cells to CD4+CD25+ cells.12,59 Chamuleau et al reported that high IDO mRNA expression levels in leukemic blasts was correlated with poor disease-free survival and OS (7.4 months vs 21.4 months) in 71 patients with AML.60 Corm et al showed that AML patients with a greater serum kynurenine/tryptophan ratio (ie, greater IDO activity) had lower survival rates.57
Programmed death-1 receptor and Tregs in AML
Programmed death-1 (PD-1) receptor is expressed on various cell types, (eg, T cells, B cells, myeloid-derived cells, and Tregs). PD-1 signaling indicates CD8+ cell exhaustion and has immunosuppressive effects in different clinical conditions, including cancer.61,62 PD-L1, a ligand for the PD-1 receptor, was shown to be present on cancer cells, including AML.11,63,64 Moreover, PD-L1 has been shown to promote the development, maintenance, and function of iTregs.65 PD-1 and PD-L1 interaction suppressed endogenous and exogenous anti-AML CTL responses in a model of AML.11,63 Moreover, tumor progression resulted in increased Treg and high PD-1 expression on CD8+ CTLs at the tumor site. PD-1–deficient mice were more resistant to AML than wild-type mice,11,63 despite the presence of a similar percentage of Tregs.11 In vitro, Treg suppression of CTL responses was dependent on PD-1 expression by T cells and Tregs and on expression of PD-L1 by APCs.11 The in vivo function of adoptively transferred AML-reactive CTLs was reduced by AML-associated Tregs.11 In addition, anti–PD-L1 mAb treatment increased the proliferation and function of adoptively transferred CTLs at tumor sites, reduced AML tumor burden, and resulted in long-term survivors. Combined Treg depletion followed by PD-L1 blockade showed superior efficacy for leukemia eradication. Thus, the interaction between PD-1 and PD-L1 can facilitate Treg-induced suppression of Teffs and dampen the antitumor immune response.
The Tim3/galectin-9 pathway, Tregs, and AML
Tim-3, a type I membrane glycoprotein, is expressed on Th1 cells and innate immune cells.66 Ligation of Tim-3 and galectin-9 (gal-9, Tim-3 ligand)67 on T cells can negatively regulate Th1 responses.66,68 In mice and humans, gal-9 expression can be found on AML cells, implicating the possible contribution of the Tim-3/gal-9 pathway along with the PD-1/PD-1 ligand pathway in regulating CD8+ CTL responses.69 Indeed, in a systemic murine AML model, endogenous CD8+ CTLs found at the sites of AML disease coexpressed Tim-3 and PD-1.69 PD-1 and Tim-3 coexpression increased during AML progression. Gal-9 deficient mice challenged with AML cells were more resistant than wild-type mice, which was associated with reduced Treg accumulation and induction of PD-1 and Tim-3 expression on CD8+ T-cells. Administration of a blocking Tim-3 fusion protein and anti–PD-L1 mAb had an additive effect in restoring the antitumor function of exhausted T cells, leading to superior survival of AML-bearing mice.69
CD200, Tregs, and AML
CD200, a type-1 transmembrane glycoprotein, has an immunosuppressive effect by interacting with its receptor CD200R expressed on immune competent T cells, B cells, and DCs.70 CD200R can induce Treg production in the murine thymus.70 Overexpression of CD200 was seen in leukemic blasts in 43% of 184 patients with AML and was associated with poor survival.71 The same group showed that in 91 AML patients CD200 expression was correlated with an increased frequency of BM CD4+CD25+FoxP3+Tregs.72 CD200-overexpressing leukemic cells directly inhibited the cytotoxic activity of NK cells.73
Tregs in AML treatment
Nontransplantation studies
The authors of several clinical studies have evaluated Treg frequencies at diagnosis and during induction chemotherapy (Table 2).35,74-77 Szczepanski et al demonstrated that 31 AML patients had a greater CD4+CD25hi Treg percentage in PB at diagnosis compared with healthy control patients.35 Tregs from patients with AML suppressed autologous responder cells more effectively than Tregs from control patients. The Treg-mediated immunosuppressive effect required both cytokines and cell-to-cell contact. Greater pretreatment Treg PB frequencies predicted poor response to induction therapy.35 There was no correlation with Treg levels and cytogenetic subgroups. Treg levels were even greater during CR than at diagnosis.
Summary of studies in which authors evaluated Treg frequencies
| Author, y . | No. of patients . | Time of sampling . | Tregs (% of CD4+ T cells) in PB . | Tregs (% of CD4+ T cells) in BM . | Comments . | ||
|---|---|---|---|---|---|---|---|
| Patients . | Controls . | Patients . | Controls . | ||||
| Wang, 2005 | 36 | At diagnosis | 4.1 | 2 | 4.9 | 1.8 | Increased Tregs caused by more production over apoptosis Tregs inhibited both autologous and controls' CD4+CD25− T cells |
| Szczepanski, 2009 | 30 | At diagnosis | 4.5 | 1.5 | Tregs were functionally immunosuppressive. Higher Treg frequencies were associated with poor prognosis. Relative Treg frequencies remained greater in complete remission | ||
| Ersvaer, 2010 | 20 | At diagnosis | ∼ 6 | Tregs were functionally immunosuppressive. Although Tregs decreased after chemotherapy, its ratio to other T-cell subsets remained high CD4/CD8 ratio remained relatively stable throughout therapy | |||
| During cytopenia | ∼ 5.5 | ||||||
| Recovery | ∼ 3 | ∼ 1 | |||||
| Shenghui, 2010 | 182 | At diagnosis | 9.2* ∼ 2† | 5.4* | 11.8* ∼ 5† | There was a strong correlation between CD25+CD127lo and CD25+FOXP3+ in CD4+ T cells whereas correlation between CD25hi and CD25+ FOXP3+ in CD4+ T cells was weak Tregs were higher in BM than in PB BM Tregs were more immunosuppressive than PB Tregs. Higher Treg frequencies were associated with poor prognosis | |
| Kanakry, 2011 | 20 | Early recovery after induction chemotherapy | 10.5 | Tregs were functionally immunosuppressive. Production of Tregs occurred in both periphery and thymus (peripheral > thymus.) Antigen stimulation appeared to be responsible for oligoclonal Treg production. Most of the T cells were CD4+ T cells, giving the ratio of 2.7/1 of CD4+/CD8+ cells | |||
| Author, y . | No. of patients . | Time of sampling . | Tregs (% of CD4+ T cells) in PB . | Tregs (% of CD4+ T cells) in BM . | Comments . | ||
|---|---|---|---|---|---|---|---|
| Patients . | Controls . | Patients . | Controls . | ||||
| Wang, 2005 | 36 | At diagnosis | 4.1 | 2 | 4.9 | 1.8 | Increased Tregs caused by more production over apoptosis Tregs inhibited both autologous and controls' CD4+CD25− T cells |
| Szczepanski, 2009 | 30 | At diagnosis | 4.5 | 1.5 | Tregs were functionally immunosuppressive. Higher Treg frequencies were associated with poor prognosis. Relative Treg frequencies remained greater in complete remission | ||
| Ersvaer, 2010 | 20 | At diagnosis | ∼ 6 | Tregs were functionally immunosuppressive. Although Tregs decreased after chemotherapy, its ratio to other T-cell subsets remained high CD4/CD8 ratio remained relatively stable throughout therapy | |||
| During cytopenia | ∼ 5.5 | ||||||
| Recovery | ∼ 3 | ∼ 1 | |||||
| Shenghui, 2010 | 182 | At diagnosis | 9.2* ∼ 2† | 5.4* | 11.8* ∼ 5† | There was a strong correlation between CD25+CD127lo and CD25+FOXP3+ in CD4+ T cells whereas correlation between CD25hi and CD25+ FOXP3+ in CD4+ T cells was weak Tregs were higher in BM than in PB BM Tregs were more immunosuppressive than PB Tregs. Higher Treg frequencies were associated with poor prognosis | |
| Kanakry, 2011 | 20 | Early recovery after induction chemotherapy | 10.5 | Tregs were functionally immunosuppressive. Production of Tregs occurred in both periphery and thymus (peripheral > thymus.) Antigen stimulation appeared to be responsible for oligoclonal Treg production. Most of the T cells were CD4+ T cells, giving the ratio of 2.7/1 of CD4+/CD8+ cells | |||
These studies revealed that patients with AML had higher Treg levels compared with HC. The frequencies of Tregs were mostly investigated in PB. One study revealed the frequency of Tregs was higher in the BM compared with PB. Two of the studies indicated higher Treg levels at diagnosis were associated with poor prognosis.
∼ indicates that absolute numbers were not provided by the articles, but the approximate numbers were estimated from figures; AML, acute myelogenous leukemia; HC, healthy controls; and PB, peripheral blood.
CD4+CD25+CD127lo T cells.
CD4+CD25high T cells.
Similarly, Ersvaer et al showed that Tregs were greater at diagnosis in 20 patients with AML compared with 30 control patients.76 The relative level of Tregs were followed from diagnosis to recovery in 6 patients and remained elevated throughout the course. The relative level of other T-cell subsets (ie, CD8+ cytotoxic T cells, CD4+ helper cells, and IL-17–secreting CD4+ cells) decreased during cytopenia, which resulted in decreased ratios of these T subsets relative to Tregs. This finding suggests that Tregs were the least affected T-cell subset after induction chemotherapy. The ratio of CD4+/CD8+ remained relatively stable during chemotherapy.
In another study, investigators evaluated Tregs in 20 patients with AML at the time of early recovery (white blood cells ≥ 0.2 × 109/L) after intensive induction chemotherapy.74 Most T cells at recovery were CD4+ cells (CD4+/CD8+ cells ratio of 2.7/1), including CD3+CD4+CD25+FoxP3+Tregs (10.5% of CD4+ cells). These Tregs had an activated phenotype (GITR+CD27+CD45RA−FoxP3hi) and were markedly more frequent than in control patients, most likely resulting from increased proliferation. Treg expansion at early recovery appeared to be mostly peripherally induced and antigen driven (CD27+). Tregs suppressed cytokine production of responder T cells.
Wang et al compared the proportion of CD4+CD25hi T cells in PB or BM in 36 untreated patients with AML with 16 healthy control patients.77 In PB, the prevalence of CD4+CD25hi T cells in patients with AML (4.1%) was substantially greater than in control patients (2.0%, P < .0001). Patients with AML also had a greater prevalence of Tregs in the BM (4.95% vs 1.83%, P < .0001). The increased Tregs did not result from decreased apoptosis because patients with AML had an increased apoptotic fraction of CD4+CD25hi T cells (7.8% vs 2.1%, P < .0001). Importantly, Treg proliferation was increased (17.5%) compared with control patients (1.9%, P < .0001). These Tregs expressed CD45RO, CD95 (Fas), and CTLA-4 and produced very low levels of TNF-α and IL-10 but not IL-2, IL-4, IL-5, and IFN-γ. In contrast, CD4+CD25− T cells produced high levels of IL-2, TNF-α, IFN-γ and low levels of IL-5 but not IL-4 and IL-10. The CD4+CD25hi Tregs inhibited proliferation of autologous CD4+CD25− T cells, as well as control CD4+CD25− T cells activated by anti-CD3 and anti-CD28 mAbs. Tregs also decreased cytokine production (IL-2 and IFN-γ) by activated CD4+CD25− T cells.
Shenghui et al showed that the frequencies of CD4+CD25+ and CD4+CD25+CD127lo cells were correlated in 182 newly diagnosed patients with AML.75 They used the CD4+CD25+CD127lo phenotype to evaluate Treg frequencies. Tregs were increased in the PB of patients with AML (mean, 9.20%) compared with control patients (mean, 5.44%). In patients with AML, Tregs were present in BM (11.8%) at a similar frequency as PB (9.2%). Both BM and PB Tregs of patients with AML inhibited allo-antigen–driven in vitro proliferation of CD4+CD25− T cells more effectively compared with control Tregs. Of interest, Tregs obtained from the BM of patients with AML were more immunosuppressive than Tregs isolated from PB. In 58 patients with AML followed for 6 months, the frequencies of PB and BM CD4+CD25+CD127lo Tregs at diagnosis were lower in patients who had achieved CR compared with those with persistent leukemia or who died.75 Tregs remained low in 5 of 7 followed for 12 months, with lasting CR yet increased dramatically in 2 patients with relapse.75
Studies in murine models have shown that the frequencies of Tregs are increased in AML in vivo, that these Tregs have suppressive functions on Teffs in vitro, and that removing/depleting Tregs improves the function of Teffs in vitro and improves treatment outcome.15 In humans, although there have been few studies with small numbers of patients, the results consistently show that the frequencies of Tregs are greater in patients with AML (PB or BM) compared with healthy control patients and that these Tregs suppress Teffs in vitro. Two of the studies indicate increased Tregs are a poor prognostic indicator.35,75
Therefore, it is time for a carefully designed phase 1/2 interventional study with a Treg-depleting agent in refractory/relapsed patients with AML. This study should not only define a maximal tolerable dose of the drug but also must evaluate the safety of the Treg-depleting agents in terms of induction or exacerbation of autoimmune disorders (eg, emergence of autoimmune antibodies) or excessive inflammatory reactions in the short and long term. Correlative studies should include repetitive assay of BM (eg, diagnostic, day 14, and at the time of recovery) Treg frequencies and functional analyses. In addition, to see the “whole picture” of this dynamic, very complex immune reaction or dysfunction, other essential parts of the immune system, should be evaluated concurrently. In this regard, the quantitative and functional status of Teffs, NK cells, DCs, DCs maturation status, AML-DCs, and IDO expression on DCs and AML blasts should be measured and analyzed. Such studies should also evaluate humoral immunity that has been neglected despite identification of specific antibodies against AML antigens (eg, antibody to Wilms' tumor gene 1 [WT1])78 and demonstration of increased plasma cells in the BM associated with residual leukemic cells on day 14.79
Treg depletion before vaccination to augment anti-AML responses
Vaccination trials with peptides or leukemic DCs in patients with AML have thus far generated short-lived responses in only a limited number of patients.80,81 Overexpression of WT1 in some patients with AML has been demonstrated.82,83 WT1 can be used as a target for vaccination because WT1-specific CD8+ CTLs can lyse myeloblasts but not HSCs.84,85 However, Tregs induced by leukemic DCs have blunted the responses of WT1-specific CD8+ T cells and leukemia-reactive CD4+ T cells in vitro.58 Lehe et al generated anti-WT1 Tregs from PBMCs of healthy people.86 These Tregs produced cytokines compatible with a Th2 cytokine profile and exerted suppressive activity on allogeneic T cells, NK cells, and CD8+ T cells.86 Tregs also induced apoptosis in APCs. In vivo, a few vaccination studies monitored Treg frequencies before and after vaccination.80,81,87,88 Vaccination with allogeneic or autologous AML cells transduced with a lentivirus expressing CD80 and IL-2 (ie, LV-CD80/IL-2) increased Treg frequencies.88
Although patient numbers were very limited in these studies, their results imply that immunologic responses might be inversely correlated with Treg frequencies.81,87 Therefore, Treg depletion before vaccination might help augment antileukemic responses. Berneman et al demonstrated that WT1-targeted DC vaccination can prevent relapse by augmenting CD8+ immune response without an increase in Tregs.89 The depletion of CD25+ T cells with anti-CD25 mAb before DC-based vaccination dramatically improved survival in mice compared with those receiving vaccination alone.90
Taken together, selective Treg depletion before vaccinations with tumor-specific peptides, proteins, and cellular vaccines may be highly useful in unleashing antitumor immune responses that are suppressed by tumor antigen-reactive Tregs.
Treg involvement in GVHD
In rodents, Treg depletion from the donor graft accelerates GVHD lethality, indicating a role of endogenous donor Treg-mediated suppression during the GVHD response.91 In humans, the authors of a prospective study showed that the frequencies of Tregs were significantly lower (40%) in 60 patients with acute GVHD than those who did not have acute GVHD or who underwent autologous HCT.92 Moreover, there was an inverse linear correlation between Treg frequencies and severity of acute GVHD. Likewise, 30 patients with chronic GVHD had a lower frequency of Tregs compared with 27 who had no chronic GVHD.93 However, there are conflicting studies relating increased Tregs with chronic GVHD in an analysis of 17 patients with chronic GVHD compared with 23 control patients without chronic GVHD assessed at time points beyond day 100 after HCT.94 Increased Treg content in the graft was found to be associated with less acute GVHD in alloHCT after myeloablative conditioning regimens95,96 but not in all studies.97 The reason for these apparent discordant results is unknown at this time.
Role of Tregs in separating graft-versus-tumor from GVHD
In alloHCT, separating from graft-versus-tumor (GVT) from GVHD has been one of the most important barriers to improving this therapy. Studies indicate that Tregs may aid in separating GVT from GVHD, at least in murine models.98-101 The Stanford group was able to show that Tregs suppress early expansion of alloreactive donor T cells and CD25 expression, decreasing GVHD but without abrogating the GVT effect.98 GVHD suppression of Tregs was primarily mediated by the perforin lysis pathway. Similarly, in elegant studies by Cai et al, it has also been shown that GVHD and GVL mechanisms are indeed different although both require cell-to-cell contact between Tregs and Teffs.99,100 Tregs can suppress GVHD through a granzyme B-independent mechanism,99 whereas the suppressive effects of Tregs on antitumor immune responses are dependent on a granzyme B/perforin-dependent cytolytic mechanism.100
Although the granzyme B and perforin pathways work closely in cell killing, Gondek et al have reported that Treg-mediated inhibition of Teff proliferation requires granzyme B, but not perforin, in vitro.38 Therefore, as a therapeutic experiment in alloHCT, disarming the granzyme B pathway in Tregs could be considered to keep the suppressive effect of Tregs on GVHD and to increase the GVT effect. Another mechanism that could separate GVT from GVHD involving T cells is related to homing receptors (ie, CC-chemokine receptors, CCR). CCR7 plays a critical role in the movement of T cells into lymph nodes and Peyer patches, where they encounter mature DCs, but not to the spleen.
CCR7 is expressed on the surface of naive T cells, memory T cells, and Tregs. Transferred CCR7-deficient Tregs failed to migrate into the lymph nodes and suppress antigen-induced T-cell responses in mice.101 CCR7−/− T cells generate attenuated GVHD responses compared with wild-type cells while generating robust GVL responses. These findings are likely because of the fact that optimal GVHD generation requires priming within the lymph nodes and Peyer patches, whereas GVL responses to the cell line used can occur in the spleen.102 Separation of GVL from GVHD through Treg modulation may be possible; however, caution should be exercised when these encouraging results are interpreted because data are from animal models, and most often authors used lymphoma cell lines, with fewer studies focused on AML cell lines. It is unclear whether similar separation occurs with human AML.
Altering Treg numbers after transplantation
In murine models, ex vivo–expanded Tregs controlled GVHD.91,103,104 In some murine studies, investigators used in vitro–expanded iTregs and found that iTregs were much less potent than nTregs at preventing GVHD.105 The lower potency of iTregs resulted from the loss of FOXP3 expression and reversion to a proinflammatory phenotype after in vivo transfer. Blocking IL-6 with antibodies in vivo reversed this process and caused GVHD suppression.105 However, in other studies, a high potency for GVHD prevention of in vitro–generated iTregs has been observed by the use of antigen-specific iTregs.106 Moreover, recent studies in a human xenogeneic GVHD model reveal comparable suppression by in vitro-expanded, polyclonally activated iTregs and ex vivo expanded nTregs.107
In humans, Tregs obtained from adult donors or umbilical cord blood were administered after transplantation to prevent GVHD.108,109 Brunstein et al showed that ex vivo–expanded Treg infusions from a third party could be used as supplemental GVHD prophylaxis after double umbilical cord blood transplantation.109 Tregs infused with the graft were detectable in PB for up to day 14 after transplantation. In a recent study, freshly isolated donor Tregs were infused (day −4) before the infusion of CD34+ cells and conventional T cells (day 0) in 28 patients with hematologic malignancies (22 patients with AML) undergoing haploidentical transplantation. Only 2 (7%) patients developed acute GVHD.108 The recovery of total CD4+ and CD8+ cells was rapid, but the frequency of Tregs was not tested after transplantation. In both trials, the GVT effect seemed not to be compromised when analyzed compared with historical controls in these very preliminary studies. Neither Tregs at the tissue level in patients with GVHD nor the immunosuppressive functions of circulating Tregs after transplantation were evaluated in these 2 studies.
In a DLI setting, low-dose IL-2 was administered concomitantly with CD4+DLI (CD8+ T cells were depleted) in 5 patients in a phase 1 trial (3 of them had AML).110 This combination led to significant in vivo expansion of Tregs, which was associated with low GVHD. However, there was no clinical response in AML. Conversely, recent other studies have indicated that depletion of Tregs from the conventional DLI graft, including CD8+ cells, improved the GVT effect in 17 patients after alloHCT.111
Obstacles and questions facing Treg research
There is no exclusively reliable marker for Tregs. CD25 can be positive in active T cells, and Tregs can be found in the CD25− subpopulation. Likewise, FOXP3 expression has also been demonstrated in activated human Teffs and in nonhematopoietic cells (normal and cancer cells). Because FOXP3 is an intracellular protein, its detection requires fixation and permeabilization of the cells. These factors hinder the use of FOXP3 as a marker to sort for viable Tregs for functional studies or in vivo expansion for cellular therapy. Phenotypic confirmation of Tregs, therefore, is not adequate, and functional analysis of Tregs should be performed to define a Treg-cell subpopulation. The inclusion of additional cell-surface determinants such as CD127, HLA-DR, CTLA-4, Lag-3, GITR, and CD45RA may be useful in identifying CD4+25+FOXP3+ subsets that are more likely to be potent suppressors, although clearly robust biomarkers are needed. The development of more rapid and direct assays to test Treg function would be warranted.
Another issue to be addressed is the stability of Treg suppressor cell function. FOXP3 protein expression may not be stable, depending on FOXP3 gene methylation, and can disappear in 10%-15% of FOXP3+ Tregs (so-called exFOXP3 cells) in mice.112 Some of these cells were permissive for interferon and IL-17 production.112 Similarly, Tregs can produce Th1 proinflammatory cytokines in patients with autoimmune disease.113 The extent to which Tregs are stable in vivo or lose suppressive and acquire effector functions has to be addressed in humans. Finally, the optimal site (BM or PB) for testing Tregs in AML is not yet known.
Because Tregs can inhibit GVHD in patients and may permit a GVL response, ex vivo–expanded Tregs may be useful in improving the outcome of patients with AML. In murine models of alloHCT, Treg infusions did not appear to impair tumor responses but did seem to decrease acute GVHD. Data in humans are limited, but trials are ongoing (Table 3). However, previously, ex vivo expansion from nTregs has been shown to be time consuming and costly, and it is difficult to achieve an adequate number of cells. In contrast, iTregs can be generated with the use of IL-2, TGF-β, and rapamycin culture conditions and rapidly expanded to high numbers107 and thus may be an alternative to nTregs. Lu et al demonstrated that a combination of IL-2, TGF-β, and retinoic acid can rapidly induce stable and fully functional CD4+CD25+FOXP3+ suppressor T cells from naive human CD4+ cells, which also resist proinflammatory stimuli.114 In this model, IL-2 and TGF-β induce the initial stages of maturation and retinoic acid completes the process, conferring resistance to the inhibitory effects of IL-6 and IL-1β (ie, proinflammatory Th17 conversion). However, under some conditions and for some types of iTregs, FOXP3 expression and the suppressive function of iTregs may diminish after a short time in vivo because they undergo conversion by proinflammatory cytokines.105 Therefore, should a longer survival time be required for optimal GVHD inhibition, repeated infusions or other interventions are necessary to maintain a thriving and functional population of infused iTregs.
Ongoing studies in AML manipulating Tregs
| NCI study . | Phase . | Center . | Primary question(s) asked . | Status of activity . |
|---|---|---|---|---|
| NCT00539695 | 2 | Baylor College of Medicine | Whether IL-2 increases Tregs as a measure of GVHD prophylaxis | Recruiting |
| NCT00675831 | 1 | Dana-Farber Cancer Institute | Feasibility and safety of CD25+ Treg-depleted DLI in patients with relapsed hematologic malignancies | Recruiting |
| NCT00602693 | 1 | University of Minnesota | MTD of UCB-derived Tregs | Suspended |
| Whether Tregs decrease GVHD | ||||
| NCT00725062 | 1/2 | University of Minnesota | What is MTD of Tregs after sibling alloHCT Whether Tregs decrease GVHD | Closed |
| NCT00987987 | 1/2 | Hôpitaux de Paris | Whether Tregs depletion before DLI improves GVT effect | Completed |
| NCT01050764 | Feasibility | Stanford University | Whether Tregs decrease GVHD after haploidentical alloHCT | Recruiting |
| NCT01096602 | 2 | Beth Israel Deaconess Medical Center | Toxicity and effect of blockade of PD-1 in conjunction with the dendritic cell/AML vaccine in AML patients in CR1 | Recruiting |
| NCT01106950 | 2 | University of Minnesota | Whether Tregs depletion with denileukin diftitox improves outcome before haploidentical NK cells infusion in relapsed/refractory AML patients | Recruiting |
| NCT01163201 | 1/2 | University of Minnesota | Determine the optimal cell dose mixture of UCB T regulatory and CD3+ T effector cells in double UCB transplantation | Not open yet |
| NCT00224354 | 1 | Baylor College of Medicine | Safety and efficacy of deleting Tregs with IL-2 immunotoxin directed to the CD25 antigen and administration of autologous gene-modified tumor cells in patients with CLL | Completed |
| NCT01067287 | 2 | Dana-Farber Cancer Institute | Safety and efficacy of the blockage of PD-1 in conjunction with the dendritic Cell/myeloma vaccines after autoHCT | Recruiting |
| NCT01251952 | 1 | Barbara Ann Karmanos Cancer Institute | Feasibility and safety of giving 2 doses of denileukin diftitox in patients with MM early after autoHCT | Recruiting |
| NCI study . | Phase . | Center . | Primary question(s) asked . | Status of activity . |
|---|---|---|---|---|
| NCT00539695 | 2 | Baylor College of Medicine | Whether IL-2 increases Tregs as a measure of GVHD prophylaxis | Recruiting |
| NCT00675831 | 1 | Dana-Farber Cancer Institute | Feasibility and safety of CD25+ Treg-depleted DLI in patients with relapsed hematologic malignancies | Recruiting |
| NCT00602693 | 1 | University of Minnesota | MTD of UCB-derived Tregs | Suspended |
| Whether Tregs decrease GVHD | ||||
| NCT00725062 | 1/2 | University of Minnesota | What is MTD of Tregs after sibling alloHCT Whether Tregs decrease GVHD | Closed |
| NCT00987987 | 1/2 | Hôpitaux de Paris | Whether Tregs depletion before DLI improves GVT effect | Completed |
| NCT01050764 | Feasibility | Stanford University | Whether Tregs decrease GVHD after haploidentical alloHCT | Recruiting |
| NCT01096602 | 2 | Beth Israel Deaconess Medical Center | Toxicity and effect of blockade of PD-1 in conjunction with the dendritic cell/AML vaccine in AML patients in CR1 | Recruiting |
| NCT01106950 | 2 | University of Minnesota | Whether Tregs depletion with denileukin diftitox improves outcome before haploidentical NK cells infusion in relapsed/refractory AML patients | Recruiting |
| NCT01163201 | 1/2 | University of Minnesota | Determine the optimal cell dose mixture of UCB T regulatory and CD3+ T effector cells in double UCB transplantation | Not open yet |
| NCT00224354 | 1 | Baylor College of Medicine | Safety and efficacy of deleting Tregs with IL-2 immunotoxin directed to the CD25 antigen and administration of autologous gene-modified tumor cells in patients with CLL | Completed |
| NCT01067287 | 2 | Dana-Farber Cancer Institute | Safety and efficacy of the blockage of PD-1 in conjunction with the dendritic Cell/myeloma vaccines after autoHCT | Recruiting |
| NCT01251952 | 1 | Barbara Ann Karmanos Cancer Institute | Feasibility and safety of giving 2 doses of denileukin diftitox in patients with MM early after autoHCT | Recruiting |
Most of the studies are at the stage of phase 2 and in the setting of allogeneic stem cell transplantation or cellular therapy.
alloHCT indicates allogeneic HSC transplantation; AML, acute myelogenous leukemia; autoHCT, autologous HSC transplantation; CLL, chronic lymphocytic leukemia; DLI, donor lymphocyte infusion; GVT, graft-versus-tumor; MM, multiple myeloma; MTD, maximal tolerated dose; NK, natural killer; PD-1, programmed death-1; and UCB, umbilical cord blood.
Another strategy to expand Tregs is IL-2 administration for in vivo expansion. Older studies used IL-2 after autologous HCT to induce the effects of GVL.115,116 Some trials resulted in clinical conditions mimicking GVHD, including in patients with AML.115
Although IL-2 is indispensable for the generation, survival, and activation of nTregs, IL-2 is also required for the expansion of CD8+ CTLs. Preferential activation of CD8+ T cells or Tregs by IL-2 is not strictly dependent on the dose of IL-2 (ie, lower and higher doses of IL-2 can activate both) but rather depends on the preactivation status of CD8+ cells because resting peripheral T cells do not express the high-affinity trimeric IL-2R until activated by specific antigens.117-120 When CD8+ CTLs are already active and express CD25, IL-2 may preferentially augment CTL-mediated responses rather than Treg-mediated responses and thus can exacerbate GVHD. However, in vivo models suggests that a greater level of local IL-2 in conditions such as inflammation may promote the induction of nTreg cell function and subsequently inhibit clonal expansion and function of Teffs. Moreover, greater doses of IL-2 are needed to activate NK cells.
Pharmacologic means to deplete Tregs are summarized in Figure 3. CD25 (denileukin diftitox),121 CTLA-4 (ipilimumab, a fully human monoclonal antibody),122 or glucocorticoid-induced TNF receptor-related protein, OX40, and toll-like receptor can each be targeted to deplete Tregs. Vaccination against FOXP3 can elicit a FOXP3-specific CTL response and depletion of Tregs in mice.123 The effect of fludarabine on Tregs is controversial.124,125 It is important to note that Treg depletion may affect the incidence and the spectrum of autoimmune diseases depending in part on the degree and duration of CD25+CD4+ Treg depletion, the age of the host (the risk is greater in neonatal mice than adult mice), the route of administration (systemic or local), and in murine models the genetic background of the host.126 Treg depletion trials are ongoing in patients, especially those with solid tumors (Table 3). To our knowledge, there has been no increase in autoimmune disorders reported during the 3-years since denileukin diftitox was approved for cutaneous T-cell lymphoma.
There are various potential ways by which the frequencies or suppressive functions of Tregs can be down-regulated. Depletion of Tregs with anti-CD25 agents has been the most common approach.
There are various potential ways by which the frequencies or suppressive functions of Tregs can be down-regulated. Depletion of Tregs with anti-CD25 agents has been the most common approach.
Conclusions
Data on Tregs and AML have been accumulating rapidly. In murine AML models, results are consistent: increased Tregs are found with AML and are associated with poor prognosis, and the depletion of Tregs improves outcomes. In humans, studies to date have been small and somewhat inconsistent. For example, Treg frequencies are greater in patients with AML compared with control patients at diagnosis; however, they remain high throughout induction therapy and are found at even greater levels in CR than at diagnosis in some studies.35,76 There are studies in which authors demonstrate that early lymphocyte recovery from chemotherapy or transplantation is associated with good prognosis.127,128 However, Kanakry et al showed that recovering T lymphocytes after induction chemotherapy were predominantly CD4+ and included a greatly expanded population of Foxp3+ T cells.74 On the other hand, all studies consistently showed that the frequency of Tregs is increased in patients with AML compared with healthy control patients and that these Tregs are suppressive on Teffs in vitro. Moreover, some of these studies indicated that increased Tregs at diagnosis of AML is poor prognostic.
On the basis of data in murine models and in limited human studies, modulating Tregs during chemotherapy, before vaccination, or pre/posttransplantation or cellular therapy seems to be an attractive option and warrants further investigation in humans. However, it is critical to acknowledge that there are potentially severe adverse effects of manipulating Tregs (eg, Treg depletion and autoimmune disorders and enhanced inflammation or Treg expansion and AML progression). Therefore, vigilance for unexpected results will be required in designing clinical trials for Treg manipulation with novel agents.
Acknowledgments
This work was supported in part by National Institutes of Health grants R01 CA72669, HL56067, AI34495, and P01 CA142106; AI056299; CA067493; and Leukemia and Lymphoma Translational Research grant R6029-07.
National Institutes of Health
Authorship
Contribution: C.U. and B.R.B. conceived the ideas expressed in the manuscript; and all authors wrote and edited the manuscript.
Conflict-of-interest disclosure: D.H.M. has intellectual property interests in the therapeutic use of IDO and IDO inhibitors and receives consulting income and research support from NewLink Genetics Inc. B.R.B has a patent and a patent pending for nTreg and iTreg generation and application to bone marrow transplantation. The remaining authors declare no competing financial interests.
Correspondence: Bruce R. Blazar, MD, MMC 109, University of Minnesota, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal