In this issue of Blood, Fu and coworkers show that shear stress–induced conformational changes in VWF allow the exposure of otherwise buried methionine residues, thereby promoting their susceptibility to oxidation.1 Methionine oxidation importantly alters the interaction between VWF and platelets and prevents degradation of the protein by ADAMTS13.
The presence of reactive oxygen species may lead to the oxidation of proteins. This process is part of our general metabolism, and several regulatory mechanisms involving natural antioxidants are in place to avoid excessive protein oxidation. However, this balance may be disturbed under several pathologic conditions and an excess of reactive oxygen species over these antioxidants may result in the uncontrolled damage of proteins that are sensitive to oxidation.2,3 Examples include atherosclerosis, diabetes, Alzheimer disease, and cancer, all of which are characterized by the presence of increased levels of oxidized proteins.
Several amino acid residues may be prone to oxidation, but in particular surface-exposed methionine residues are easily converted into methionine sulfoxide derivatives. Often, generation of methionine sulfoxide results in loss of protein function. For instance, cigarette smoke–induced oxidation of methionine residues in α1-antitrypsin renders this protein inactive toward neutrophil elastases in lungs, which may contribute to the pathogenesis of chronic obstructive pulmonary disease.4
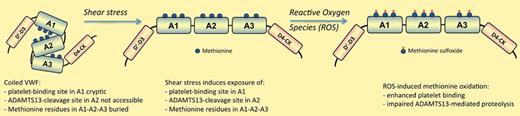
VWF is a multimeric protein that is pivotal to the recruitment of platelets to the injured vessel wall and subsequent thrombus growth. The multimeric size of VWF is an important parameter in this regard, because the hemostatic potential of VWF increases with its size. VWF multimers may be reduced in size enzymatically by the protease ADAMTS13. Interestingly, both VWF-platelet interactions and ADAMTS13-mediated proteolysis are regulated by shear stress. In the normal circulation, VWF is present in a closed globular conformation, being unable to bind platelets and resistant to ADAMTS13-mediated proteolysis. Under conditions of increased shear rates, VWF is converted into an open stretched conformation, which coincides with the exposure of the platelet-binding site and the ADAMTS13-cleavage site tyrosine1605-methionine1606 in the VWF A1 and A2 domains, respectively.5
Recently, it has been demonstrated by 2 different research groups that oxidation of methionine1606 impairs proteolysis by ADAMTS13.6,7 What was difficult to understand, however, is that methionine1606 is deeply buried within the A2 domain, and not easily accessible to oxidizing agents, raising questions at the physiologic relevance of this finding. Fu and colleagues have addressed this issue in a very elegant manner.1 Based on the notion that VWF is susceptible to shear stress–induced conformational changes, they have investigated how oxidation of methionine residues in VWF is affected by shear stress. This study revealed a number of interesting findings. First, they indeed found that shear stress accelerates oxidation of methionine1606. In addition, they observed that other methionine residues within the A1-A2-A3 domain region of VWF are being oxidized after shear-induced unfolding of VWF. Apparently, shear stress induces conformational changes allowing the exposure of methionine residues not only within the VWF A2 domain, but also in the A1 and A3 domains, both of which are of functional importance for VWF. A third intriguing finding by Fu and coworkers is that oxidized VWF appears more efficient than its native counterpart in supporting ristocetin-dependent platelet agglutination. This is unexpected given that it is more common that methionine-oxidation makes the protein less rather than more active. It seems reasonable to assume that oxidation of VWF may serve a physiologic purpose, and could be considered akin to phosphorylation: alteration of a side chain to change the function of a protein. Perhaps we should not only measure VWF levels and multimer size to assess its hemostatic potential in the various patient cohorts, but also its state of oxidation.
Altogether, Fu et al show that shear stress–induced unfolding of the VWF molecule not only promotes platelet binding and ADAMTS13-mediated proteolysis, but also facilitates oxidation of methionine residues (see figure). These exciting observations pave the way for a series of follow-up studies to investigate this phenomenon in more detail. For instance, how does methionine oxidation impair ADAMTS13-mediated proteolysis? Methionine is unique in that its hydrophobic side chain is relatively flexible, and its oxidation generates a less flexible and more hydrophilic sulfoxide.8 It is possible that the increased hydrophilicity and reduced flexibility of the sulfoxide variant prevent optimal penetration into the ADAMTS13 active center. Another intriguing issue relates to deoxidation of methionine sulfoxides. The mammalian proteome contains several methionine sulfoxide reductases that are capable of reversing methionine oxidation, most of which are located inside cells.4 It would be of interest to investigate whether these enzymes may also act on oxidized VWF, and if they do, how their action is affected by shear stress. Finally, a key question is how oxidation affects VWF function in more complex settings, such as in in vitro whole-blood perfusion or in in vivo thrombosis models. Does oxidation of VWF convert the molecule into an active mode similar to that seen in thrombocytopenic thrombotic purpura or VWD–type 2B?
Shear stress–induced unfolding of VWF exposes buried, oxidation-sensitive methionine residues. Indicated are methionine residues in the A1 (M1303, M1304, M1385), A2 (M1495, M1521, M1606), and A3 (M1761, M1860) domains of VWF that are susceptible to oxidation. Except for M1495, their conversion into methionine sulfoxide proceeds much more efficient following shear stress–induced unfolding of the molecule, as they are buried in the coiled VWF conformation. Methionine oxidation results in enhanced platelet binding and reduced ADAMTS13-mediated proteolysis.1
Shear stress–induced unfolding of VWF exposes buried, oxidation-sensitive methionine residues. Indicated are methionine residues in the A1 (M1303, M1304, M1385), A2 (M1495, M1521, M1606), and A3 (M1761, M1860) domains of VWF that are susceptible to oxidation. Except for M1495, their conversion into methionine sulfoxide proceeds much more efficient following shear stress–induced unfolding of the molecule, as they are buried in the coiled VWF conformation. Methionine oxidation results in enhanced platelet binding and reduced ADAMTS13-mediated proteolysis.1
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal