Abstract
Allogeneic hematopoietic cell transplantation (HCT) is effective therapy for hematologic malignancies through T cell–mediated GVL effects. However, HCT benefits are frequently offset by the destructive GVHD, which is also induced by donor T cells. Naive Th can differentiate into Th1 and Th17 subsets and both can mediate GVHD after adoptive transfer into an allogeneic host. Here we tested the hypothesis that blockade of Th1 and Th17 differentiation is required to prevent GVHD in mice. T cells with combined targeted disruption of T-bet and RORγt have defective differentiation toward Th1 and Th17 and skewed differentiation toward Th2 and regulatory phenotypes, and caused ameliorated GVHD in a major MHC-mismatched model of HCT. GVL effects mediated by granzyme-positive CD8 T cells were largely preserved despite T-bet and RORγt deficiency. These data indicate that GVHD can be prevented by targeting Th1 and Th17 transcription factors without offsetting GVL activity.
Introduction
Separation of GVHD from GVL effects is the major challenge of allogeneic hematopoietic cell transplantation (HCT) that is used for the treatment of hematologic malignancies. On Ag stimulation, T-cell precursors can differentiate into distinct functional cell subsets including Th1 and Th17 cells. Understanding the role of each subset in the development of GVHD is critical to develop effective therapy and improve HCT outcome.
The cytokine storm caused by the conditioning regimen and Th1-cell cytokines is key to initiating the inflammatory cascade and amplifying immune responses that cause GVHD.1-3 However, studies using IFN-γ gene knockout (KO) mice as donors showed that deficiency of IFN-γ is paradoxically associated with more severe acute GVHD.4,5 Our group and others found that Th17 cells can augment GVHD in some circumstances,6,7 and in vitro–generated Th17 cells alone are sufficient to mediate lung and skin GVHD.8 IFNγ blockade promotes Th17 differentiation, while IL-17 blockade promotes Th1 differentiation and each blockade alone is ineffective for preventing GVHD,9 suggesting that Th1 and Th17 cells are mutually inhibitory, and that each Th type alone is sufficient to induce GVHD.
The transcription factor T-bet is required for the differentiation of Th1 cells10 and RORγt is necessary for Th17 cells.11 Therefore, we hypothesized that targeted disruption of both T-bet and RORγt factors would block Th1 and Th17 differentiation and prevent GVHD. In the current study, we used mice deficient for T-bet, RORγt, or both as T-cell donors to test T-bet and RORγt as targets to prevent GVHD after allogeneic HCT.
Methods
Mice
C57BL/6 (B6; H-2b), B6.Ly5.1, BALB/c (H-2d), and B6D2F1 (H-2b/d) were purchased from the National Cancer Institute/National Institutes of Health (NCI/NIH). T-bet and RORγt KO mice on B6 background were purchased from The Jackson Laboratory and RORγt/T-bet double knockout (dKO) mice were bred at Moffitt Cancer Center. All animals were housed in the American Association for Laboratory Animal Care–accredited Animal Resource Center at Moffitt Cancer Center. Experiments were all carried out under protocols approved by the Institutional Animal Care and Use Committee.
Abs and flow cytometry
The following Abs were used for cell-surface staining: anti-CD4–FITC, or -allophycocyanin (L3T4), anti-CD8α–FITC, –allophycocyanin, –allophycocyanin-cy7 or –Alexa Fluor 700(Ly-2), anti-CD45.1–FITC, or –allophycocyanin (A20), anti-B220–PE (RA3-6B2), anti-H-2Kb–FITC, –PE, or –biotin (AF6), purchased from eBioscience; anti-CD4–Pacific Blue (RM4-5) purchased from BD Biosciences. Detection of biotinylated Abs was performed using allophycocyanin-cy7 or allophycocyanin conjugated to streptavidin (BD Biosciences). Intracellular staining was carried out using anti–IFN-γ–PE or Per-cp 5.5 (XMG1.2; BD Biosciences), anti–IL-17–allophycocyanin (17B7; eBioscience), anti–IL-4–PE (11B11; BD Pharmingen), anti–IL-5–PE (TRFK5; BD Pharmingen), anti-TNFα–PE, or PE-Cy7 (MP6-XT22; BD Pharmingen), anti-Foxp3–PE (FJK-16s; eBioscience), anti-Granzyme B–PE (16G6; eBioscience) and the appropriate isotype controls. Cells were analyzed on a LSR II (BD Biosciences). Data were analyzed using FlowJo (TreeStar).
Cell preparation
T cells were purified through negative selection using magnetic bead depletion of non-T cells. Briefly, after red cell lysis, spleen, and lymph node cells were incubated with biotin-conjugated Ab anti-CD11b, anti-B220, anti-DX5, and anti-Ter119 for 15 minutes. All of the Abs were purchased from eBioscience. Cells were subsequently incubated with biotin beads (Miltenyi Biotec) for 15 minutes at 4°C, and Ab-bound cells were removed magnetically.
In vitro generation of Th1 and Th17 cells
CD4+CD25− cells isolated from WT, T-bet−/−, RORγt−/− or RORγt−/−/T-bet−/− mice were stimulated in the presence of APCs with 1 μg/mL anti-CD3 mAb. The cytokine stimuli for Th17 cell differentiation includes 5 ng/mL TGFβ, 10 ng/mL IL-6, 10 μg/mL anti–IL-4, 10 μg/mL anti-IFNγ mAbs; 10 ng/mL IL-12, 1000 U/mL IFNγ, 10 μg/mL anti–IL-4 were used for generation of Th1 cells. Cell phenotype was confirmed on day 4 by intracellular cytokine staining of IFNγ and IL-17 expression.
HCT
BM was flushed from donor femurs and tibias with Dulbecco modified media (DMEM; Invitrogen) and passed through sterile mesh filters to obtain single-cell suspensions. T cells in BM were depleted with anti-Thy1.2 mAb plus low-toxicity rabbit complement (C-6 Diagnostics). T cell–depleted BM cells, referred to as TCD-BM, were used in all transplantation experiments throughout. Host mice were conditioned with total body irradiation administered at 800 cGy (a single dose) for BALB/c and 1200 cGy (split doses) for B6D2F1 using a Shepherd Mark I Cesium Irradiator (J. L. Shepherd and Associates). Irradiated recipients received a single IV injection in the lateral tail vein of TCD-BM (BM alone group) with or without added T cells.
Histologic analysis
Representative samples of liver, colon, small intestines, and lung were obtained from transplanted recipients and fixed in 10% neutral-buffered formalin. Samples were then embedded in paraffin, cut into 5-μm-thick sections, and stained with H&E. A semi-quantitative scoring system was used to account for histologic changes consistent with GVHD in the colon, liver, and lung as previously described.12 Data were presented as individual GVHD target organ scores as well as a composite score from all 4 tissues. All slides for GVHD analysis were coded and read in a blinded fashion. Images were visualized with an Olympus BX45 microscope. Image acquisition was performed with an Olympus DP70 digital camera (×400) and software package.
Leukemia/lymphoma models
To examine the GVL effects of donor T cells, we performed studies using the B6→BALB/c and B6→B6D2F1 BMT models. In BALB/c recipients, A20 B-cell lymphoma line transduced with a luc/neo plasmid (A20-luc) was used to allow for visualization of tumor dissemination. Mice received 800 cGy TBI on day −1. On day 0, B6 recipients received grafts containing 5 × 106 TCD-BM, with 2 × 106 T cells and 2 × 103 A20-Luc tumor cells. For the tumor titration experiment, BALB/c recipients received 5 × 106 TCD-BM and 0.5 × 106 T cells with 2 × 103, 1 × 104, or 5 × 104 A20-Luc tumor cells. Mortality because of GVHD or tumor relapse was distinguished by bioluminescent imaging (BLI). In the B6→B6D2F1 models, mice received 1200 cGy TBI on day −1. On day 0, B6D2F1 recipients received grafts containing 5 × 106 TCD-BM, with 2 ∼ 4 × 106 T cells and 1 × 104 host type P815 (DBA/2-derived, H-2Dd) tumor cells.
In vivo BLI
Mice were given an IP injection of luciferin (150 mg/kg body weight) and then anesthetized with isoflurane gas using a Xenogen XGI Gas Anesthesia System and imaged using the IVIS Imaging system (Xenogen) to assess bioluminescence 10 minutes after injection of the substrate. Imaging data were analyzed and quantified with Living Image Software (Xenogen) as described in our previous work.12
Per cell-based CTL assay
Splenocytes from recipient mice transplanted with WT, T-bet−/−, RORγt−/−, or RORγt−/−/T-bet−/− donor T cells were used as effecter cells and P815 cells (1 × 104) were used as a target cells. EL4 cells were used as a negative control. Cytolysis was measured with a standard 4-hour assay. Target cells were labeled with 5 μCi/mL 3H-thymidine, incubated overnight, washed, and dispensed into the wells of U-bottom 96-well plates. Different numbers of the effector cells (1 × 104 to 5 × 105) were added to generate different E:T ratios of 6:1 to 100:1. The radioactivity released into supernatants was measured in a scintillation counter. Negative controls (spontaneous release) were supernatants from 3H-labeled target cell culture without effector cells. CTL was normalized by percentage of T cells in recipient spleen.
Lymphocyte isolation from liver, gut, and lung
Isolation of lymphocytes from liver and gut was done as in our previous work.12 Briefly, small intestine was dissected from the gastric-duodenal junction to the ileocecal junction. Intestines were flushed with 2% FBS/PBS, cut into 0.5-cm-long pieces, and incubated in complete medium containing 0.5 mg/mL collagenase D (Roche) and 1 μg/mL DNase for 1 hour at 37°C with continuous shaking. Intestinal pieces were then vortexed for 15 seconds, and the supernatant was strained and centrifuged at 325g for 5 minutes. Pellets were resuspended in 40% Percoll overlaid on 80% Percoll, and centrifuged at 1300g for 30 minutes. Lymphocytes were then recovered from the interphase. Livers were homogenized and passed through a 70-μm cell strainer. Pellets were resuspended in PBS, overlaid on Ficoll, and centrifuged at 1300g for 20 minutes. Lymphocytes were then recovered from the interphase. We adapted the protocol established by others to isolate lymphocytes from lung.13 The lung tissues were minced finely and incubated in RPMI 1640 with 5% FCS, penicillin/streptomycin, 10mM HEPES, 50μM 2-ME, 20mM l-glutamine containing 20 U/mL collagenase D and 1 μg/mL DNase. After incubation for 60 minutes at 37°C, cells were collected by centrifugation. The cell pellet was suspended in 40% Percoll and layered onto 80% Percoll. The cells at the interphase were then collected for further analysis.
Intracelluar cytokine staining and serum cytokine analysis
For intracellular cytokines staining, splenocytes from recipient mice at the time specified were stimulated in vitro with 50 ng/mL PMA (Sigma-Aldrich), 500 ng/mL ionomycin (Sigma-Aldrich) and 1 μL of Golgi Plug (BD Biosciences), and incubated at 37°C for 4 to 5 hours before staining. The procedure was described in a previous publication.8
Blood samples were obtained from TCD-BM transplant recipients at the time specified, and cytokine analysis was performed as described previously.14,15 Briefly, IFNγ, TNFα, IL-17, IL-4, IL-10, and IL-6 were measured in recipient serum using a cytometric bead array kit according to the manufacturer's instructions (BD Biosciences).
Statistic analysis
For comparison of recipient survival among groups in GVHD experiments, the log-rank test was used to determine the statistical significance. To compare the engraftment and expansion of donor T cells, cytokine levels, and pathology scores, a Student t test was used
Results
Deficiency of T-bet and RORγt transcription factors in donor T cells significantly reduces GVHD after allogeneic BMT
To evaluate the contributions of Th1 and Th17 subsets in the development of GVHD, we used wild-type (WT), T-bet−/−, RORγt−/−, and RORγt−/−/T-bet−/− mice of B6 background as donors. We first measured the phenotypes of these 4 different strains by testing expression of CD4, CD8, CD25, and Foxp3 in spleen. The percentages of CD4 and CD8 cells were moderately reduced in RORγt−/− and RORγt−/−/T-bet−/− mice compared with WT or T-bet−/− mice (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). After T-cell purification, there was no significant difference in the percentage of CD4 and CD8 T cells. The percentages of Tregs (CD4+CD25+) in spleen of RORγt−/−/T-bet−/− mice was slightly higher than that of the 3 other strains. To exclude the possibility that Tregs would affect the outcome of GVHD induced by RORγt−/−/T-bet−/− T cells, we also purified non-Tregs CD4+CD25− T cells from RORγt−/−/ T-bet−/− mice and mixed with the purified RORγt−/−/T-bet−/− T cells to obtain the same ratio of Tregs as the 3 other strains (supplemental Figure 1). To confirm the effect of T-bet and RORγt on T-cell differentiation, we polarized CD4 T cells from each strain into Th1 or Th17 cells in vitro. As expected, T cells deficient for T-bet failed to differentiate into Th1 cells, while T cells deficient for RORγt failed to differentiate into Th17 cells and the double-deficient T cells essentially failed to differentiate into either lineage (supplemental Figure 2).
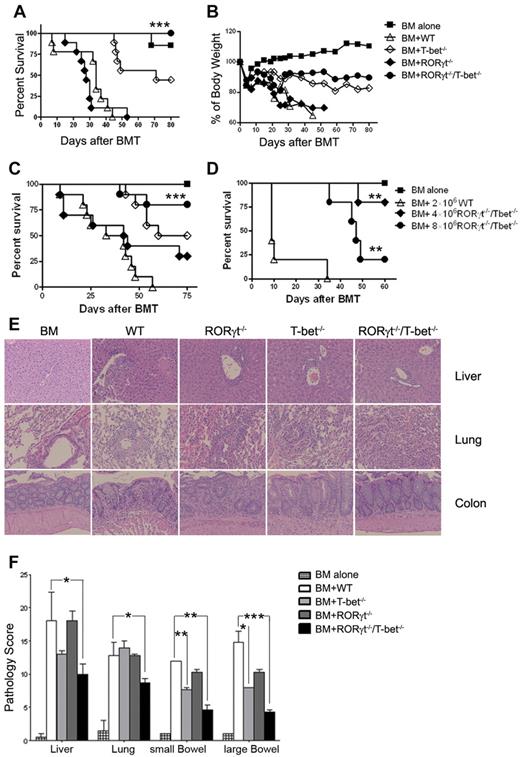
We compared the ability of purified T cells from WT, T-bet−/−, RORγt−/−, and RORγt−/−/T-bet−/− mice to induce GVHD when transplanted together with TCD-BM from WT B6 donors into fully MHC-mismatched, lethally irradiated BALB/c recipients. As shown in Figure 1, RORγt−/− T cells had comparable ability to cause GVHD as WT T cells, whereas T-bet−/− T cells were less pathogenic. Mice transplanted with T cells deficient for T-bet showed attenuated GVHD and improved survival (Figure 1A, P = .0003). Furthermore, T cells deficient for both RORγt and T-bet failed to induce GVHD lethality because 100% of recipients with RORγt−/−/T-bet−/− T cells survived long-term with only moderate loss of body weight, which was significantly better than those of T-bet−/− T cells alone (P = .015; Figure 1A-B). Consistent with the absence of GVHD, recipients of RORγt−/−/T-bet−/− had similar reconstitution and function of engrafted donor T and B cells as the recipients with TCD-BM alone. In contrast, reconstitution and function of engrafted donor T and B cells was impaired in the survived recipients with T-bet−/− T cells (supplemental Figure 3).
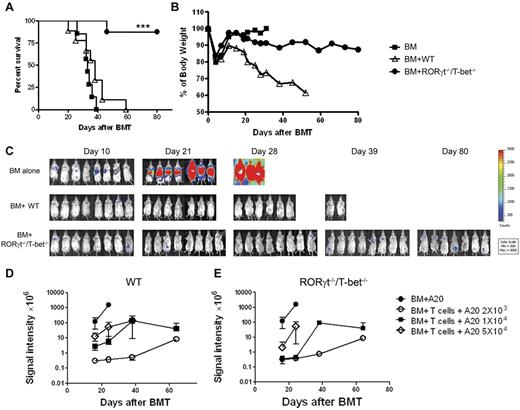
Absence of T-bet and RORγt is sufficient to protect mice from lethal GVHD. Lethally irradiated BALB/c mice (n = 10 per group) were transplanted with 5 × 106 TCD-BM alone or with 2 × 106 purified T cells from WT, T-bet−/−, RORγt−/−, or RORγt−/−/T-bet−/− mice. Overall survival (A) and the percentage of original body weight over time (B) are depicted. Data are shown from 2 replicate experiments combined. (C) Lethally irradiated BALB/c mice (n = 11 per group) were transplanted with 5 × 106 TCD-BM alone or with 1 × 106 purified T reg-depleted T cells from WT, T-bet−/−, RORγt−/−, or RORγt−/−/T-bet−/− mice. Survival data are shown from 2 replicate experiments combined. (D) Lethally irradiated BALB/c mice (n = 6 per group) were transplanted with 5 × 106 TCD-BM alone or plus total T cells at the doses indicated from WT or RORγt−/−/T-bet−/− mice. Survival is shown from 1 experiment with 5 recipients in each group. (E) H&E staining of colon, liver, and lung sections of recipients 20 to 25 days after HCT. (F) Pathologic score mean ± SE indicates the damage in small intestine, colon, liver, and lung using a semi-quantitative scoring system. Data are shown from 2 replicate experiments combined. Asterisk indicates statistical significance between WT and RORγt−/−/T-bet−/− groups: *P < .05; **P < .01; ***P < .001.
Absence of T-bet and RORγt is sufficient to protect mice from lethal GVHD. Lethally irradiated BALB/c mice (n = 10 per group) were transplanted with 5 × 106 TCD-BM alone or with 2 × 106 purified T cells from WT, T-bet−/−, RORγt−/−, or RORγt−/−/T-bet−/− mice. Overall survival (A) and the percentage of original body weight over time (B) are depicted. Data are shown from 2 replicate experiments combined. (C) Lethally irradiated BALB/c mice (n = 11 per group) were transplanted with 5 × 106 TCD-BM alone or with 1 × 106 purified T reg-depleted T cells from WT, T-bet−/−, RORγt−/−, or RORγt−/−/T-bet−/− mice. Survival data are shown from 2 replicate experiments combined. (D) Lethally irradiated BALB/c mice (n = 6 per group) were transplanted with 5 × 106 TCD-BM alone or plus total T cells at the doses indicated from WT or RORγt−/−/T-bet−/− mice. Survival is shown from 1 experiment with 5 recipients in each group. (E) H&E staining of colon, liver, and lung sections of recipients 20 to 25 days after HCT. (F) Pathologic score mean ± SE indicates the damage in small intestine, colon, liver, and lung using a semi-quantitative scoring system. Data are shown from 2 replicate experiments combined. Asterisk indicates statistical significance between WT and RORγt−/−/T-bet−/− groups: *P < .05; **P < .01; ***P < .001.
To further exclude the role of Tregs in GVHD, we depleted Tregs and compared the ability of Treg-depleted T cells from 4 different strains in the induction of GVHD. Recipients with RORγt−/−/T-bet−/− T cells had a significant advantage in survival over other groups of recipients (Figure 1C). To more quantitatively evaluate the defect of RORγt−/−/T-bet−/− T cells in GVHD, a donor T-cell dose response study was performed. GVHD lethality induced by RORγt−/−/T-bet−/− T cells was 20% at 4 × 106 per mouse and 80% at 8 × 106 (Figure 1D). Given that 1 × 106 WT T cells can readily induce 100% GVHD lethality in this BMT model (Figure 1C, data not shown), we conclude that the ability of RORγt−/−/T-bet−/− T cells in inducing GVHD was ∼ 8-fold reduced compared with that of WT T cells.
To confirm the development of GVHD in separate experiments, we examined GVHD pathology in lung, liver, small intestine, and colon 20 to 25 days after HCT, and found that pathology scores were significantly lower in the recipients transplanted with RORγt−/−/T-bet−/− T cells than the recipients of WT and RORγt T cells (Figure 1E-F). There was also significant overall reduction in pathologic damage in the colon in recipients of T-bet−/− donor T cells compared with WT recipients. However, the overall pathology score in the lung, liver, and colon was lower in the recipients of RORγt−/−/T-bet−/− T cells than the recipients of T-bet−/− donor T cells.
The liver sections of WT T-cell recipients showed inflammatory cells in the portal tract and other changes of severe GVHD such as endothelialitis and apoptosis, but were absent in recipients of TCD-BM alone (Figure 1D). The portal tract infiltration by inflammatory cells was moderate in RORγt−/− T-cell recipients, mild in T-bet−/− T-cell recipients, and minimal in RORγt−/−/T-bet T-cell recipients. The lung of WT T-cell recipients showed intense perivascular inflammatory cell infiltration and scattered inflammatory cells in the alveolar space, but no infiltration was noted in the lungs of recipients of TCD-BM alone. The peribronchial infiltration by inflammatory cells was obvious in recipients of either RORγt−/− or T-bet−/− T cells, but was minimal in recipients of RORγt−/−/T-bet−/− T cells. The large intestine showed intense inflammation and crypt destruction in recipients of WT T cells, none of which were present in recipients of TCD-BM alone. Inflammatory cells were present in the mucosa and there was apoptosis of the crypts in the recipients of either RORγt−/− or T-bet−/− T cells, but infiltration of inflammatory cells was minimal and there was no crypt apoptosis in the mucosa of RORγt−/−/T-bet−/− T-cell recipients. Taken together, these results indicate that expression of T-bet and RORγt is required for donor T cell-medicated GVHD in vivo.
Absence of RORγt/T-bet in donor T cells is associated with reduced Th1 and Th17 differentiation
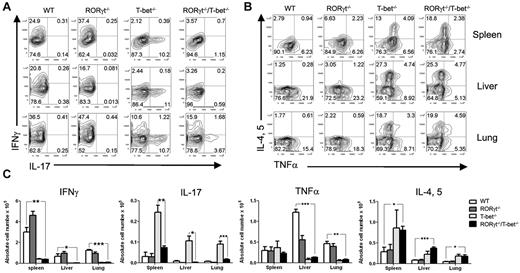
To address the role of T-bet and RORγt in the pathogenesis of GVHD, we tested how the absence of T-bet and/or RORγt affected the generation of Th1 and Th17 cells in vivo 5 days after allogeneic HCT. To this end, lethally irradiated BALB/c mice were transplanted with purified T cells from WT, T-bet−/−, RORγt−/−, or RORγt−/−/T-bet−/− mice. We observed that the absence of T-bet resulted in a significant increase in CD4+ T cells secreting IL-17 but not IFNγ in recipient spleen, liver and lung, whereas the absence of RORγt resulted in a significant increase in CD4+ T cells secreting IFNγ but not IL-17 (Figure 2A). As expected, the absence of both RORγt and T-bet resulted in a significant decrease in T cells that produce IL-17, IFNγ, and TNFα. The same pattern of cytokine production was also observed in CD8+ T cells (supplemental Figure 3). The cytokine profile in the serum showed similar results as intracellular cytokine staining; but IL-10, a negative regulator,16 was elevated in the recipient of T-bet−/−/RORγt−/− mice (supplemental Figure 4). These data demonstrate that the absence of T-bet and RORγt blocks Th1 and Th17 differentiation after adoptive transfer in allogeneic HCT.
Deficiency in T-bet and RORγt results in a significant reduction in Th1 and Th17 cells, but enhancement in Th2 cells. (A) Lethally irradiated (800 cGy) BALB/c mice (n = 4) were transplanted with 2 × 106 purified B6 WT, T-bet−/−, RORγt−/−, or RORγt−/−/T-bet−/− T cells. Intracellular cytokine profiles of splenic CD4+ T cells are shown in 5 days after BMT. Representative contour plot depicts the percentage of IL-17– and/or IFN-γ–secreting cells from spleen, liver and lung in the gated H-2Kb+CD4+ cell population. (B) IL-4, IL-5–, and/or TNFα–secreting cells from spleen, liver, and lung in the gated H-2Kb+ CD4+ donor T cells. (C) Absolute number of IFN-γ, IL-17, TNFα, and IL-4/IL-5–secreting cells from spleen, liver, and lung. Four mice in each group were used, and the experiment was repeated 3 times. Asterisk indicates statistical significance: *P < .05; **P < .01; ***P < .001.
Deficiency in T-bet and RORγt results in a significant reduction in Th1 and Th17 cells, but enhancement in Th2 cells. (A) Lethally irradiated (800 cGy) BALB/c mice (n = 4) were transplanted with 2 × 106 purified B6 WT, T-bet−/−, RORγt−/−, or RORγt−/−/T-bet−/− T cells. Intracellular cytokine profiles of splenic CD4+ T cells are shown in 5 days after BMT. Representative contour plot depicts the percentage of IL-17– and/or IFN-γ–secreting cells from spleen, liver and lung in the gated H-2Kb+CD4+ cell population. (B) IL-4, IL-5–, and/or TNFα–secreting cells from spleen, liver, and lung in the gated H-2Kb+ CD4+ donor T cells. (C) Absolute number of IFN-γ, IL-17, TNFα, and IL-4/IL-5–secreting cells from spleen, liver, and lung. Four mice in each group were used, and the experiment was repeated 3 times. Asterisk indicates statistical significance: *P < .05; **P < .01; ***P < .001.
RORγt−/−/T-bet−/− donor T cells predominantly differentiated into Th2 and T regulatory cells
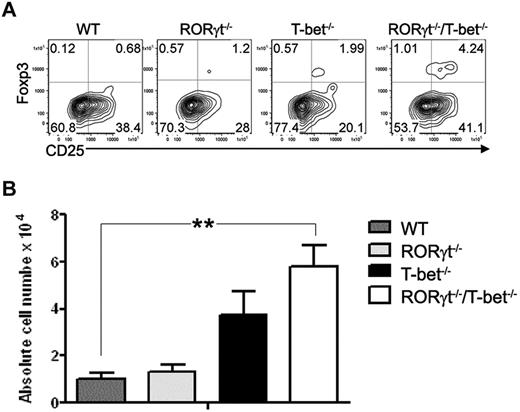
In the same transplant experiments, we tested the effects of T-bet and RORγt deficiency on donor T-cell differentiation into Th2 cells 5 days after HCT, and found that T-bet–deficient or RORγt/T-bet doubly deficient T cells differentiated into Th2 cells (IL-4+ or IL-5+) significantly more than WT or RORγt-deficient T cells, as detected in the recipient spleen, liver, and lung (Figure 2B). Since transplantation of in vitro–polarized murine Th2 cells is associated with an increased survival after T-replete HCT across MHC barriers, we speculate that more IL-4– and IL-5–positive cells contribute to the reduction of GVHD in the absence of T-bet. Donor-derived, alloreactive T cells contribute to lung injury early after HCT and TNFα is a significant mediator in this process.17-24 Therefore, we compared TNFα production in the lung, liver, and spleen of recipients given WT, T-bet−/−, RORγt−/−, and RORγt−/−/T-bet−/− T cells. Blocking T-bet and RORγt significantly reduced TNFα production and ameliorated pathologic injury in the lung. In addition, significantly more Tregs were detected in the spleens of RORγt−/−/T-bet−/− T-cell recipients than in those transplanted with WT and RORγt−/− T cells (P < .01; Figure 3). There was no significant difference in Treg numbers between the T-bet−/− and RORγt−/−/T-bet−/− groups. Given that Tregs play an important role in the suppression of GVHD,25-29 accumulation of Tregs likely contributed to the diminished GVHD in the recipients of RORγt−/−/ T-bet−/− T cells.
Absence of RORγt/T-bet of donor T cells leads to augmented differentiation of T regulatory cells. Fourteen days after injection of donor TCD-BM (ly5.1+) with WT, T-bet−/−, RORγt−/−, or RORγt−/−/T-bet−/− T cells into lethally irradiated BALB/c mice (n = 4), recipient spleen was removed and measured for expression of CD4, CD25, Foxp3, ly5.1, and H-2Kb. (A) Expression of CD25 and Foxp3 is shown on gated donor CD4+ T cells (H2K b+ly5.1−). (B) Absolute number of donor Tregs (CD4+CD25+Foxp+) in recipient spleen. Data are shown from 1 representative experiment of 3 replicates. Asterisk indicates statistical significance: **P < .01.
Absence of RORγt/T-bet of donor T cells leads to augmented differentiation of T regulatory cells. Fourteen days after injection of donor TCD-BM (ly5.1+) with WT, T-bet−/−, RORγt−/−, or RORγt−/−/T-bet−/− T cells into lethally irradiated BALB/c mice (n = 4), recipient spleen was removed and measured for expression of CD4, CD25, Foxp3, ly5.1, and H-2Kb. (A) Expression of CD25 and Foxp3 is shown on gated donor CD4+ T cells (H2K b+ly5.1−). (B) Absolute number of donor Tregs (CD4+CD25+Foxp+) in recipient spleen. Data are shown from 1 representative experiment of 3 replicates. Asterisk indicates statistical significance: **P < .01.
Absence of T-bet/ RORγt inhibits expression of chemokine receptor on donor T cells homing to GVHD-targeted organs
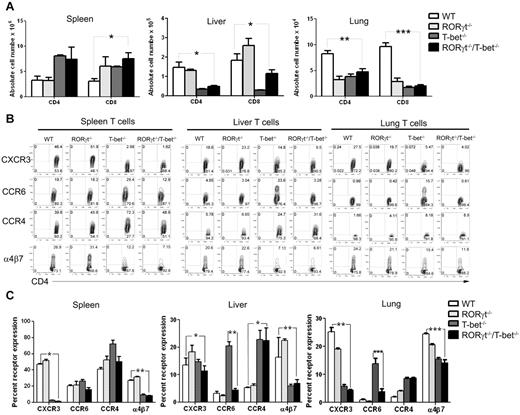
The data presented in Figure 2 showed that WT and RORγt−/− donor CD4+ T cells preferentially differentiated into Th1 cells, the T-bet−/− T cells preferentially differentiated into Th17 cells, while the RORγt−/−/T-bet−/− T cells preferentially differentiated into Th2 and Tregs. We also observed a significant decrease in the absolute number of CD4+ and CD8+ T cells deficient for T-bet and RORγt in liver and lung 14 days after HCT (Figure 4A). CD4+ T cells secreting IFN-γ were also reduced in the liver and lung, although there was no significant difference in the absolute numbers of these cells in the spleen. Overall, these studies demonstrated that the absence of T-bet and RORγt significantly decreased the number of proinflammatory CD4 and CD8 T cells. Although the total numbers of T-bet−/− or RORγt−/−/ T-bet−/− T cells in recipient spleen were higher than those of WT and RORγt−/− T cells, the total numbers of donor T cells in the lung (P < .01) or liver (P < .05) were significantly reduced in the absence of T-bet or RORγt/T-bet. In separate experiments, we isolated lymphocytes from liver, lung, and gut and compared T-cell infiltration to these GVHD target organs. Within 5 and 14 days after transplantation, we found that the number of donor T cells that infiltrated each of these organs was reduced with RORγt−/−/T-bet−/− compared with WT T cells (supplemental Figure 6).
Absence of RORγt/T-bet of donor T cells is associated with a distinct pattern of chemokine expression. (A) The absolute number of H-2b+CD4+ T and H-2b+CD8+ T cells in spleen, liver, and lung of recipients given WT, T-bet−/−, RORγt−/−, or RORγt−/−/T-bet−/− T cells donor cells 14 days after HCT. Mean ± SE is shown (n = 9), and data are from combined 3 replicate experiments. (B) Five days after BMT, splenocytes from recipients were stained for H-2Kb, CD4, and chemokine receptors. Gated H-2Kb+CD4+ T cells are shown in CD4 versus chemokine receptors. The expression profile of chemokine receptors on CD8+ T cells was similar to that of CD4+ T cells (data not shown). (C) Summary of the expression of chemokine receptors on CD4+ T cells. Representative 1 of 3 replicate experiments is shown. Asterisk indicates statistical significance: *P < .05; **P < .01; ***P < .001.
Absence of RORγt/T-bet of donor T cells is associated with a distinct pattern of chemokine expression. (A) The absolute number of H-2b+CD4+ T and H-2b+CD8+ T cells in spleen, liver, and lung of recipients given WT, T-bet−/−, RORγt−/−, or RORγt−/−/T-bet−/− T cells donor cells 14 days after HCT. Mean ± SE is shown (n = 9), and data are from combined 3 replicate experiments. (B) Five days after BMT, splenocytes from recipients were stained for H-2Kb, CD4, and chemokine receptors. Gated H-2Kb+CD4+ T cells are shown in CD4 versus chemokine receptors. The expression profile of chemokine receptors on CD8+ T cells was similar to that of CD4+ T cells (data not shown). (C) Summary of the expression of chemokine receptors on CD4+ T cells. Representative 1 of 3 replicate experiments is shown. Asterisk indicates statistical significance: *P < .05; **P < .01; ***P < .001.
Because chemokine receptors and their ligands play a critical role in donor T-cell migration into GVHD target tissues, we compared the expression of chemokine receptors by donor T cells in recipient spleen, liver and lung. We found that WT and RORγt−/− donor T cells from all tissues expressed much higher levels (mean percentage of positive cells) of the gut-homing receptor α4β7, as well as the liver-homing receptor CXCR3 in spleen (P = .024), liver (P = .011) and lung (P = .004) compared with WT and RORγt−/− T cells (Figure 4B). We also checked the expression of Th17 homing marker CCR630 and Th2-associated chemokine receptor CCR4.31 T-bet−/− donor T cells expressed higher levels of CCR6 than other types of T cells (P < .01), and T-bet−/− and RORγt−/−/T-bet−/− T cells expressed relatively higher CCR4 than WT or RORγt−/− T cells (P < .05 in liver but no difference in spleen and lung). Data were repeated 3 times. Because chemokine receptors are required for infiltration of alloreactive T cells into GVHD targeted organ, the distinct expression of those receptors on different types of cells likely contributed to the significantly reduced migration of RORγt−/−/T-bet−/− T cells in recipient liver and lung compared with WT T cells.
GVL activity is largely preserved in RORγt−/−/T-bet−/−T cells
When HCT is used as a therapy for hematologic malignances, an important role for donor T cells is to prevent relapse of the original disease through GVL effects. Therefore, it is critically important to determine whether T cells lacking T-bet and RORγt retain the beneficial GVL effect. To this end, we studied HCTs from B6 donors into BALB/c recipients harboring the A20 B cell lymphoma that had been transduced with a luc/neo plasmid for in vivo monitoring by BLI. Because only RORγt−/−/T-bet−/− T cells had severely impaired ability to cause GVHD, we focused our attention to compare the ability of WT and RORγt −/−/T-bet−/− T cells in mediating GVL activity. Mortality because of GVHD or tumor relapse was distinguished by BLI. As expected, all A20-negative recipients of TCD-BM alone survived. However, if A20 cells were infused before HCT, all recipients of TCD-BM alone died within 40 days without weight loss but with very strong BLI signals (Figure 5A), indicating that tumor growth was the cause of death. TCD-BM plus WT T cells induced severe GVHD with significant weight loss (Figure 5B) but little or no BLI signals before death (Figure 5C). In contrast, the majority of recipients of RORγt−/−/T-bet−/− T cells survived through the 80-day observation period (Figure 5B) with mild to modest body-weight loss, but with very little if any BLI signal (Figure 5C), indicating that these recipients were largely free from tumor. To quantitatively evaluate GVL activity of RORγt −/−/T-bet−/− T cells versus WT T cells, we used a low dose of donor T cells with titrated doses of tumor cells from 2 × 103 to 4 × 104. We observed that RORγt −/−/T-bet−/− T cells had comparable GVL effects as WT T cells against A20 cells up to 1 × 104, although the GVL activity was compromised against excessive load of tumor (Figure 5D-E). These results suggest that T cells deficient for T-bet and RORγt largely preserved GVL activity with severely compromised ability to induce GVHD.
GVL activity is largely preserved in RORγt−/−/T-bet−/− T cells. Lethally irradiated BALB/c mice received TCD-BM cells alone or plus 2 × 106 naive T cells from WT or RORγt−/−/T-bet−/− donors. Recipients were given 2 × 103 A20 tumor cells with luciferase transgene at the same time of transplantation. Recipient survival (A) and body weight changes (B) are shown. (C) Tumor growth in recipients was monitored with in vivo bioluminescent imaging. Data shown were combined from 2 replicate experiments with 8 mice in each group. (D-E) Lethally irradiated BALB/c mice received TCD-BM cells alone or plus 5 × 105 naive T cells from WT or RORγt−/−/T-bet−/− donors. Recipients were given 2 × 103, 1 × 104 or 5 × 104 A20 tumor cells with luciferase transgene at the same time of transplantation. The summary of BLI signal intensity in WT (D) or RORγt−/−/T-bet−/− (E) recipients are shown at multiple time points after BMT. Six recipients were included in all the groups, except 5 in the TCD-BM alone group. Asterisk indicates statistical significance between WT and RORγt−/−/T-bet−/− recipients; ***P < .001.
GVL activity is largely preserved in RORγt−/−/T-bet−/− T cells. Lethally irradiated BALB/c mice received TCD-BM cells alone or plus 2 × 106 naive T cells from WT or RORγt−/−/T-bet−/− donors. Recipients were given 2 × 103 A20 tumor cells with luciferase transgene at the same time of transplantation. Recipient survival (A) and body weight changes (B) are shown. (C) Tumor growth in recipients was monitored with in vivo bioluminescent imaging. Data shown were combined from 2 replicate experiments with 8 mice in each group. (D-E) Lethally irradiated BALB/c mice received TCD-BM cells alone or plus 5 × 105 naive T cells from WT or RORγt−/−/T-bet−/− donors. Recipients were given 2 × 103, 1 × 104 or 5 × 104 A20 tumor cells with luciferase transgene at the same time of transplantation. The summary of BLI signal intensity in WT (D) or RORγt−/−/T-bet−/− (E) recipients are shown at multiple time points after BMT. Six recipients were included in all the groups, except 5 in the TCD-BM alone group. Asterisk indicates statistical significance between WT and RORγt−/−/T-bet−/− recipients; ***P < .001.
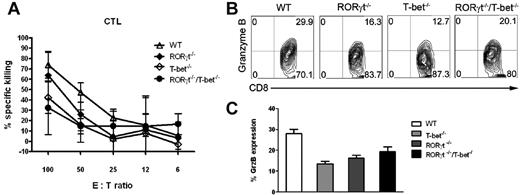
To investigate the molecular mechanisms of GVL effects mediated by T-bet−/− and/or RORγt−/− T cells, we examined the cytolytic activity of donor T cells after transplantation into the recipient. Fourteen days after HCT, splenocytes isolated from BALB/c recipients of WT donor T cells demonstrated strong CTL activity against host-type P815 (H-2d) targets (Figure 6A), but not at all to donor-type EL4 (H-2b) targets (data not shown). The strength of CTL activity against P815 targets ranged from the highest to lowest in the sequence of WT, RORγt−/−, T-bet−/−, and RORγt−/−/T-bet−/− T cells. CTL activity of RORγt−/−/T-bet−/− T cells was reduced but not eliminated and the expression of granzyme B was also partially preserved on those T cells (Figure 6B,C). Perforin and granzyme are the dominant cytotoxic pathways involved in GVL effects after transplantation. High levels of granzyme B expression contribute to the CTL activity of RORγt−/−/T-bet−/− T cells as well.
Absence of RORγt/T-bet preserves CTL function after BMT. (A) Splenocytes from recipients given WT, RORγt−/−, T-bet−/−, and RORγt−/−/T-bet−/− T cells were harvested 2 weeks after BMT. Equal numbers of donor splenocytes were set up in a conventional 4-hour 3H-release assay with p815 cells as a target cell, as described in “Methods.” EL-4 cells were used as a negative control, but no killing activity was observed (data not shown). Data are presented as means ± SEM (from n = 4/group). (B) Granzyme B expression of donor T cells is shown gated in H-2Kb+Ly5.1-CD8+ T cells. (C) Granzyme B expression is shown on gated H-2Kb+Ly5.1−CD8+ T cells from 3 mice.
Absence of RORγt/T-bet preserves CTL function after BMT. (A) Splenocytes from recipients given WT, RORγt−/−, T-bet−/−, and RORγt−/−/T-bet−/− T cells were harvested 2 weeks after BMT. Equal numbers of donor splenocytes were set up in a conventional 4-hour 3H-release assay with p815 cells as a target cell, as described in “Methods.” EL-4 cells were used as a negative control, but no killing activity was observed (data not shown). Data are presented as means ± SEM (from n = 4/group). (B) Granzyme B expression of donor T cells is shown gated in H-2Kb+Ly5.1-CD8+ T cells. (C) Granzyme B expression is shown on gated H-2Kb+Ly5.1−CD8+ T cells from 3 mice.
To evaluate the effects of T-bet and RORγt in GVHD and GVL activity in other mouse strains and tumor combinations, we tested B6→(B6×DBA2)F1 (B6D2F1) transplants in recipients with P815 leukemia. In the absence of leukemia, most recipients of WT T cells died from GVHD whereas the majority of recipients of RORγt−/−/T-bet−/− T cells survived without GVHD (Figure 7A), confirming the impaired ability of RORγt−/−/T-bet−/− T cells to induce GVHD. In mice inoculated with leukemia, recipients of TCD-BM plus T cells had significantly better survival than recipients of TCD-BM alone (Figures 7B, P < .01), and 50% of the mice were protected from leukemia mortality regardless for the T-cell type transplanted (Figure 7C), indicating that WT and RORγt−/−/T-bet−/− T cells had equivalent GVL activity against p815.
Absence of T-bet and ROR[gamma]t does not alter donor antitumor (p815) response. Lethally irradiated (1200 cGy) B6D2F1 mice were transplanted with grafts from WT or RORγt−/−/T-bet−/− donors, containing 5 × 106 TCD-BM cells and 2-4 × 106 T cells from WT, T-bet−/−/RORγt−/− donors. No (A) or 4 × 103 P815 tumor cells (B-C) were added into donor graft. Survival by Kaplan-Meier analysis of B6D2F1 recipients (A-B) and mortality because of tumor relapse (C) are shown. Data are combined from 2 separate experiments (n = 13, TCD-BM + T-cell groups; n = 4, TCD-BM control group). Asterisk indicates statistical significance: *P < .05 and **P < .01.
Absence of T-bet and ROR[gamma]t does not alter donor antitumor (p815) response. Lethally irradiated (1200 cGy) B6D2F1 mice were transplanted with grafts from WT or RORγt−/−/T-bet−/− donors, containing 5 × 106 TCD-BM cells and 2-4 × 106 T cells from WT, T-bet−/−/RORγt−/− donors. No (A) or 4 × 103 P815 tumor cells (B-C) were added into donor graft. Survival by Kaplan-Meier analysis of B6D2F1 recipients (A-B) and mortality because of tumor relapse (C) are shown. Data are combined from 2 separate experiments (n = 13, TCD-BM + T-cell groups; n = 4, TCD-BM control group). Asterisk indicates statistical significance: *P < .05 and **P < .01.
Discussion
We show here that T cells negative for T-bet and RORγt fail to differentiate into Th1 and Th17 and cause GVHD in allogeneic recipients. Using 2 distinct GVL models, we also demonstrate that T cells lacking T-bet and RORγt retain GVL activity after HCT. These findings validate T-bet and RORγt as potentially unique therapeutic targets required for the detrimental but not the beneficial functions of donor T cells after HCT.
Our data indicate that WT T cells predominately differentiate into Th1 cells during GVHD, much less into Th2 or Th17 cells, and minimally into Tregs. T-bet−/− T cells preferentially differentiate into Th2 and Th17 cells consistent with results in experimental colitis, where they switch to Th2.32 Foxp3+ Tregs are also increased in recipients of T-bet−/− T cells compared with WT T cells. RORγt−/− T cells differentiate into Th1 cells in larger proportion than WT T cells, but little or not at all into Th17 cells. Consistent with no evidence indicating that blockade of Th17 differentiation at the DNA transcription level affects Treg generation, Foxp3+ Tregs were comparable in recipients of WT or RORγt−/− T cells. RORγt−/−/T-bet−/− T cells failed to differentiate into Th1 and Th17 cells, but did differentiate into Th2 and Tregs. These data support our hypothesis that both Th1 and Th17 cells contribute to GVHD development, each lineage alone is sufficient to induce GVHD, and a combined blockade is required to prevent GVHD. Previous work by Yi et al showed that blockade of Th1 and Th17 differentiation using IFNγ- and IL-17-KO T cells could alleviate GVHD moderately.9 Because IFNγ and IL-17 are not the only cytokines secreted by Th1 and Th17 cells, eliminating IFNγ and IL-17 is not expected to completely block Th1 and Th17 differentiation. Our strategy was to block Th1 and Th17 transcription factors T-bet and RORγt, which could effectively prevent GVHD after allogeneic BMT. The other major difference is that Yi et al tested CD4 T cells only9 whereas we tested CD4 and CD8 T cells, a strategy more representative to clinical HCT. Thus, although the ideas were similar to target T-cell differentiation, strategies were different and outcomes were distinct.
We demonstrated that blocking T-bet, RORγt, or both regulates T-cell differentiation and affects GVHD development. Expression of chemokine receptors is regulated during T-cell differentiation: CXCR3 is expressed on Th1 cells, CCR2 and CCR4 on Th2 T cells, and CCR6 on Th17 cells.33,34 Because GVHD requires donor T-cell infiltration into target organs, and T-cell differentiation regulates chemokine expression, targeting T-bet−/− and RORγt−/− may also contribute to GVHD development by affecting T-cell migration. Indeed, we found that fewer RORγt−/−/T-bet−/− T cells migrated into recipient liver and lung (Figure 4A), resulting in significantly less tissue injury compared with WT T cells (Figure 1D). In addition, less colon pathology was observed in the recipients of RORγt−/−/T-bet−/− T cells in comparison to those of WT T cells (Figure 1D), likely the result of lower expression of the α4β7 leukointegrin, required for intestinal-specific trafficking (Figure 4B). At the same time, higher numbers of CD4 and CD8 T cells were observed in the spleens of the recipients of RORγt−/−/T-bet−/− T cells compared with recipients of WT cells (Figure 4A), indicating that RORγt−/−/T-bet−/− T cells expanded as normally as WT T cells but had altered migration, presumably a consequence of differential chemokine expression.
We reasoned that Th2 cells are not pathogenic or even protective in the development of GVHD, as suggested by many published works.35-40 Neutralizing IFNγ or IL17 does not eliminate the effectors induced by other cytokines produced by Th1 and Th17, such as TNFα, IL-21, IL-23, and others. In fact, Yi et al found that IFNγ−/−/IL17−/− donor T cells still induced severe idiopathic pneumonia.9 Their data showed that even though donor T cells were deficient in IL-17 and IFNγ, up to 50% TNFα producing cells were still present in the transplant recipients. TNFα is a key cytokine in the effector phase of GVHD after experimental and clinical allogeneic HCT.41,42 High TNFα was found in the serum of patients who developed lung injury after SCT43 and in the lungs of animals with GVHD.17,20-23 Neutralization of TNFα by etanercept after BMT significantly reduced the severity of experimental or clinical idiopathic pneumonia syndrome.20 Therefore, high levels of TNF-α contribute to the pathogenesis of GVHD even though IFN-γ and IL-17 are deficient. Yi et al observed that IFNγ−/−IL-17−/− T cells still caused severe lung GVHD, in which blocking TNFα-signaling with TNFR-IgG failed to significantly reduce lung pathology. However, other groups previously showed that TNFα contributes to lung GVHD.44,45 Therefore, we reasoned that essential absence of TNFα when T-bet and RORγt were blocked was attributable to the lack of lung GVHD in our study. Alternatively or additionally, Yi, et al reported that IFNγ−/−IL-17−/− T cells caused severe lung injury primarily because lung tissues failed to up-regulate B7H1 because of the lack of IFNγ. In our study, IFNγ production was not completely blocked for T-bet−/− or RORγt−/−/T-bet−/− CD8 T cells. Thus, it is possible that a low level of IFNγ was sufficient to up-regulate B7H1 in lung tissue that might play a protective role.
Our data in genetically modified mice show that disrupting T-bet increases the production of IL-17+ CD4 and IL-17+ CD8 T cells and induces GVHD, albeit less than WT T cells. Based on findings from our group and others15,16 that polarized Th17 cells cause GVHD particularly in lung tissues, we expected that eliminating Th17 transcription factor RORγt would block Th17 development and ameliorate GVHD. Our data show that RORγt−/− T cells indeed failed to differentiate into Th17 and Tc17 cells in vitro and in vivo, but can still differentiate into Th1 and Tc1 cells after allogeneic HCT and cause GVHD. Conversely, IL-17, IFNγ, and TNFα production are severely impaired in recipients of RORγt−/−/T-bet−/− T cells where GVHD is almost completely prevented. In addition, higher recruitment and/or conversion of Tregs could contribute to reduced GVHD in recipients of RORγt−/−/ T-bet−/− T cells.
Given that allogeneic HCT is primarily used to treat hematologic malignancies, it is important to evaluate the contribution of donor T cells to GVL effects because transplantation would not be as beneficial for patients with a malignant disease if T cells had no activity against malignant cells. Our study shows that T cells deficient for T-bet and RORγt have preserved GVL activity against A20 lymphoma and p815 leukemia (Figures 5,7). The preserved CTL activity is likely mediated by the granzyme and perforin pathway (Figure 6). In addition, because IFNγ production by dKO CD8+ T cells was not completely eliminated, and Th2 cells may also mediate antitumor immunity,46 we further reasoned that residual IFNγ and enhanced Th2 cytokines might contribute to preserved GVL effects of dKO T cells. The present study indicates that T cells deficient for T-bet and RORγt fail to induce severe GVHD while maintaining GVL effects, validating T-bet and RORγt as targets to improve safety of allogeneic HCT for the treatment of hematologic malignancies. We envision several strategies that can be applied to block Th1 and Th17 differentiation or effector function in clinical practice: (1) targeting T-bet and RORγt transcription factors through specific siRNAs or small molecule inhibitors47 ; (2) inhibiting Th1- and Th17-promoting microRNAs (eg, miR-155 and miR-326)48,49 ; and (3) neutralizing multiple Th1- and Th17-priming and effector cytokines (eg, IL-12, IL-6, IFNγ, and IL-17).1,50
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful for the technical assistance provided by Flow Cytometry and Mouse Core Facility at the Moffitt Cancer Center.
This work was supported in part by National Institutes of Heath grants CA118116, CA143812 and Melanoma Research Alliance pilot award (X.-Z.Y.), and CA132197, AI082498, CA076292 (C.A.).
National Institutes of Health
Authorship
Contribution: Y.Y. participated in experimental design, performed research, collected, analyzed, and interpreted data, performed statistical analysis, and drafted and revised the manuscript; D.W., K.K., and K.S. performed research, collected and analyzed data, and edited the manuscript; C.L. performed pathologic analysis; C.A. participated in experimental design, interpreted data, and revised the manuscript; and X.-Z.Y. designed research, collected, analyzed, and interpreted data, performed statistical analysis, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xue-Zhong Yu, MD, H. Lee Moffitt Cancer Center & Research Institute, SRB-2, 12902 Magnolia Dr, Tampa, FL 33612-9497; e-mail: xue.yu@moffitt.org.

![Figure 7. Absence of T-bet and ROR[gamma]t does not alter donor antitumor (p815) response. Lethally irradiated (1200 cGy) B6D2F1 mice were transplanted with grafts from WT or RORγt−/−/T-bet−/− donors, containing 5 × 106 TCD-BM cells and 2-4 × 106 T cells from WT, T-bet−/−/RORγt−/− donors. No (A) or 4 × 103 P815 tumor cells (B-C) were added into donor graft. Survival by Kaplan-Meier analysis of B6D2F1 recipients (A-B) and mortality because of tumor relapse (C) are shown. Data are combined from 2 separate experiments (n = 13, TCD-BM + T-cell groups; n = 4, TCD-BM control group). Asterisk indicates statistical significance: *P < .05 and **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/18/10.1182_blood-2011-03-340315/4/m_zh89991179650007.jpeg?Expires=1767698740&Signature=N2jZ7v7flelFaPO4I1CusIM5oEv9JafxnYy~vd3fo71lo1WHOQGlNCk7UQpRwf9Xn7z11i77Pqh9E~OYLVWsJtX3A0aXCJEix530W1bJN5BW7564AATLuk8IhRabINRBeyTNewNV1BDmAB3hJj~jypcaqjuFPgtFTYKOLcxfSHyvdo3cLcaGnr3heEIkJ6OdPJx1UmFJ7unwe~RgP8jtAf4hdoK~JNghZzPxpAWb3WyhBREStIRUMdBl9cgznyBrmA~aFnYfei9qMLRU~D9duuiGHwNaCvjt7q5AzVnFZihyUOWmgIUdKNIk-hrGspbH1dYXQbLlcDymO3d2VDHanQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal