Abstract
To selectively modulate human complement alternative pathway (CAP) activity implicated in a wide range of acute and chronic inflammatory conditions and to provide local cell surface and tissue-based inhibition of complement-induced damage, we developed TT30, a novel therapeutic fusion protein linking the human complement receptor type 2 (CR2/CD21) C3 fragment (C3frag = iC3b, C3dg, C3d)-binding domain with the CAP inhibitory domain of human factor H (fH). TT30 efficiently blocks ex vivo CAP-dependent C3frag accumulation on activated surfaces, membrane attack complex (MAC) formation and hemolysis of RBCs in a CR2-dependent manner, and with a ∼ 150-fold potency gain over fH, without interference of C3 activation or MAC formation through the classic and lectin pathways. TT30 protects RBCs from hemolysis and remains bound and detectable for at least 24 hours. TT30 selectively inhibits CAP in cynomolgus monkeys and is bioavailable after subcutaneous injection. Using a unique combination of targeting and effector domains, TT30 controls cell surface CAP activation and has substantial potential utility for the treatment of human CAP-mediated diseases.
Introduction
The mammalian complement system is an essential component of the innate immune response that plays a central pathophysiologic role in human diseases by using a variety of effector mechanisms including anaphylatoxin generation, opsonization of targets for recognition by professional phagocytes, cell lysis, and pro-inflammatory intracellular signaling after the generation and insertion of the membrane attack complex (MAC).1-3
The complement system is comprised of > 30 soluble and membrane-bound proteins that can be activated by 3 distinct biochemical mechanisms – the classic, lectin and alternative pathways.4 The classic and lectin pathways are activated through engagement by specific target recognition molecules such as IgM, IgG, mannose-binding protein and ficolins.3 In contrast, the activation of the complement alternative pathway (CAP) is based on a different type of mechanism (see Figure 1A) a thioester bond in C3 protein slowly spontaneously hydrolyses (the “tickover” process), leading to formation of the conformationally altered C3(H2O) form of C3.5,6 C3(H2O) can now be bound by factor B (fB), which is itself conformationally altered when bound and cleaved by the protease factor D (fD).7 The complex of C3(H2O)Bb can act as a potent C3 cleavage and activation enzyme designated C3 convertase, which is capable of cleaving additional C3 molecules to the small anaphylatoxin C3a and much larger C3b. The structural changes on C3a removal convert the thioester group of the C3b fragment to an exposed reactive acyl-imidazole group that can react with nucleophilic surfaces of cells in its proximity.8 Notably, all 3 pathways can generate C3 convertases using unique mechanisms of recognition and early activation, although the lectin pathway intersects with the classic pathway when C4 and then C2 are activated to form the shared C4b2a C3 convertase.3
Mechanism of TT30 activity, structure, and functional assays. (A) Complement alternative pathway (detailed description is provided in the Introduction). (B-C) TT30 structure and selective inhibition of human CAP and CCP in vitro. (B) TT30 is a fusion protein that combines the first 4 short consensus repeats (SCRs) of Complement Receptor type 2 (CR2) with the first 5 SCRs of factor H. The CR2 domain binds iC3b and C3dg/C3d, while the factor H domain inactivates the CAP. (C) ELISA-based complement pharmacodynamic (PD) assays for assessment of TT30 activity ex vivo. For CAP testing (top panel), serum samples were loaded onto LPS-coated wells under conditions promoting CAP activation, which leads to MAC deposition on surface with expression of activated C9 neo-epitope. Addition of mouse anti-human C9 neo-epitope IgG mAb-AP and an alkaline peroxidase substrate resulted in colorimetric reaction where the amount of complement activation correlated with the color intensity and was measured in terms of absorbance at 405 nm using ELISA Plate Reader. Similar process was followed for the CCP activation (bottom panel), with the exception that the wells were coated with IgM and the buffer diluent contained 0.5mM MgCl2, and 2mM CaCl2.
Mechanism of TT30 activity, structure, and functional assays. (A) Complement alternative pathway (detailed description is provided in the Introduction). (B-C) TT30 structure and selective inhibition of human CAP and CCP in vitro. (B) TT30 is a fusion protein that combines the first 4 short consensus repeats (SCRs) of Complement Receptor type 2 (CR2) with the first 5 SCRs of factor H. The CR2 domain binds iC3b and C3dg/C3d, while the factor H domain inactivates the CAP. (C) ELISA-based complement pharmacodynamic (PD) assays for assessment of TT30 activity ex vivo. For CAP testing (top panel), serum samples were loaded onto LPS-coated wells under conditions promoting CAP activation, which leads to MAC deposition on surface with expression of activated C9 neo-epitope. Addition of mouse anti-human C9 neo-epitope IgG mAb-AP and an alkaline peroxidase substrate resulted in colorimetric reaction where the amount of complement activation correlated with the color intensity and was measured in terms of absorbance at 405 nm using ELISA Plate Reader. Similar process was followed for the CCP activation (bottom panel), with the exception that the wells were coated with IgM and the buffer diluent contained 0.5mM MgCl2, and 2mM CaCl2.
Surface bound C3b can also now bind factor B and the resulting C3bB complex is cleaved by factor D into C3bBb, a C3 convertase, leading to further production of C3b and C3a.9 This autocatalytic mechanism of continuous C3b deposition is called “the amplification loop” (red arrows in Figure 1A), and plays a critical role in signal amplification regardless of which pathway initiated the complement response.10 Additional surface deposited C3b can form a C3bBbC3b C5 convertase, which reacts with further components of complement to create the MAC.
The complement system has to interact in precisely balanced way to minimize the damage of “self” cells. Host organisms express several regulators of complement activity (RCA) that can function on cell surfaces or in fluid phase; for example factor H, CR1 (CD35), decay acceleration factor (DAF, CD55), membrane cofactor protein (MCP, CD46), membrane inhibitor of reactive lysis (MIRL, CD59), and C4-binding protein (C4bp).11 Several of these proteins can promote factor I mediated proteolysis of C3b (termed cofactor activity),12 leading to subsequently processed proteins iC3b, C3dg, and C3d.13 C3b and its proteolytic products elicit several different physiologic responses via specific interaction of individual fragments with different receptors (CR1, CR2, CR3, CR4, and CRIg).8
Uncontrolled CAP activity has been shown to be involved in several chronic human diseases, for example, age-related macular degeneration (AMD), dense deposit disease, thrombotic microangiopathies, and paroxysmal nocturnal hemoglobinuria (PNH).2 Several types of complement inhibitors have been previously developed which rely on traditional strategies of systemic interruption of protein-protein interactions using monoclonal antibodies or peptides.14,15 However, because there are typically large quantities of circulating complement factors, and complete systemic complement inhibition of a particular circulating factor can increase the risk of infection and/or autoimmune diseases, we sought to develop a targeted strategy whereby a therapeutic protein would preferentially accumulate at sites undergoing complement-mediated attack. Such a strategy would in principle minimize the potential for systemic side effects of the inhibitor, potentially lower the dose required for clinical benefit and increase the target tissue residency time of the inhibitor. To accomplish this task, we created a therapeutic molecule, denoted TT30, using a targeting mechanism based on the physiologic interaction between the tissue-fixed C3 fragments (C3frag) iC3b, C3dg, and C3d and the C3 frag ligand-binding recombinant portion of the B-cell receptor CR2 (CD21),16 coupled to the CAP inhibitory domain of factor H. Thus, TT30 is designed to be targeted to and act locally at sites of ongoing complement activation.
Methods
Design and expression of TT30
A plasmid incorporating full-length TT30 cDNA was synthesized by Blue Heron Biotechnology based on codon optimization of human sequences listed in GenBank. Sequence encoding SCRs 1-4 of human CR2 (NP_001006659.1, residues 21-275) was fused 5′ to the sequence encoding SCRs 1-5 of human complement fH (NP_000177.2, residues 19-323). The production cell line was developed by Catalent Pharma Systems using proprietary retroviral (GPEx) technology.17 TT30 was expressed and secreted into the protein-free Hyclone culture medium containing l-glutamine and 0.1% pluronic F68 (Logan).
Wieslab complement system alternative/classical ELISA assays
The Wieslab Complement System Alternative and Classic Pathway Enzyme ELISA kits (Euro-Diagnostica) do not rely on traditional RBC hemolytic assays for complement activation (ie, a requirement for formation of active C5b-9), but assess the degree of CAP or classical complement pathway (CCP) activity by measuring by ELISA the amount of active C5b-9 formation. During incubation of serum in the wells, the CAP/CCP is activated in a buffer containing pathway-specific metal ions, and C5b-9 is deposited on the coated wells. Bound C5b-9 is detected with alkaline phosphatase–conjugated mouse monoclonal antibody mAb aE11, recognizing the C9 neoantigen formed during MAC formation. The amount of C9 neoantigen generated is directly proportional to the functional activity of the CAP or CCP. These kits were used to assess the effect of TT30, purified factor H (Quidel) and anti–human C5 mAbs (A217; Quidel) on each pathway, according to manufacturers' instructions. The wells of the microtiter strips were precoated with pathway-specific activators (endotoxin-LPS for CAP and IgM for CCP). TT30 and other compounds were diluted in pooled normal human serum (Bioreclamation) and incubated in presence of pathway-specific buffer diluent (5mM MgCl2 and 10mM EGTA for CAP or 0.5mM MgCl2 and 2mM CaCl2 for CCP) at 37°C. To assess the requirement for binding of CR2 to its C3frag ligands in the activity of TT30, the molecule was incubated with excess inhibitory anti-human CR2 monoclonal antibody (clone 1048; Green Mountain) or anti-KLH isotype control (BioLegend). Aliquots of complement-preserved pooled normal serum from each species (representing 100% activity) and heat-inactivated complement-depleted pooled normal serum (representing 0% activity) were used as positive and negative controls for the assay, respectively. During incubation of serum-diluted compounds in the wells, the CAP/CCP was activated and C5b-9 was deposited on the LPS/IgM-coated wells. The wells were then washed and the bound C5b-9 was detected with alkaline phosphatase–conjugated mAb aE11, recognizing the C9 neoantigen formed during MAC assembly. After a further washing step, detection of the bound mAb was obtained by incubation with alkaline phosphatase substrate solution. The amount of complement activation correlated with the color intensity and was measured in terms of absorbance at 405 nm using ELISA Plate Reader (BioTech Instruments). GraphPad Prism 5 software was used for curve fitting and the estimation of the IC50 values.
Rabbit RBC hemolysis assay
This assay measures the release of hemoglobin from rabbit RBCs lysed on exposure to CAP-activated serum. Water and 42mM EDTA were used as positive and negative controls for the assay, respectively. This assay was used for assessing TT30 activity, that is, the extent to which TT30 inhibits CAP-mediated hemolysis of rabbit RBCs. As serum C3 is activated, C3 convertases, C3 activation fragments and C5 convertases are deposited on rabbit RBCs. Serum CAP activity in the presence of TT30 was evaluated in a concentration-dependent manner. Rabbit RBC (Bioreclamation) were washed, adjusted to 2.9 × 109 erythrocytes/mL and incubated with human complement-preserved serum containing serial dilutions of TT30 under experimental conditions promoting CAP activity (Mg-EGTA) and subsequent hemolysis. After 30 minutes at 37°C, 25mM EDTA was added to stop the reaction, followed by centrifugation and removal of the supernatant to a new plate that was read at 415 nm. Percent lysis was calculated as (A415ser ×- A415ser × bkgd)/(A415water) × 100.
Flow cytometry
RBC left after hemolysis assay were washed and stained with anti-C3 fragment specific biotinylated monoclonal anti–human C3d antibodies (clone A702; Quidel Corp) and with FITC-conjugated monoclonal HB5 antibodies (Santa Cruz Biotechnology) at 37°C for 30 minutes, followed by incubation with streptavidin-conjugated APC (BD Biosciences) for additional 30 minutes at ambient temperature. Isotype-matched controls were from BD Biosciences. Cells were analyzed on Accuri C6 cytometer using CFlow Version 1.0227.4 software (Accuri Cytometers Inc).
Immunohistochemistry
Fresh, unfixed tissue samples from human biopsy (normal lung) and autopsy (inflamed asthmatic lung from a 69-year-old white female with a diagnosis of Alzheimer disease and a history of asthma, who died of respiratory failure) were obtained from Asterand. Tissues were placed in molds and frozen in Tissue-Tek OCT (Optimal Cutting Temperature) Compound. Tissues were stored and maintained at −85 to −70°C. Sections were cut at ∼ 5 μm and fixed in 10% neutral buffered formalin (NBF) for 10 seconds at room temperature. Cryosections were stained with mouse anti–human iC3b mAb (clone A209; Quidel), mouse anti–human C3 mAb (clone 6C9; LifeSpan Biosciences) and TT30 precomplexed with polyclonal sheep anti–human factor H antibody (Abcam), followed by donkey anti-sheep IgG-HRP (Jackson ImmunoResearch). Mouse anti–human CD21 antibody (1048; BD Pharmingen) was used to block TT30 binding. For precomplexing mixture and specificity (blocking) control, 5 μg/mL TT30 was mixed with 5 μg/mL sheep anti-fH antibody (1:1). Slides were incubated overnight at 2-8°C on a rocker mechanism, and before use on the subsequent day, purified factor H was added to achieve a final concentration of 50 μg/mL to absorb excess anti-fH. For immunostaining, slides were rinsed twice with TBS followed by protein block for 20 minutes and subsequent incubation with the precomplexing mixture and the antibodies for 2 hours at room temperature. Slides were then rinsed twice with TBS before and after the application of peroxidase blocking solution for 5 minutes. Next, peroxidase-conjugated donkey anti–sheep IgG (diluted at 1:500) was applied for 30 minutes, followed by slide rinsing with TBS twice, application of 3,3′-diaminobenzidine (DAB) peroxidase substrate (Sigma-Aldrich) for 4 minutes and rinsing with tap water. Slides were counterstained with hematoxylin, washed, blued in saturated lithium carbonate, washed, dehydrated through alcohols, cleared in xylene, and coverslipped for interpretation.
TT30 inhibition of CAP and CCP activity in human serum
TT30 in increasing amounts was added into human whole blood from 9 donors that had been processed to serum and serum CAP and CCP activities were assessed in these samples using Wieslab Complement System Alternative or Classic Pathway Enzyme ELISA kits. Briefly, blood was collected into serum collection tubes (BD Vacutainer, red top) with 21g needle, and the various TT30 dilutions were injected into the collection tubes without breaking the vacuum by injection with the 0.3 mL syringe. Tubes were placed at ambient temperature for 30-60 minutes, then on wet ice, and centrifuged to separate serum at 1500g, 4°C for 15 minutes. Supernatant was removed and frozen at −80°C.
TT30 pharmacodynamic activity in cynomolgus monkeys
Male and female cynomolgus monkeys received a single intravenous bolus injection of 20 mg/kg TT30, or a single subcutaneous (SC) injection of 20 mg/kg TT30, or single intravenous injections of 15, 30 and 60 mg/kg TT30. Blood samples were collected from each animal into blood collection tubes at nominal sampling timepoints, including 0 (before dose) and at incremental time intervals after TT30 administration (after dose), then placed on wet ice and processed for plasma or serum. Samples were assayed for TT30 concentrations in plasma and CAP- or CCP-mediated MAC formation in serum. TT30 concentrations in plasma were determined by a sandwich ELISA assay in which TT30 was captured on plates coated with mouse anti–human CR2 mAb and detected with a goat anti–human factor H polyclonal antiserum followed by a peroxidase-conjugated donkey anti–goat IgG. A peroxidase substrate (ABTS) was added and color development was stopped after the incubation, followed by measurement of color intensity at 405 nm. CAP- or CCP-mediated MAC formation in serum was detected by the Wieslab Complement Alternative or Classic Pathway Enzyme ELISA kits, as described in “Wiselab complement system alternative/classical ELISA assays.” The method was tested and found applicable for the monkey serum.
Data analysis
All data calculations and analysis were performed using Microsoft Office Excel 2007 (Microsoft Corp) and GraphPad Prism Version 5. Serum CAP and CCP activities were reported as % activity (Sample – Neg. Control)/(Pos. Control – Neg. Control) × 100 and normalized to the activity in neat serum. For rabbit RBC hemolysis assays, % lysis was calculated as [(OD415ser ×- OD415ser × bkgd)/(OD415water) × 100].
Results
Design and characterization of TT30
TT30 is a 560-amino acid, 65 kDa recombinant human fusion protein of SCR1-4 of human CR2 and SCR1-5 of fH (Figure 1B). TT30 directly joins the sequence of CR2 with that of fH, without the introduction of an intervening linker sequence. Since it has been shown that only first 2 short consensus repeat (SCR) domains (of 15/16, depending on splicing) of CR2 are necessary for this ligand-binding function,18 we used these 2 and added additional 2 SCR domains as a spacer. The CAP regulatory function of TT30 was provided by the first 5 SCR domains of fH (of 20), since it has been shown that SCR1-4 domains are sufficient and necessary for cofactor activity to inactivate C3b in both the C3 and C5 convertase (sites of action designated by yellow stars in Figure 1A), as well as to provide decay-acceleration function that physically disrupts the C3bBb interaction and blocks further convertase activity.19 SCR5 was added to stabilize the first 4 SCRs and protect against carboxypeptidase attack. TT30 was expressed and produced in CHO cells using fed-batch culture and purified to homogeneity via a series of chromatography and membrane process steps. Additional information regarding TT30 expression, purification, and biophysical characterization is included in supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Inhibitory activity, selectivity, and potency of TT30
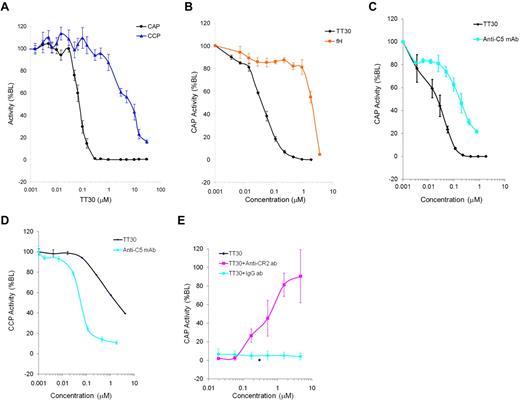
The Wieslab Complement System Alternative and Classic Pathway Enzyme ELISA kits (assay format shown on Figure 1C) were used to access the effect of TT30 on CAP or CCP pathways. TT30 was added to human whole blood immediately after being collected from 9 donors, and its CAP/CCP inhibitory activity was measured in processed serum. Figure 2A shows potent and selective inhibition of CAP by TT30 (IC50 values for inhibition of CAP and CCP were 0.08 ± 0.03μM and 9.8 ± 7.6μM, respectively, while IC90 values for CAP and CCP were 0.14μM and > 29.4μM, respectively), suggesting that TT30 is a potent and selective inhibitor of CAP-mediated MAC formation and a weak inhibitor of CCP activity.
Inhibition of CAP and CCP activity by TT30, fH and an anti–human C5 monoclonal antibody. (A) Inhibition of CAP and CCP after spiking TT30 into human whole blood from 9 donors and measuring activity in processed serum using Wieslab Complement System Alternative ELISA kit (Euro-Diagnostica). Results are expressed as percent baseline level of activity and represent mean ± SEM of duplicates (n = 9). IC50 values for inhibition of CAP and CCP were 0.08 ± 0.03μM and 9.8 ± 7.6μM, respectively, while IC90 values for inhibition of CAP and CCP were 0.14μM and > 29.4μM, respectively. (B) Potency and specificity of targeted CAP inhibition by TT30 compared with factor H. Potency of TT30 and fH spiked into human serum was measured as inhibition of CAP using Wieslab Complement System Alternative ELISA kit. TT30 showed high potency (IC50 = 0.04 ± 0.005 μM) while fH was less effective (IC50 = 3.0 ± 1.6μM). Potency of TT30 and anti–human C5 monoclonal antibody from Quidel Corp. was measured as inhibition of CAP (C) or CCP (D) using Wieslab Complement System Alternative or Classic Pathway ELISA kit. TT30 exhibits selectivity for CAP (IC50 = 0.03 ± 0.007μM in CAP compared with 2.1 ± 0.7μM in CCP) while anti-C5 antibody showed similar activity in either pathway (IC50 = 0.16 ± 0.07μM in CAP compared with IC50 = 0.05 ± 0.009μM in CCP). (E) TT30 was spiked into human serum at 0.3μM alone or in the presence of serial 3-fold dilutions of anti-CR2 monoclonal antibody (clone 1048, starting concentration 6.66μM). Isotype control anti-KLH antibody (BioLegend) was used at the same dilution range to confirm specificity of targeted TT30 activity. TT30-mediated inhibition of MAC formation was unaffected by nonspecific isotype control antibody, while anti-CR2 antibody fully abrogated TT30 activity. Results represent mean ± SEM of duplicate values from 3 independent experiments.
Inhibition of CAP and CCP activity by TT30, fH and an anti–human C5 monoclonal antibody. (A) Inhibition of CAP and CCP after spiking TT30 into human whole blood from 9 donors and measuring activity in processed serum using Wieslab Complement System Alternative ELISA kit (Euro-Diagnostica). Results are expressed as percent baseline level of activity and represent mean ± SEM of duplicates (n = 9). IC50 values for inhibition of CAP and CCP were 0.08 ± 0.03μM and 9.8 ± 7.6μM, respectively, while IC90 values for inhibition of CAP and CCP were 0.14μM and > 29.4μM, respectively. (B) Potency and specificity of targeted CAP inhibition by TT30 compared with factor H. Potency of TT30 and fH spiked into human serum was measured as inhibition of CAP using Wieslab Complement System Alternative ELISA kit. TT30 showed high potency (IC50 = 0.04 ± 0.005 μM) while fH was less effective (IC50 = 3.0 ± 1.6μM). Potency of TT30 and anti–human C5 monoclonal antibody from Quidel Corp. was measured as inhibition of CAP (C) or CCP (D) using Wieslab Complement System Alternative or Classic Pathway ELISA kit. TT30 exhibits selectivity for CAP (IC50 = 0.03 ± 0.007μM in CAP compared with 2.1 ± 0.7μM in CCP) while anti-C5 antibody showed similar activity in either pathway (IC50 = 0.16 ± 0.07μM in CAP compared with IC50 = 0.05 ± 0.009μM in CCP). (E) TT30 was spiked into human serum at 0.3μM alone or in the presence of serial 3-fold dilutions of anti-CR2 monoclonal antibody (clone 1048, starting concentration 6.66μM). Isotype control anti-KLH antibody (BioLegend) was used at the same dilution range to confirm specificity of targeted TT30 activity. TT30-mediated inhibition of MAC formation was unaffected by nonspecific isotype control antibody, while anti-CR2 antibody fully abrogated TT30 activity. Results represent mean ± SEM of duplicate values from 3 independent experiments.
Potency and specificity of targeted CAP inhibition by TT30 was further compared with fH, by spiking each of the proteins separately into human serum. A 75-fold gain of potency of TT30 over fH was observed (IC50 = 0.04 ± 0.005μM compared with 3.0 ± 1.6 μM, respectively; Figure 2B), and a ∼ 150-fold gain when considering the level of fH already in the serum used in the assay (∼ 3μM). Next, the inhibitory capacity of the tissue targeted candidate therapeutic (TT30) was compared with a systemic non-pathway-specific terminal complement inhibitor (anti-C5 antibody) in CAP and CCP assays. In contrast to an anti-C5 mAb, which showed complete and comparable inhibition of both complement pathways as expected (IC50 = 0.18 ± 0.05μM in CAP vs IC50 = 0.05 ± 0.009μM in CCP), TT30 demonstrated preferential inhibition of CAP over CCP (IC50 = 0.03 ± 0.003μM in CAP vs IC50 = 2.1 ± 0.7μM in CCP, see Figure 2C-D).
Specificity of targeted CAP inhibition was confirmed by spiking TT30 into human serum in the presence of serial dilutions of anti-CR2 monoclonal antibody (clone 1048). Anti-KLH monoclonal antibody was used as isotype control. High inhibitory potency of TT30 (0% CAP activity at 0.3μM) was unaffected by nonspecific isotype control antibody, while anti-CR2 antibody fully abrogated TT30 activity (Figure 2E).
TT30 protects rabbit RBC from hemolysis via targeting by CR2 domain
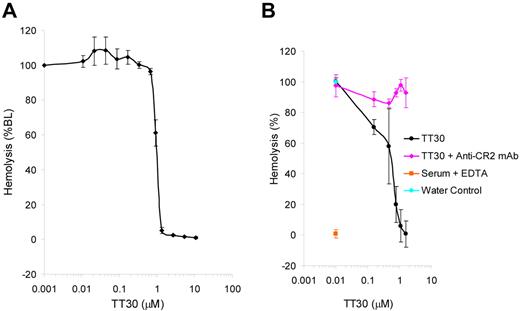
Erythrocyte lysis can be studied in an ex vivo system in which rabbit RBC are exposed to CAP-activated human serum leading to activation of C3 on the cells and decoration by C3 fragments, insertion of the MAC into the cell membrane and subsequent hemolysis measured by absorbance of the released hemoglobin. This assay was used to access the effect of TT30 on RBC lysis under CAP activation conditions. The results (Figure 3A) showed inhibition of hemolysis by TT30 (IC50 = 0.9 ± 0.08μM), while addition of anti-CR2 inhibitory antibody (clone 1048) led to reversal of TT30-mediated inhibition of CAP-induced RBC lysis (Figure 3B).
TT30 inhibits CAP-mediated hemolysis of rabbit RBC and this inhibition is reversed by anti-CR2 antibody. (A) TT30 inhibits CAP-mediated lysis of rabbit RBC by human complement serum. Rabbit RBC were incubated with human complement-preserved serum containing serial dilutions of TT30 under experimental conditions promoting CAP activity and subsequent hemolysis. The amount of free hemoglobin correlated with the color intensity and was measured as absorbance at 415 nm. Percent lysis was calculated as (A415ser ×- A415ser × bkgd)/(A415water) × 100 and the data were graphed using Excel. TT30 showed high potency (IC50 = 0.9 ± 0.008μM). Results represent mean ± SEM of duplicate values from 5 independent experiments. (B) Reversal of TT30-mediated inhibition of CAP-induced hemolysis of rabbit RBC by anti-CR2 monoclonal antibody (clone 1048). TT30 was serially diluted and spiked into human serum in presence of excess anti-CR2 antibody (1 mg/mL) and added to rabbit RBC under conditions promoting CAP activation in the ELISA assay format. Results represent mean ± SEM of duplicates from 3 independent experiments.
TT30 inhibits CAP-mediated hemolysis of rabbit RBC and this inhibition is reversed by anti-CR2 antibody. (A) TT30 inhibits CAP-mediated lysis of rabbit RBC by human complement serum. Rabbit RBC were incubated with human complement-preserved serum containing serial dilutions of TT30 under experimental conditions promoting CAP activity and subsequent hemolysis. The amount of free hemoglobin correlated with the color intensity and was measured as absorbance at 415 nm. Percent lysis was calculated as (A415ser ×- A415ser × bkgd)/(A415water) × 100 and the data were graphed using Excel. TT30 showed high potency (IC50 = 0.9 ± 0.008μM). Results represent mean ± SEM of duplicate values from 5 independent experiments. (B) Reversal of TT30-mediated inhibition of CAP-induced hemolysis of rabbit RBC by anti-CR2 monoclonal antibody (clone 1048). TT30 was serially diluted and spiked into human serum in presence of excess anti-CR2 antibody (1 mg/mL) and added to rabbit RBC under conditions promoting CAP activation in the ELISA assay format. Results represent mean ± SEM of duplicates from 3 independent experiments.
Detection of TT30 on protected RBC by flow cytometry
Rabbit RBCs protected from hemolysis by TT30 were further examined for the presence of TT30 on their surface by flow cytometric staining. Simultaneous detection of C3 fragments and bound TT30 was performed using anti-C3d (A702) and anti-TT30 (HB5) mAbs. Dot plots of the double staining including isotype controls are presented in Figure 4A. TT30 added to the RBC at 0.46μM exhibited highest levels of staining (70%, middle panel), while further increase in TT30 concentration to 3.7μM led to lack of detectable TT30 and C3d because of blockade of C3 activation and amplification on RBCs by TT30 (right panel). Control conditions in the presence of EDTA resulted in no staining, as expected (left panel). To study the duration of TT30 binding to C3 fragments of the RBC, a sample with the highest bound TT30 (from 0.46μM treatment) was selected and tested for C3/TT30 staining for up to 24 hours of incubation. Figure 4B shows that after protecting from hemolysis, TT30 remains bound to C3 fragments on rabbit RBC for over 24 hours, exhibiting strong staining and high mean fluorescence intensity (MFI) throughout this time period.
Detection of TT30 on C3d-decorated rabbit RBC after hemolysis induced by CAP activation in human serum. Residual RBC protected from hemolysis were stained with biotinylated anti-C3d (A702; Quidel) and FITC-conjugated anti-CR2 (clone HB5; Santa Cruz Biotechnology) monoclonal antibodies to detect C3d fragment deposition and TT30, respectively. Streptavidin-conjugated APC (BD Biosciences) was used as a secondary detection reagent. Isotype controls were from BD Biosciences. Cells were analyzed on Accuri C6 cytometer (Accuri Cytometers Inc) using CFlow software. (A) TT30 bound to rabbit RBC protected from hemolysis at 0.46μM can be detected by flow cytometry (middle panel) in contrast to a higher bound TT30 concentration of 3.7μM (right panel). In presence of EDTA, there is no C3 fragment decoration of rabbit RBC and thus no binding of TT30 (EDTA control, left panel). Results represent dot plots of double staining of one representative experiment. (B) TT30 binding is retained on the RBC surface for at least 24 hours. Histograms represent overlays of 1-color staining of isotype control (black) over TT30 staining (red).
Detection of TT30 on C3d-decorated rabbit RBC after hemolysis induced by CAP activation in human serum. Residual RBC protected from hemolysis were stained with biotinylated anti-C3d (A702; Quidel) and FITC-conjugated anti-CR2 (clone HB5; Santa Cruz Biotechnology) monoclonal antibodies to detect C3d fragment deposition and TT30, respectively. Streptavidin-conjugated APC (BD Biosciences) was used as a secondary detection reagent. Isotype controls were from BD Biosciences. Cells were analyzed on Accuri C6 cytometer (Accuri Cytometers Inc) using CFlow software. (A) TT30 bound to rabbit RBC protected from hemolysis at 0.46μM can be detected by flow cytometry (middle panel) in contrast to a higher bound TT30 concentration of 3.7μM (right panel). In presence of EDTA, there is no C3 fragment decoration of rabbit RBC and thus no binding of TT30 (EDTA control, left panel). Results represent dot plots of double staining of one representative experiment. (B) TT30 binding is retained on the RBC surface for at least 24 hours. Histograms represent overlays of 1-color staining of isotype control (black) over TT30 staining (red).
Bioavailability and pharmacokinetics of TT30
TT30 pharmacokinetic (PK) and pharmacodynamic (PD) activity was tested in male and female cynomolgus monkeys, being the most pharmacologically relevant species to humans because of their 89% sequence identity in CR2 and fH domains and similar TT30 inhibition in in vitro CAP activity assays. The monkeys received 20 mg/kg TT30 either by single intravenous bolus injection or a single subcutaneous (SC) injection and then plasma or serum samples were assayed for TT30 concentrations and CAP-mediated MAC formation (Figure 5A-B). TT30 administered by intravenous infusion produced complete inhibition of CAP-mediated MAC formation (CAP activity), whereas CCP-mediated MAC formation (CCP activity) was only partially inhibited (33.3% residual CCP activity). After a SC injection, plasma TT30 concentrations at 15 minutes ranged from 0.9-3.1 μg/mL (1.6 ± 0.77 μg/mL), reaching peak levels at 6-12 hours. There was a complete inhibition of CAP-mediated MAC formation with 0.1 ± 0.29% residual CAP activity at 6 hours. TT30 maintained ∼ 0% CAP activity from 3 through 24 hours. As plasma TT30 concentrations declined, serum CAP activity increased, returning to baseline levels by 72-96 hours.
TT30 selectively inhibits CAP activity in vivo and is bioavailable after SC injection in cynomolgous monkeys, with longer duration of activity. (A) Plasma TT30 concentrations, (B) serum CAP activity, and (C) relationship of plasma TT30 concentrations to serum CAP activity independent of time, in cynomolgous monkeys after a single TT30 dose of 20 mg/kg IV bolus injection. A single SC compared with intravenous injection of TT30 provides 3-fold longer duration of inhibition of CAP activity (24 vs 8 hours, respectively). As plasma TT30 concentrations declined, serum CAP activity increased, returning to baseline values by 72-96 hours. Results are presented through 120 hours after dosing. (D) CAP- and CCP-mediated MAC formation after IV bolus TT30 administration of 15, 30, or 60 mg/kg TT30. Each dosing group consisted of 5 male and 5 female cynomolgous monkeys. Serum samples were assayed for CAP activity. Results (% of baseline [%BL]) are presented through 48 hours after dosing.
TT30 selectively inhibits CAP activity in vivo and is bioavailable after SC injection in cynomolgous monkeys, with longer duration of activity. (A) Plasma TT30 concentrations, (B) serum CAP activity, and (C) relationship of plasma TT30 concentrations to serum CAP activity independent of time, in cynomolgous monkeys after a single TT30 dose of 20 mg/kg IV bolus injection. A single SC compared with intravenous injection of TT30 provides 3-fold longer duration of inhibition of CAP activity (24 vs 8 hours, respectively). As plasma TT30 concentrations declined, serum CAP activity increased, returning to baseline values by 72-96 hours. Results are presented through 120 hours after dosing. (D) CAP- and CCP-mediated MAC formation after IV bolus TT30 administration of 15, 30, or 60 mg/kg TT30. Each dosing group consisted of 5 male and 5 female cynomolgous monkeys. Serum samples were assayed for CAP activity. Results (% of baseline [%BL]) are presented through 48 hours after dosing.
The relationship of plasma TT30 concentrations to serum CAP activity over time, after an IV bolus injection of 20 mg/kg, is presented in Figure 5C. Complete (∼ 100%) inhibition of CAP-mediated MAC formation was achieved from 5 minutes through 8-12 hours. Despite complete inhibition of CAP activity, TT30 induced only a modest (∼ 60%) and short duration (< 4 hours) inhibition of CCP-mediated MAC formation. Relative to administration of the same dose (20 mg/kg) of TT30 as an IV bolus injection, administration by SC injection resulted in comparable complete (∼ 100%) inhibition of CAP activity, with a 3-fold longer duration of inhibition (24 vs 8 hours, respectively). This observation suggests that SC administration may be a feasible route for clinical studies.
The analysis of TT30 PK and PD activity in cynomolgus monkeys that received IV bolus injections of 15, 30, or 60 mg/kg TT30 is shown in Figure 5D. Four groups of 5 males (M) and 5 females (F) received 0 (saline), 15, 30, or 60 mg/kg TT30, resulting in serum TT30 concentrations of ∼ 666, 947, and 2739 μg/mL, respectively, on day 1, at 15 minutes (data not shown), which provided complete (∼ 100%) inhibition of CAP activity at all doses, and a dose-dependent duration of inhibition.
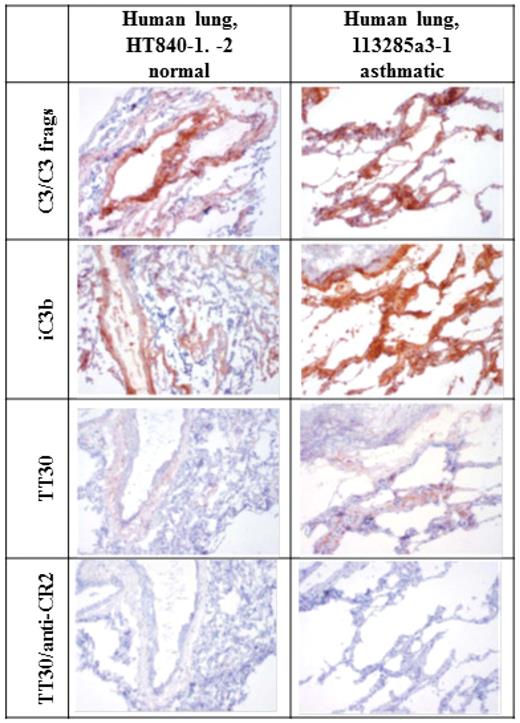
Representative immunostaining of cryosections of normal and inflamed human asthmatic lung for C3/C3 fragments, iC3b and sC5b-9, and immunostaining with TT30 (precomplexed with a sheep polyclonal anti–human-fH IgG) is illustrated Figure 6. Staining for iC3b and C3/C3 fragments in normal human lung tissue was characterized by weak to strong signal in frequent intravascular, interstitial, and perivascular extracellular proteinaceous material, as well as peribronchiolar interstitium and/or alveolar septae. Within inflamed human lung tissue, staining of iC3b and C3/C3 fragments coincided with TT30 staining and was of greater intensity in peribronchiolar interstitium and/or alveolar septa, extending further into smaller airways than in the normal lung. TT30 immunostaining was prevented by an anti-CD21 (CR2) mAb, indicating dependence of TT30 binding on CR2 recognition of C3 fragments in tissues. Thus, TT30 immunostaining patterns were generally similar to those for iC3b. This is consistent with the spontaneous tickover of CAP activity and with normal capacity of cells to convert C3b to iC3b. Of importance is that the TT30 staining patterns (tissue elements stained and subcellular localization) were similar in the human and cynomolgous monkey tissue panels (data not shown), indicating that cynomolgus monkeys are a relevant animal species for nonclinical evaluation of the pharmacologic and toxicologic properties of TT30.
Staining for iC3b and C3/C3 fragments in inflamed and normal human lung. Left column. Lung from control “normal” human (HT840-1, -2). 10×. Staining for iC3b and C3/C3 fragments in normal human lung tissue was characterized by weak to strong staining of frequent intravascular, interstitial, and perivascular extracellular proteinaceous material, as well as peribronchiolar interstitium and/or alveolar septae. This is consistent with the spontaneous tickover of CAP activity and with normal methods of inactivating C3b to iC3b. Staining for C3/C3 fragments was more intense than staining for iC3b. Staining with TT30 is less intense (likely because of differences in concentration and signal amplification, as well as binding avidity) but follows the same pattern as for iC3b and C3/C3 fragments. TT30 staining is blocked by inclusion of an anti-CR2 mAb. Right column. Inflamed lung from asthmatic human (113285a3-1). 10×. Staining for iC3b and C3/C3 fragments in peribronchiolar interstitium and/or alveolar septa is of greater intensity and extends further into smaller airways than in the normal lung. Staining with TT30 is more intense and more broadly distributed, after the iC3b and C3/C3 fragment staining patterns. This is consistent with the targeting of TT30 to sites of C3 fragment deposition by binding of the CR2 portion. This binding is blocked by addition of an anti-CR2 mAb.
Staining for iC3b and C3/C3 fragments in inflamed and normal human lung. Left column. Lung from control “normal” human (HT840-1, -2). 10×. Staining for iC3b and C3/C3 fragments in normal human lung tissue was characterized by weak to strong staining of frequent intravascular, interstitial, and perivascular extracellular proteinaceous material, as well as peribronchiolar interstitium and/or alveolar septae. This is consistent with the spontaneous tickover of CAP activity and with normal methods of inactivating C3b to iC3b. Staining for C3/C3 fragments was more intense than staining for iC3b. Staining with TT30 is less intense (likely because of differences in concentration and signal amplification, as well as binding avidity) but follows the same pattern as for iC3b and C3/C3 fragments. TT30 staining is blocked by inclusion of an anti-CR2 mAb. Right column. Inflamed lung from asthmatic human (113285a3-1). 10×. Staining for iC3b and C3/C3 fragments in peribronchiolar interstitium and/or alveolar septa is of greater intensity and extends further into smaller airways than in the normal lung. Staining with TT30 is more intense and more broadly distributed, after the iC3b and C3/C3 fragment staining patterns. This is consistent with the targeting of TT30 to sites of C3 fragment deposition by binding of the CR2 portion. This binding is blocked by addition of an anti-CR2 mAb.
Discussion
Although a large number of attempts to develop therapeutic modalities to treat complement-mediated diseases over the past 20 years, including broad inhibition directed at C3 or C5 proteins and bioactive fragments, specific inhibition of anaphylatoxin receptors and replacement or supplementation of endogenous complement inhibitors.14,20-25 the success rate has been disappointingly low with the exception of the 2007 approval of Eculizumab, a humanized antibody against complement component C5 (Soliris) by the US Food and Drug Administration and European regulatory authorities.26 The central challenge in developing complement blocking therapeutic agents has been to find a balance between providing sufficient inhibition of systemic complement activity to prevent local tissue damage without impairing the critical protection functions (ie, C3b-mediated pathogen opsonization and immune clearance) of this important part of the innate immune system. Similar strategies to limit systemic inhibition of complement by specifically targeting inhibitors to sites of complement activation and disease include the use of fusion proteins consisting of a complement inhibitor linked to a C3 binding region of CR2. In each case, inhibitory activity of the fusion protein was shown to be significantly greater than for the free, untargeted form of the inhibitor.27 Targeting and protection at specific locations of complement damage was also observed in the kidney for a mouse model of lupus nephritis,28 in the lung and intestines for mouse models of ischemia/reperfusion injury,29 in the retina for a mouse model of age-related macular degeneration,30 and in joints and cartilage in a mouse model of collagen induced arthritis.31
To provide specific local inhibition of complement on affected cells and tissues, TT30 was designed as a human fusion protein with the dual function of binding and inhibition, where binding is specifically targeted to complement breakdown products on the surface of affected cells. Functional inhibition by TT30 is provided by fH domains that inhibit C3 and C5 convertases – key activators and amplifiers of the complement pathway.
In our hands, TT30 specifically inhibited CAP-induced MAC formation, as evident by higher inhibitory activity in assays of CAP compared with CCP, when TT30 was spiked into whole human blood, supporting the rational for design of TT30 as CAP-specific inhibitor. Moreover, TT30 was more potent than fH in inhibiting CAP activity when either protein was spiked into human serum. Of note is that TT30 accomplishes this inhibition in the presence of at least 5-fold higher endogenous fH concentrations present in the serum in the assay. This observation is in line with earlier studies showing higher potency of targeted fH linked via CR2 domain that is acting on the cell surface compared with soluble phase fH,29 providing proof to the concept that TT30 works on cell surface and not in the fluid phase.
A monoclonal CR2-specific antibody, HB5 (generated by immunizing mice with the human B cell line SB32 ) that binds to an epitope on CR2 that is not on the domains comprising the CR2-C3d interface33 was used in this study to detect TT30 bound to C3 fragments on rabbit RBC protected from hemolysis, along with anti-C3d antibodies to detect C3-decorated RBC. It should be noted that commercial anti-C3d antibody, clone A702, is specific to C3d fragments but can also recognize C3d in the context of other C3 fragments (Quidel Corp; A702 certificate of analysis). Therefore, it can be stated that TT30 was detected as bound to C3d-containing fragments but not solely to C3d.
CR2-mediated targeting mechanism of TT30 was confirmed by reversal of TT30 inhibition using an anti-CR2 antibody (clone 1048) that is known to interfere with CR2-C3d interactions.34 In this study, clone 1048 was shown to reverse both TT30-mediated inhibition of MAC formation in a Wieslab assay, and protection from hemolysis in a rabbit RBC hemolysis assay. RBCs coincubated with TT30 and 1048 in the course of hemolysis assay also exhibited significantly reduced levels of TT30 on their surface as detected by flow cytometry using antibody HB5 (data not shown), compared with TT30-treated RBCs in the absence of 1048.
It should be noted that the highest levels of TT30 binding were detected by HB5 antibody when 0.46μM TT30 were added to the rabbit RBC, while increasingly higher amounts of added TT30 resulted in reduced to undetectable TT30 levels. This is most probably the result of the greatly reduced levels of C3 fragments bound to the surface of RBC in presence of increased TT30 concentrations, in line with the mechanism of TT30 activity of inactivating C3 and C5 AP convertases and thus inhibiting breakdown of C3 and subsequent opsonization of C3 fragments. The lack of TT30 detection at higher concentrations on the surface of RBC was not because of the insufficient availability of HB5 antibody, since when higher amounts of HB5 were added to stain RBC there was still no TT30 detection (data not shown).
Our preclinical data represent a strong rationale for therapeutic development using targeted complement modulation by TT30 in several complement-mediated inflammatory conditions and other diseases in which this pathway has been shown by genetic and other experimental means to be markedly dysregulated in patients.
As a novel biologic fusion protein, TT30 takes a targeted inhibition strategy not previously used in any class of compounds. The paired processes of cell surface binding to C3 fragments by CR2 domains and subsequent inhibition of complement activity by the linked fH domains, result in markedly improved site-specific local control of complement activation. The inhibitory capacity of TT30 was shown to be greatly enhanced compared with soluble fH in a CR2-dependent manner in several functional assays, including membrane attack complex formation and inhibition of red blood cell lysis. In addition, TT30 was detected by flow cytometry as durably bound to C3 fragments on rabbit red blood cells that were protected from hemolysis. In in vivo experiments, PK/PD assays conducted in primates demonstrated bioavailability after both intravenous and subcutaneous delivery of TT30. Finally, TT30 was found to be substantially more efficient than endogenous wild-type fH, and also to demonstrate no comparable inhibitory effects on the classic and lectin pathways. Thus, TT30 is a selective inhibitor of the complement alternative pathway with the capability to provide durable local tissue binding and protection with only minimal transient systemic inhibition, making it a novel therapeutic with a unique mechanism of pathway-specific activity and broad applications for complement alternative pathway-associated diseases.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: V.M.H. conceived the strategy, supervised the project, and prepared the manuscript; M.F.H. designed and carried out the inhibition and flow cytometry studies, analyzed data, and prepared the manuscript; M.S. prepared the manuscript; C.J.H. designed PK/PD studies and analyzed data; I.M. designed protein characterization studies, analyzed data, and prepared the manuscript; A.M.R. provided unpublished background data and helped to design experimental methods; and A.S.L. provided information regarding clinical pathologic correlation and reviewed the manuscript.
Conflict-of-interest disclosure: M.F.H., M.S., C.J.H., and A.S.L. are employed by Alexion Pharmaceuticals. I.M. is a former employee of Taligen Therapeutics (Alexion Pharmaceuticals). V.M.H. is a consultant to Alexion, has had royalty and stock option income, and has been an employee of Alexion Pharmaceuticals during the completion of this study. A.R. has been a consultant and received research support from Alexion Pharmaceuticals.
Correspondence: Masha Eridkis-Hareli, Alexion Pharmaceuticals, 245 1st street, Cambridge, MA 02142; e-mail: fridkis-harelim@alxn.com.



![Figure 5. TT30 selectively inhibits CAP activity in vivo and is bioavailable after SC injection in cynomolgous monkeys, with longer duration of activity. (A) Plasma TT30 concentrations, (B) serum CAP activity, and (C) relationship of plasma TT30 concentrations to serum CAP activity independent of time, in cynomolgous monkeys after a single TT30 dose of 20 mg/kg IV bolus injection. A single SC compared with intravenous injection of TT30 provides 3-fold longer duration of inhibition of CAP activity (24 vs 8 hours, respectively). As plasma TT30 concentrations declined, serum CAP activity increased, returning to baseline values by 72-96 hours. Results are presented through 120 hours after dosing. (D) CAP- and CCP-mediated MAC formation after IV bolus TT30 administration of 15, 30, or 60 mg/kg TT30. Each dosing group consisted of 5 male and 5 female cynomolgous monkeys. Serum samples were assayed for CAP activity. Results (% of baseline [%BL]) are presented through 48 hours after dosing.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/17/10.1182_blood-2011-06-359646/4/m_zh89991179810005.jpeg?Expires=1765884195&Signature=bYX9-cxN58yEMqXvw3-NRzAvmZ3NL2f1wqOVZaMhk8csGNY2wdimMAOUBV3kC-iKlS~rcHdPfnqH4~dvqmU11mbKb3dlwrSL-zXspRU9xYRqdbbMukEEwZHVuWZyerVZ68b2CKF4RVfrBXsgN0DwfUk0r6YMD60f0yhgfXuQUxbXrivn56WeeyZbG8TE~TrKtEi18jSsWjtT8hu4jG4eY4mBTc6dUFn7RXG4U7Uw~1Z5pqn7wxdCZNxMdd77ASF3yI0Ka7IfLNlW2ae8z6eeXQ6g5DYJei5tfY9y4YEsWe7k9ZZWeV57z3J3RYQPWcSf~AdXVQ9Vrx6Ji6qs4aMNng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)