Abstract
The three-amino-acid loop extension (TALE) class homeodomain proteins MEIS1 and PKNOX1 (PREP1) share the ability to interact with PBX and HOX family members and bind similar DNA sequences but appear to play opposing roles in tumor development. Elevated levels of MEIS1 accelerate development of HOX- and MLL-induced leukemias, and this pro-tumorigenic property has been associated with transcriptional activity of MEIS1. In contrast, reduction of PKNOX1 levels has been linked with cancer development despite the absence of an identifiable transactivating domain. In this report, we show that a chimeric protein generated by fusion of the MEIS1 C-terminal region encompassing the transactivating domain with the full-length PKNOX1 (PKNOX1-MC) acquired the ability to accelerate the onset of Hoxa9-induced leukemia in the mouse bone marrow transduction/transplantation model. Gene expression profiling of primary bone marrow cells transduced with Hoxa9 plus Meis1, or Hoxa9 plus Pknox1-MC revealed perturbations in overlapping functional gene subsets implicated in DNA packaging, chromosome organization, and in cell cycle regulation. Together, results presented in this report suggest that the C-terminal domain of MEIS1 confers to PKNOX1 an ectopic transactivating function that promotes leukemogenesis by regulating expression of genes involved in chromatin accessibility and cell cycle progression.
Introduction
There is a compelling body of evidence implicating the HOX transcription factors and their cofactors PBX and MEIS/PKNOX(PREP) in leukemogenesis (reviewed by Argiropoulos et al1 ). In particular, deregulated expression of HOXA9 has been detected in a large proportion of human acute myeloid (AML) and lymphoid leukemias (ALLs)2-5 and is associated with poor prognosis for refractory AML.2 A significant proportion of these leukemias, especially those harboring MLL rearrangements, also overexpress MEIS1,3-6 indicating that activation of MEIS1 may represent a key collaborating genetic event in leukemia development.
MEIS1 is a member of the TALE class of homeoproteins, which includes the PBX and MEIS/PKNOX protein families characterized by an atypical homeodomain (HD) containing a three-amino acid loop extension (TALE) between the first 2 α-helices.7 MEIS1/PKNOX1 and PBX1 proteins physically interact with each other using the bipartite Homothorax-MEIS domain (HMA/HMB) and the PBC-A domain of PBX.8,9 This interaction was reported to enhance the stability of the heterodimers and to promote their nuclear localization.10-12 MEIS1 and PBX1 also form heterodimers with HOX proteins in a DNA-dependent manner and have been reported to form triple complexes composed of MEIS1/PKNOX1, PBX1, and HOX in both a DNA-independent13,14 and DNA-dependent manner,15 suggesting that, in some cellular contexts, a triple HOX-PBX-MEIS/PKNOX complex is required for HOX-mediated gene regulation.15,16 The combinatorial diversity generated by 39 different Hox, 3 Meis, 2 Pknox, and 4 Pbx gene products probably enables formation of distinct regulatory complexes targeting numerous genomic loci in a cell context-dependent manner.
Using a bone marrow transduction/transplantation-based experimental model, we showed that overexpression of Meis1 significantly accelerates the onset of Hoxa9-induced AML,17,18 and similar collaborative Meis1 effects were reported for other Hox and Hox-fusion genes tested.19,20 High levels of Meis1 expression also appeared to be the rate-limiting factor for development of Mll-induced leukemias,21 implying that Meis1 activity is necessary to create and/or maintain cellular context required for tumor development. Pknox1 overexpression, in contrast, failed to accelerate but rather slightly delayed the occurrence of Hox9-induced AML.18 Supporting the possibility that PKNOX1 exerts tumor suppressive activity, Longobardi et al reported that Pknox1 deficiency creates a tumor-prone phenotype and accelerates EμMyc lymphomagenesis.22
Structural and functional analyses identified 3 distinct MEIS1 domains required for leukemogenesis, namely, the PBX-interacting domain composed of the HMA/HMB motifs, the DNA binding HD, and the C-terminal domain (CTD).23-25 The HD and HMA/HMB have been highly conserved during evolution of MEIS and PKNOX families. In contrast, the regions separating their HMA/HMB and the HD, and most notably their C-termini, are highly divergent. The CTD of MEIS1 is required for its transcriptional and transforming activity.23,24 Observations that the transactivating domain of VP-16 can rescue both the transactivating and the transforming functions in MEIS1 mutants lacking the CTD23,24 lends further support to the model predicting that the transforming function of MEIS1 reflects its ability to actively participate in regulation of gene expression. An equivalent transactivating domain of PKNOX1 has not yet been identified, but genetic evidence demonstrates requirement for PKNOX1 in positive regulation of Hox,26 TALE,27 somatostatin,28 and follicle-stimulating hormone29 gene expression.
We postulated that the proposed opposing roles of MEIS1 and PKNOX1 in leukemogenesis map to their distinct carboxy termini. To test this hypothesis, we generated fusion PKNOX1 proteins composed of MEIS1 CTD, in the presence or absence of the PKNOX1 CTD, and tested their transforming functions in the in vivo leukemia-initiating assays. In this report, we demonstrate that MEIS1 CTD confers to PKNOX1 the ability to accelerate the onset of Hoxa9-induced leukemia and that, in the absence of PKNOX1 CTD, this function can be provided for by the transactivating domain of VP-16. We also show that the transforming ability conferred to PKNOX1 by MEIS1 CTD correlates with a distinct gene expression profile associated with wild-type Meis1, but not Pknox1, overexpression.
Methods
Animals
(C57Bl/6J-Ly5.2 × C3H/HeJ) F1-recipient and (C57Bl/6-Ly5.1 × C3H/HeJ) F1 congenic donor mice were bred in a specific pathogen-free animal facility. Animal handling followed the guidelines of the Canadian Council on Animal Care, and the experimental procedures were approved by the University of Montreal Deontology Committee on Animal Experimentation.
Retroviral vectors
The retroviral and expression vectors MSCV-Hoxa9-PGK-NEO (#412),17 MSCV-pKOF-PGK-GFP (#1932),30 MSCV-PGK-GFP (#652),18 and pCS2-Pbx1a-HA (#1452)24 have been described. The MSCV-Meis1a-PGK-GFP (#1031) carries full-length Meis1a cDNA, and the MSCV-FLAG-Meis1a-PGK-GFP (#1514) encodes N-terminal FLAG (DYKDDDDK) tagged MEIS1A. The cDNAs coding Pknox1-SW, Pknox-MC, and Pknox-VP16 mutants were generated by Overlap Extension PCR using Pknox1 (NM_016670) or Meis1a (NM_001193271) cDNA as templates, and the following primers: (1) Pknox1-SW (#2102): Pknox1 (forward 5′-agttatcgaagatctaccatgatggcgacacagacg-3′, and reverse 5′-ctcggttggactggtctattggctggagaattcgtc-3′) and Meis1 (forward 5′-gacgaattctccagccaatagaccagtccaaccgag-3′, and reverse 5′-aggtattgaagatctttacatgtagtgccactgccc-3′); (2) Pknox1-MC (#2100): Pknox1 (forward as for Pknox1-SW, and reverse 5′-gttggactggtctatcatctgaagggagtcgct-3′), and Meis1 (forward 5′-cagcgactcccttcagatgatagaccagtccaacc-3′, and reverse as for Pknox1-SW); and (3) Pknox1-VP16 (#2106): Pknox1 (forward as for Pknox1-SW, and reverse 5′-acatcggtcgggggggctggctggagaattcgtc-3′), and VP1613 (forward 5′-gacgaattctccagccagcccccccgaccgatgt-3′ and reverse 5′-agttatcgaagatctctagaattccccaccgtactcgtcaa-3′). The final PCR product was amplified using the forward Pknox1 primer, and the reverse Meis1 or VP16 primers. The Pknox1-ΔC (#2104) was generated by PCR amplification of Pknox1 cDNA as a template using forward primer as for Pknox1-SW and reverse 5′-agttatcgaagatctctatggctggagaattcgtc-3′. The products of amplification reactions were sequenced, and the engineered cDNAs were subcloned in MSCV-PGK-GFP vector. Retroviral vectors coding for N-terminal FLAG-tagged mutants were generated by subcloning the cDNAs in the pKOF vector.30 Details of vector construction are available on request.
Bone marrow cell culture, retroviral infection, and transplantation
DNA and RNA analyses
Southern and Northern blot analyses were performed as described.17 The GFP probe was a 730-bp Gfp fragment, and the Pknox1 probe is composed of the 5′ 610 bp of the wild-type Pknox1 cDNA.
Coimmunoprecipitation and Western blotting
Preparation of cytoplasmic and nuclear protein extracts,32 immunoprecipitations, and Western blotting were performed as described.24 Primary antibodies used were anti-FLAG (Sigma-Aldrich), anti-hemagglutinin (HA; Abcam), α-tubulin (Cell Signaling Technology), histone H2A (Abcam), and isotype control antibodies (Jackson ImmunoResearch Laboratories). Secondary anti–rabbit and anti–mouse HRP-conjugated antibodies were from Santa Cruz Biotechnology.
Morphologic characterization of leukemias and flow cytometry
Morphologic features24 and flow cytometric analyses of leukemic cell populations18 were performed as described. Images were acquired using a Leica DMIRB microscope with an HCXPL FluotarL 40×/0.6 numeric aperture objective (Leica) and a Retiga EKI camera (Q-Imaging). Images were transformed directly into TIFF files using Adobe Photoshop Version 6.0 (Adobe Systems). For the phenotypical evaluation of leukemias, the following antibodies were used: allophycocyanin-conjugated CD11b (BD Biosciences PharMingen), TER119 (BioLegend), and CD45R[B220] (Beckman Coulter); PE-conjugated CD11b and CD45R[B220] (BD Biosciences PharMingen); biotin-conjugated CD3, IgM and TER119 (BD Biosciences PharMingen); PE-CY7 conjugated CD117[c-Kit] (eBioscience); streptavidin-conjugated PE-CY7 (eBioscience) and allophycocyanin (BD Biosciences PharMingen); and PE-CY5-conjugated Ly6A/E [Sca-1] (eBioscience). Data were acquired using BD LSRII cytometer and FACSDiva Version 4.1 software (BD Biosciences PharMingen). Analyses were performed using the FlowJo Version 7.6.4 software (TreeStar).
Gene expression arrays
Primary BM cells isolated from 5-fluorouracil-treated mice were infected with MSCV-Hoxa9-pgk-Neor. After 12 days, cells were distributed among cocultures of Flag-Meis1, or Flag-Pknox1, or Flag-Pknox1-MC retrovirus-producing cells. After 2-day incubation, the nonadherent cell populations were harvested, and RNA was extracted using Trizol reagent according to the manufacturer's protocol (Invitrogen). RNA was reverse-transcribed and labeled as previously described.33 A total of 6 μg of Cy5-labeled cDNA were hybridized in biological duplicate to a Mouse Gene Expression 385K Array (A4543-00-01; Roche/NimbleGen) as recommended by the manufacturer. The microarrays were scanned using a GenePix 4000B scanner (Molecular Devices), and the data were extracted and normalized (Robust Multichip Average) using NimbleScan Version 2.5 software (Roche/NimbleGen), and expression data were analyzed using GeneSpring.GX Version 7.3 software (Agilent Technologies). Genes were included in the analysis if their probes' signal intensities were above the values determined for random GC controls, and the threshold for differential expression of genes between datasets was a 2-fold difference. All microarray data are available in ArrayExpress under accession number E-MTAB-671.
DAVID functional annotation clustering
The gene lists obtained by comparing the test cell population to the parental Hoxa9 cell population were investigated for enriched gene ontology (GO) term-based biological processes using the DAVID Bioinformatics Resources 6.7 internet application (http://david.abcc.ncifcrf.gov/).34,35 The significance of the ascertained biological processes was calculated using the “Ease score,” a modified 1-tailed Fisher Exact Probability Value, which was further corrected for false discovery rate using the Benjamini correction.
Unique dataset determination
Datasets of genes uniquely modulated (up and down) in Meis1-expressing cells were retrieved by identifying genes whose expression levels were commonly up- (or down)–regulated compared with both the parental Hoxa9 and the Pknox1 + Hoxa9 cell populations. The same analysis, but comparing to the parental Hoxa9 and the Meis1 + Hoxa9 cells, was performed to ascertain genes uniquely modulated in the Pknox1-cell population. Both Meis1 and Pknox1 exclusive datasets were then analyzed for similarity with the Pknox1-MC dataset, as obtained by comparison with the parental Hoxa9-cells.
Results
CTD of MEIS1 converts PKNOX1 to a HOXA9-collaborating oncoprotein
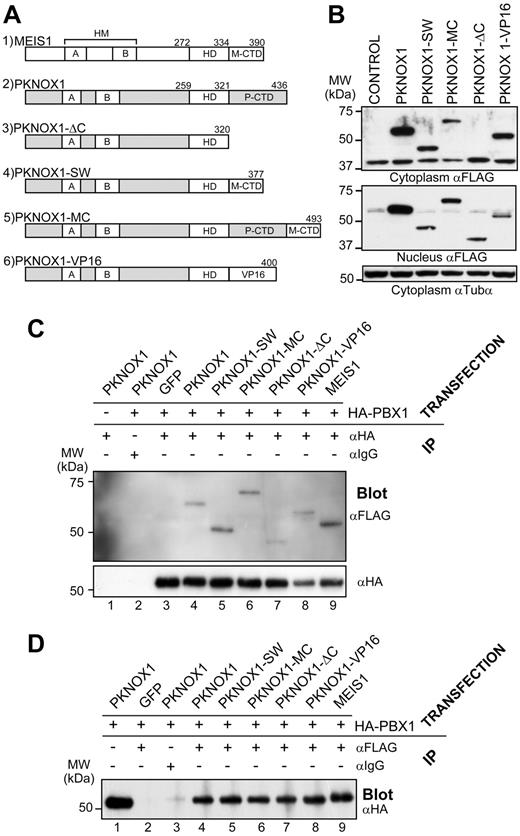
The CTD of MEIS1 is necessary for its transcriptional activity23,36,37 and required for acceleration of the Hox-induced leukemia.24,25 To examine whether MEIS1 CTD is sufficient to confer these 2 functions to the highly related, but nontransforming PKNOX1, we created a series of 4 PKNOX1 mutants (Figure 1A) introducing the following modifications: (1) deletion of the PKNOX1 CTD (PKNOX1-ΔC); (2) replacement of PKNOX1 CTD by the MEIS1 CTD (PKNOX1-SW); (3) addition of the MEIS1A CTD to the wild-type PKNOX1 (PKNOX1-MC); and (4) replacement of PKNOX1 CTD by the VP16-derived transactivation domain (PKNOX1-VP16). The wild-type and mutated cDNAs produced proteins of the predicted molecular weights as determined by Western blot analyses (Figure 1B). Coimmunoprecipitation experiments demonstrated that these PKNOX1 mutants carrying modifications introduced into the C-terminal region of the protein retained their ability to interact with PBX1 (Figure 1C-D), and no impairment of its nuclear import could be detected by confocal microscopy (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Structural and biochemical properties of wild-type and mutant PKNOX1 proteins. (A) Schematic representation of the mutants used in this study. Numbers above the constructs indicate amino acid positions. HMA/HMB indicates Homothorax-Meis domain A/B; M-CTD, MEIS1 CTD; and P-CTD, PKNOX1 CTD. (B) Western blot analysis of FLAG-tagged wild-type and mutant PKNOX1 expression in cytoplasmic (top) and nuclear (middle) lysates of GP-E + 86 producer cells infected with retroviruses expressing the indicated constructs. Tubulin-α levels are shown as a loading control (bottom panel). (C) Immunoprecipitation of lysates from NIH-3T3 cells transfected with the constructs indicated above the horizontal line was carried using anti-HA or an isotypic antibody, as shown above the top panel. The amount of interacting FLAG-tagged mutants was determined by Western blot analysis using anti-FLAG antibody (top panel), whereas the amount of precipitated HA-tagged PBX1A was determined by anti-HA antibody (bottom panel). (D) Immunoprecipitation of lysates from NIH-3T3 cells transfected with the constructs indicated above the horizontal line was performed using anti-FLAG or isotypic antibody, as shown. The amount of interacting HA-tagged PBX1A was determined using anti-HA antibody. Input level is shown in lane 1 and corresponds to 30 μg of protein.
Structural and biochemical properties of wild-type and mutant PKNOX1 proteins. (A) Schematic representation of the mutants used in this study. Numbers above the constructs indicate amino acid positions. HMA/HMB indicates Homothorax-Meis domain A/B; M-CTD, MEIS1 CTD; and P-CTD, PKNOX1 CTD. (B) Western blot analysis of FLAG-tagged wild-type and mutant PKNOX1 expression in cytoplasmic (top) and nuclear (middle) lysates of GP-E + 86 producer cells infected with retroviruses expressing the indicated constructs. Tubulin-α levels are shown as a loading control (bottom panel). (C) Immunoprecipitation of lysates from NIH-3T3 cells transfected with the constructs indicated above the horizontal line was carried using anti-HA or an isotypic antibody, as shown above the top panel. The amount of interacting FLAG-tagged mutants was determined by Western blot analysis using anti-FLAG antibody (top panel), whereas the amount of precipitated HA-tagged PBX1A was determined by anti-HA antibody (bottom panel). (D) Immunoprecipitation of lysates from NIH-3T3 cells transfected with the constructs indicated above the horizontal line was performed using anti-FLAG or isotypic antibody, as shown. The amount of interacting HA-tagged PBX1A was determined using anti-HA antibody. Input level is shown in lane 1 and corresponds to 30 μg of protein.
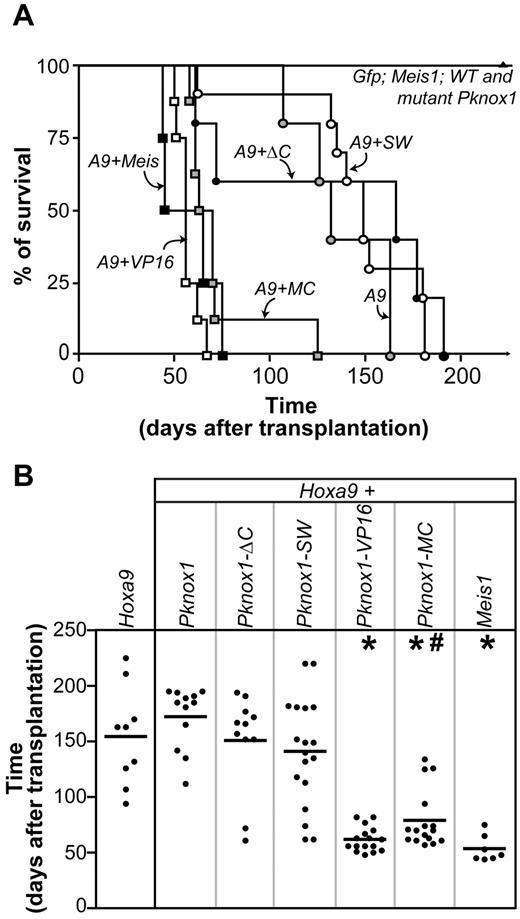
The ability of wild-type and chimeric Pknox1 gene products to accelerate Hoxa9-induced leukemia was evaluated in bone marrow transplantation assays. In agreement with our previously published observations,17,18,24 recipients of Hoxa9 + Meis1–transduced cells died of leukemia within 53 ± 5 days compared with 166 ± 14 days determined for Hoxa9 chimeras, whereas the latency of AML development in mice transplanted with Hoxa9 + Pknox1–transduced cells (172 ± 8 days, P > .2) was similar to that determined for the Hoxa9 group (Figure 2).
Identification of the Pknox1 mutants capable of accelerating Hoxa9-induced leukemia development. (A) Survival curve of mice that received a transplantation of bone marrow cells transduced with Hoxa9, or Hoxa9 plus Pknox1 mutants, or Hoxa9 plus wild-type Meis1 or Pknox1, or control (Gfp) and various TALE cDNAs alone. (B) AML latency in groups of mice identified on the top. *P < .0001, as determined by unpaired 2-tailed Student t test, compared with mice that received Hoxa9-transduced cells. #P = .024, as determined by unpaired 2-tailed Student t test, comparing mice that received Hoxa9 + Meis1 and Hoxa9 + Pknox1-MC-transduced cells.
Identification of the Pknox1 mutants capable of accelerating Hoxa9-induced leukemia development. (A) Survival curve of mice that received a transplantation of bone marrow cells transduced with Hoxa9, or Hoxa9 plus Pknox1 mutants, or Hoxa9 plus wild-type Meis1 or Pknox1, or control (Gfp) and various TALE cDNAs alone. (B) AML latency in groups of mice identified on the top. *P < .0001, as determined by unpaired 2-tailed Student t test, compared with mice that received Hoxa9-transduced cells. #P = .024, as determined by unpaired 2-tailed Student t test, comparing mice that received Hoxa9 + Meis1 and Hoxa9 + Pknox1-MC-transduced cells.
None of the PKNOX1 mutants was capable of inducing leukemia in the absence of Hoxa9 co-overexpression, and recipients remained healthy during an extended period of observation (> 8 months, Figure 2A). We first evaluated the pro-tumorigenic ability of PKNOX1 lacking its CTD (PKNOX1-ΔC) and the mutant in which the PKNOX1 CTD was replaced with MEIS1 CTD (PKNOX1-SW), and found that these 2 PKNOX1 mutants were not capable of collaborating with Hoxa9 in AML development (Figure 2). In all groups of recipients, the transplantation inocula comprised similar proportions of doubly transduced cells as determined by evaluating the proportions of GFP+[Pknox1] plus Neor[Hoxa9] progenitor cell subpopulations (supplemental Table 1), and GFP+ cells represented, on average, > 50% of leukemic cell populations (Table 1). Moreover, Southern blot analyses of genomic DNA isolated from leukemic cells demonstrated the presence of the integrated wild-type Pknox1, or Pknox1-ΔC, or Pknox1-SW proviruses (Figure 3A), which were transcribed (Figure 3B) and translated (Figure 3C) in the products of the predicted sizes. The failure of PKNOX1-ΔC or PKNOX1-SW to accelerate development of Hoxa9-induced leukemia therefore could not be attributed to a poor gene transfer or silencing of the integrated proviruses. These observations rather suggested that the PKNOX1 CTD is not involved in prevention of Pknox1 and Hoxa9 collaboration in AML development and that MEIS1 CTD positioned adjacent to the PKNOX1 HD was not capable of conferring the transforming function to the PKNOX1-SW chimera. Identical position of the VP16-transactivating domain in the PKNOX1-VP16 fusion created, however, a HOXA9-collaborating oncoprotein, and recipients of Hoxa9 + Pknox1-VP16–transduced cells rapidly died of AML (62 ± 3 days; Figure 2), suggesting an absence of intrinsic determinants preventing conversion of PKNOX1 into a leukemia-promoting HOXA9 partner.
Characteristics of AML resulting from genetic interactions between Hoxa9 and wild-type or mutant Pknox1
| Genotype . | AML latency, days . | Spleen weight, mg . | WBC, cells × 109/L . | Blasts, % of total . | GFP+ cells, % BMC . | CD11b+ cells, % BMC . | CD117+ cells, % BMC . | |
|---|---|---|---|---|---|---|---|---|
| PBL . | BM . | |||||||
| Control | NA | 120 ± 13 (20) | 6 ± 1 (5) | NA | 18 ± 3 (1) | NA | 48 (1) | 6 (1) |
| Hoxa9 | 166 ± 14 (8) | 394 ± 77 (4) | 83 ± 41 (6) | 45 ± 6 (6) | 55 ± 6 (5) | NA | ND | ND |
| Hoxa9 + Meis1 | 53 ± 5 (7) | 288 ± 38 (5) | 146 ± 83 (4) | 64 ± 2 (4) | 70 ± 7 (6) | 63 ± 17 (3) | 91 ± 2 (3) | 40 ± 13 (3) |
| Hoxa9 + Pknox1 | 172 ± 8 (12) | 528 ± 51 (9) | 350 (1) | 71 (1) | 60 ± 7 (2) | 50 ± 18 (7) | 91 ± 5 (7) | 17 ± 5 (7) |
| Hoxa9 + Pknox1-ΔC | 155 ± 13 (12) | 547 ± 25 (4) | 193 ± 29 (3) | 39 ± 10 (3) | 60 ± 9 (4) | 78 ± 19 (5) | 91 ± 3 (5) | 34 ± 9 (5) |
| Hoxa9 + Pknox1-SW | 141 ± 11 (18) | 527 ± 53 (10) | 478 ± 195 (6) | 57 ± 6 (6) | 65 ± 5 (6) | 87 ± 5 (6) | 93 ± 2 (6) | 16 ± 5 (6) |
| Hoxa9 + Pknox1-VP16 | 53 ± 5 (15) | 291 ± 21 (15) | 89 ± 30 (13) | 56 ± 4 (13) | 80 ± 5 (11) | 94 ± 2 (5) | 88 ± 2 (5) | 40 ± 4 (4) |
| Hoxa9 + Pknox1-MC | 80 ± 6 (16) | 379 ± 33 (14) | 182 ± 51 (13) | 56 ± 4 (13) | 70 ± 5 (5) | 74 ± 14 (6) | 90 ± 3 (6) | 22 ± 8 (6) |
| Genotype . | AML latency, days . | Spleen weight, mg . | WBC, cells × 109/L . | Blasts, % of total . | GFP+ cells, % BMC . | CD11b+ cells, % BMC . | CD117+ cells, % BMC . | |
|---|---|---|---|---|---|---|---|---|
| PBL . | BM . | |||||||
| Control | NA | 120 ± 13 (20) | 6 ± 1 (5) | NA | 18 ± 3 (1) | NA | 48 (1) | 6 (1) |
| Hoxa9 | 166 ± 14 (8) | 394 ± 77 (4) | 83 ± 41 (6) | 45 ± 6 (6) | 55 ± 6 (5) | NA | ND | ND |
| Hoxa9 + Meis1 | 53 ± 5 (7) | 288 ± 38 (5) | 146 ± 83 (4) | 64 ± 2 (4) | 70 ± 7 (6) | 63 ± 17 (3) | 91 ± 2 (3) | 40 ± 13 (3) |
| Hoxa9 + Pknox1 | 172 ± 8 (12) | 528 ± 51 (9) | 350 (1) | 71 (1) | 60 ± 7 (2) | 50 ± 18 (7) | 91 ± 5 (7) | 17 ± 5 (7) |
| Hoxa9 + Pknox1-ΔC | 155 ± 13 (12) | 547 ± 25 (4) | 193 ± 29 (3) | 39 ± 10 (3) | 60 ± 9 (4) | 78 ± 19 (5) | 91 ± 3 (5) | 34 ± 9 (5) |
| Hoxa9 + Pknox1-SW | 141 ± 11 (18) | 527 ± 53 (10) | 478 ± 195 (6) | 57 ± 6 (6) | 65 ± 5 (6) | 87 ± 5 (6) | 93 ± 2 (6) | 16 ± 5 (6) |
| Hoxa9 + Pknox1-VP16 | 53 ± 5 (15) | 291 ± 21 (15) | 89 ± 30 (13) | 56 ± 4 (13) | 80 ± 5 (11) | 94 ± 2 (5) | 88 ± 2 (5) | 40 ± 4 (4) |
| Hoxa9 + Pknox1-MC | 80 ± 6 (16) | 379 ± 33 (14) | 182 ± 51 (13) | 56 ± 4 (13) | 70 ± 5 (5) | 74 ± 14 (6) | 90 ± 3 (6) | 22 ± 8 (6) |
Values in parentheses are the number of mice analyzed in each category.
NA indicates not applicable; and ND, not done.
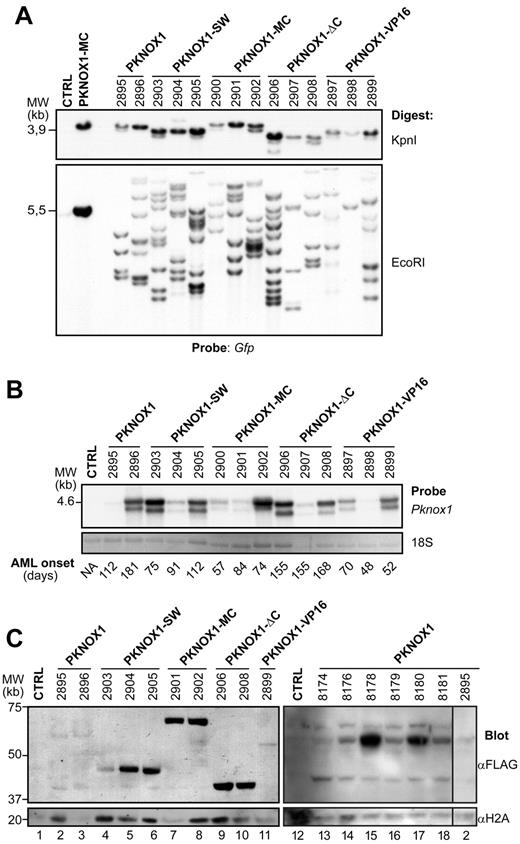
Leukemias comprise the wild-type and mutant Pknox1-transduced cells. (A) Southern blot analysis of proviral integrations in genomic DNA isolated from the bone marrow of leukemic mice. Top panel: DNA was digested with KpnI to release the integrated wild-type or mutant Pknox1 (3.8-4.2 kb) proviruses detectable with a gfp-specific probe. Bottom panel: Clonal analyses of leukemic cell populations. DNA was digested with EcoRI, which cuts inside the provirus and generates a unique DNA fragment for each integration event. Membrane was hybridized with a gfp-specific probe. The identity of the various Pknox1 cDNAs cotransduced together with Hoxa9 is indicated on top, and the numbers represent individual mice analyzed. Note that material obtained from this representative group of mice was used for all analyses shown in this figure. Control lane contains genomic DNA isolated from untransduced bone marrow cells. Digest of the Pknox1-MC retroviral vector is shown as a positive control. (B) Top panel: Northern blot analysis of wild-type and mutant Pknox1 mRNA levels in total RNA isolated from bone marrow cells of leukemic mice. The membranes were hybridized with a 610-bp wild-type Pknox1 cDNA. The double Pknox1 band originates from the presence of an alternative splice site in the retroviral vector.38 Bottom panel: 18S RNA levels are shown as a loading control. Control lane contains RNA isolated from untransduced bone marrow cells. The average AML latency in days is indicated below. (C) Western blot analysis of PKNOX1 levels in nuclear extracts obtained from bone marrow cells of leukemic mice. Top panels: The membranes were hybridized with anti-FLAG to detect expression of the FLAG-tagged PKNOX1 mutants. Bottom panels: Histone H2A levels are shown as a loading control. Lanes 1 to 12 and lanes 13 to 18 contain samples from 2 distinct experiments.
Leukemias comprise the wild-type and mutant Pknox1-transduced cells. (A) Southern blot analysis of proviral integrations in genomic DNA isolated from the bone marrow of leukemic mice. Top panel: DNA was digested with KpnI to release the integrated wild-type or mutant Pknox1 (3.8-4.2 kb) proviruses detectable with a gfp-specific probe. Bottom panel: Clonal analyses of leukemic cell populations. DNA was digested with EcoRI, which cuts inside the provirus and generates a unique DNA fragment for each integration event. Membrane was hybridized with a gfp-specific probe. The identity of the various Pknox1 cDNAs cotransduced together with Hoxa9 is indicated on top, and the numbers represent individual mice analyzed. Note that material obtained from this representative group of mice was used for all analyses shown in this figure. Control lane contains genomic DNA isolated from untransduced bone marrow cells. Digest of the Pknox1-MC retroviral vector is shown as a positive control. (B) Top panel: Northern blot analysis of wild-type and mutant Pknox1 mRNA levels in total RNA isolated from bone marrow cells of leukemic mice. The membranes were hybridized with a 610-bp wild-type Pknox1 cDNA. The double Pknox1 band originates from the presence of an alternative splice site in the retroviral vector.38 Bottom panel: 18S RNA levels are shown as a loading control. Control lane contains RNA isolated from untransduced bone marrow cells. The average AML latency in days is indicated below. (C) Western blot analysis of PKNOX1 levels in nuclear extracts obtained from bone marrow cells of leukemic mice. Top panels: The membranes were hybridized with anti-FLAG to detect expression of the FLAG-tagged PKNOX1 mutants. Bottom panels: Histone H2A levels are shown as a loading control. Lanes 1 to 12 and lanes 13 to 18 contain samples from 2 distinct experiments.
To further explore this possibility, we next examined the leukemogenic properties of PKNOX1 chimera carrying the MEIS1 CTD fused to the C-terminal amino acid (Q436) of PKNOX1 (PKNOX1-MC). In contrast to PKNOX1-SW, PKNOX1-MC noticeably accelerated the onset of Hoxa9-induced leukemia, and all recipients of doubly transduced cells died of AML after latency comparable with that determined for Hoxa9 + Meis1 mice (Figure 2). Southern blot analysis of proviral integrations in DNA extracted from BM also revealed that, in all experimental groups, the leukemias arose from multiple independent integration events (Figure 3A), whereas Northern and Western blot analyses confirmed that the proviruses were transcribed (Figure 3B) and translated (Figure 3C). Together, these observations suggested that MEIS1 CTD is sufficient to confer to PKNOX1 the ability to accelerate the Hoxa9-induced leukemia.
PKNOX1-MC accelerates the development but has no impact on the phenotype of HOXA9-induced leukemia
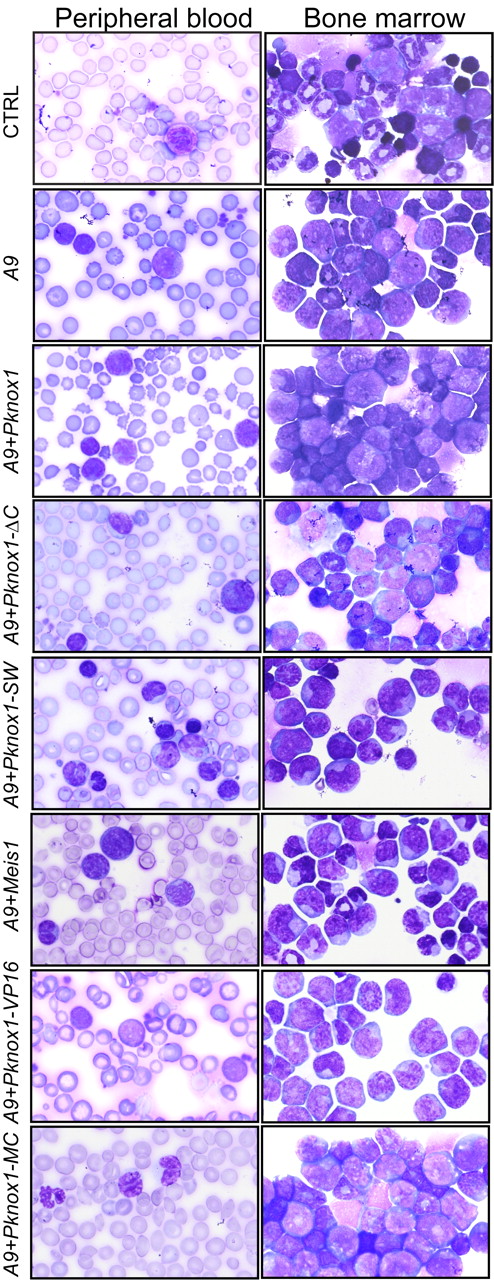
Supporting our previously reported observations,17,18,24 all recipients of Hoxa9 + Meis1– and Hoxa9 + Pknox1–transduced cells died of AML. The PKNOX mutants evaluated in this study had no noticeable effect on the phenotype of the Hoxa9-induced disease. All sick mice had elevated WBC counts (Table 1) and high proportions of immature/blastic myeloid cells in the peripheral blood and bone marrow (Figure 4; Table 1). Analyses of bone marrow cell surface markers showed that, in all experimental groups, the leukemic cell populations expressed high levels of myeloid marker CD11b and various levels of CD117 (Table 1) and were negative for stem cell antigen Ly-6A/E, erythroid TER119, and lymphoid markers CD45R and CD3, suggesting that majority of leukemias were composed of immature myeloid cells (data not shown).
Morphologic characterization of leukemias. Representative samples of Wright-stained peripheral blood smears (right column) and bone marrow cytospins (left column). Original magnification ×100. Genotypes of leukemias are shown on the left.
Morphologic characterization of leukemias. Representative samples of Wright-stained peripheral blood smears (right column) and bone marrow cytospins (left column). Original magnification ×100. Genotypes of leukemias are shown on the left.
MEIS1 and PKNOX1-MC regulate an overlapping subset of target genes
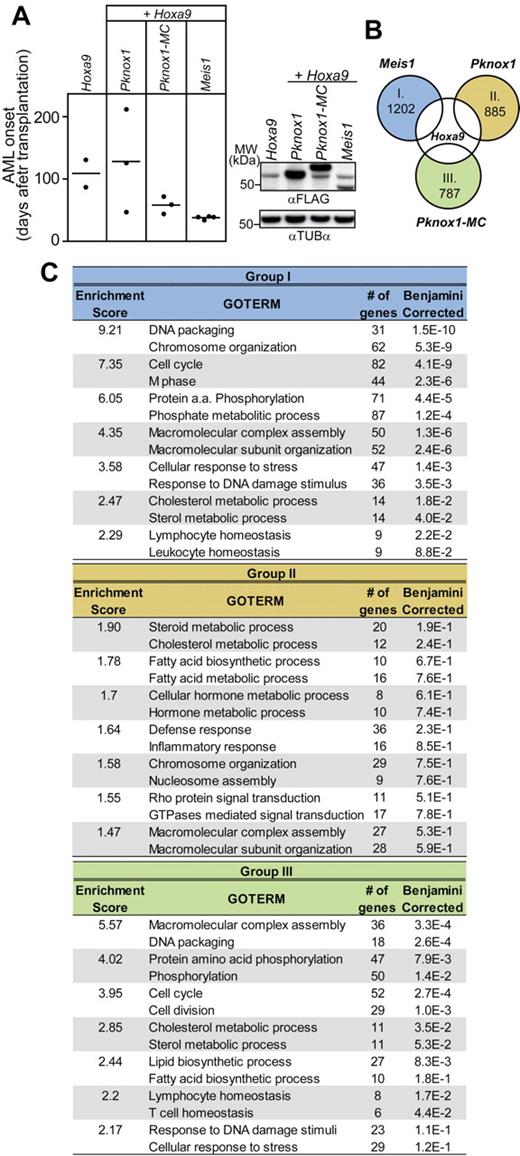
Results of our in vivo studies suggested that MEIS1 CTD is sufficient to convert PKNOX1 into a HOXA9-collaborating oncoprotein. Given the overlap between the transactivating and transforming functions attributed to MEIS1 CTD,23,24,36,39 we postulated that MEIS1 and PKNOX1-MC chimera also regulate an overlapping subset of target genes required for acceleration of Hoxa9-induced leukemia. To assess this possibility, we analyzed the global gene expression profiles of primary bone marrow cell populations engineered to co-overexpress Hoxa9 plus Meis1, or Pknox1, or Pknox1-MC. Briefly, the Hoxa9-transduced cell populations recovered from 2-day coculture with producers of Meis1-Gfp, or Pknox1-Gfp, or Pknox1-MC-Gfp recombinant retroviruses were immediately divided between requirements for flow cytometry, transplantation, and RNA extraction. The recovered cell populations comprised similar proportions of GFP+ cells (45%-50%, data not shown), expressed the corresponding protein products (Figure 5A right panel), and led to AML development within the previously established time frame (Figure 5A left panel).
Impact of Pknox1-MC, Meis1, and Pknox1 expression on global gene expression profile of Hoxa9-transduced cells. (A) Left panel: Survival plot of mice transplanted with 2 × 105 cells used for expression profiling. The genotypes of the transplanted cell populations are shown on top. Right panel: Western blot analysis of FLAG-tagged protein expression in lysates from recovered cells. Tubulin-α levels are shown as a loading control. (B) Venn diagram depicting strategy for identification of genes exhibiting in response to Meis1 (group I), Pknox1 (group II), and Pknox1-MC (group III) at least 2-fold increase in expression levels compared to the parental Hoxa9 cell population. The number of genes found in each of these datasets is indicated inside the circle. (C) Identification of transcript clusters enriched in GO terms associated with distinct biological processes from the gene datasets shown in panel B, as determined by the DAVID Version 6.7 bioinformatics software. Only the top 2 enriched GO terms are shown for each functional cluster. The Enrichment Score refers to the negative log transformation on the geometric mean of the P values obtained for each of the clustered enriched term. The Benjamini-corrected P value provided for each biologic process represents a 1-tailed Fisher Exact Probability Value further corrected for false discovery rate using the Benjamini correction.
Impact of Pknox1-MC, Meis1, and Pknox1 expression on global gene expression profile of Hoxa9-transduced cells. (A) Left panel: Survival plot of mice transplanted with 2 × 105 cells used for expression profiling. The genotypes of the transplanted cell populations are shown on top. Right panel: Western blot analysis of FLAG-tagged protein expression in lysates from recovered cells. Tubulin-α levels are shown as a loading control. (B) Venn diagram depicting strategy for identification of genes exhibiting in response to Meis1 (group I), Pknox1 (group II), and Pknox1-MC (group III) at least 2-fold increase in expression levels compared to the parental Hoxa9 cell population. The number of genes found in each of these datasets is indicated inside the circle. (C) Identification of transcript clusters enriched in GO terms associated with distinct biological processes from the gene datasets shown in panel B, as determined by the DAVID Version 6.7 bioinformatics software. Only the top 2 enriched GO terms are shown for each functional cluster. The Enrichment Score refers to the negative log transformation on the geometric mean of the P values obtained for each of the clustered enriched term. The Benjamini-corrected P value provided for each biologic process represents a 1-tailed Fisher Exact Probability Value further corrected for false discovery rate using the Benjamini correction.
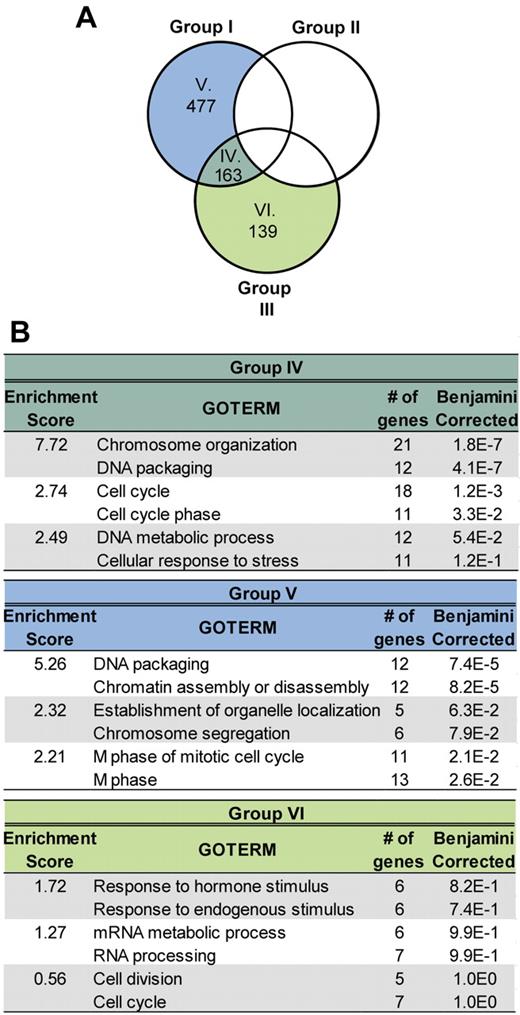
To determine the molecular profile of these doubly transduced cells, we first identified sets of genes whose expression levels were up-regulated at least 2-fold compared to the parental Hoxa9 population (supplemental Table 2) and found 1202 genes up-regulated in response to Meis1, 885 in response to Pknox1, and 787 in response to Pknox1-MC (Figure 5B). To identify potential enrichments for clusters of functionally related annotations associated with various biological processes, these datasets were then analyzed using the DAVID (the database for annotation, visualization, and integrated discovery) Bioinformatics Resources.34,35 No significant enrichment (P ≤ .05) for distinct GO terms could be determined in response to Pknox1 (Figure 5C group II). The Meis1 dataset comprised annotation clusters highly enriched in GO terms for genes associated with processes, such as DNA packaging, chromosomal organization, cell cycle, cell division, and protein phosphorylation (Figure 5C group I), and the same functional gene subsets were activated in response to Pknox1-MC (Figure 5C group III). This expression pattern also characterized the subset of genes shared between the Pknox1-MC and Meis1 (Figure 6 group IV), was not detectable among the unique Pknox1-MC up-regulated genes (Figure 6 group VI), but was partially recapitulated in the Meis1 dataset (Figure 6 group V). The same analytical approach was then applied to identify and classify subsets of genes down-regulated in response to overexpression of Meis1, or Pknox1-MC, or Pknox1 (supplemental Table 3). In each experimental group, expression levels of > 1000 genes were reduced compared with the parental Hoxa9 cells, but no enrichments for transcripts denoting suppression of distinct biological processes could be detected in the unique or the Meis1/Pknox1-MC–shared gene subsets (data not shown).
Comparison between datasets representing genes up-regulated in cells expressing Hoxa9 + Meis1 and Hoxa9 + Pknox1-MC. (A) The overlap between genes up-regulated in the Meis1 + Hoxa9 and Pknox1-MC + Hoxa9, but not in the Pknox1 + Hoxa9 cells, compared with the parental Hoxa9 cells is shown by Venn diagram. (B) Enrichment for GO terms associated with various biological processes in the gene datasets defined in A (group IV, V, and VI), was performed as described for Figure 5C.
Comparison between datasets representing genes up-regulated in cells expressing Hoxa9 + Meis1 and Hoxa9 + Pknox1-MC. (A) The overlap between genes up-regulated in the Meis1 + Hoxa9 and Pknox1-MC + Hoxa9, but not in the Pknox1 + Hoxa9 cells, compared with the parental Hoxa9 cells is shown by Venn diagram. (B) Enrichment for GO terms associated with various biological processes in the gene datasets defined in A (group IV, V, and VI), was performed as described for Figure 5C.
The functional annotation clustering suggested a correlation between the enrichments in GO terms associated with cell proliferation and the ability of MEIS1 and PKNOX1-MC to accelerate the Hoxa9-induced leukemia. Examination of the highest scoring annotations also revealed that MEIS1 and PKNOX1-MC shared the ability to activate sets of genes with similar functions, although not necessarily the same genes. The unique Meis1 + Hoxa9 dataset, as determined by an additional comparison between wild-type Meis1 and Pknox1 datasets (supplemental Table 4; supplemental Figure 2 top panel, A), for example, comprised the previously identified Meis1 targets Nrip1, Mylk, Tet1, Meis1, and Neto2.23,25,39 The intersection between the unique Meis1 + Hoxa9 and Pknox1-MC datasets (supplemental Table 4; supplemental Figure 2 top panel, D) comprised 23 shared genes, including Meis1, Mylk, and Neto2, but not Nrip1 or Tet1. This observation suggested that ectopic MEIS1 CTD retained most, but not all, functions required for MEIS1 activity and was consistent with slightly longer AML latency observed in Hoxa9 + Pknox1-MC recipients compared with the Hoxa9 + Meis1 group (80 ± 6 and 53 ± 5 days, respectively; P = .024, as determined by unpaired 2-tailed Student t test, Figure 2B). Additional interesting findings were strong up-regulation of Vhr (Dusp3) in the Meis1 dataset but down-regulation of this gene and of the previously identified MEIS1 target Flt3 in the Pknox1 group (supplemental Table 4; supplemental Figure 2 bottom panel, F), suggesting that MEIS1 and PKNOX1 may play opposing roles in regulation of some target genes.
Discussion
The CTD of MEIS1 is essential for its transactivating and pro-tumorigenic functions.23-25,36 In this report, we demonstrate that MEIS1 CTD is sufficient to convert the nontransforming PKNOX1 into a HOXA9-collaborating oncoprotein. Results of our experiments show that the wild-type MEIS1 and the chimeric PKNOX1 composing the MEIS1 CTD regulate an overlapping functional gene subsets, and suggest that leukemogenic potential of MEIS1 reflects its ability to deregulate multiple pathways implicated in control of cellular proliferation and division.
Results presented in this report identify MEIS1 CTD as a functional module capable of activating oncogenic pathways, even when presented in a heterologous PKNOX1 context. Fusion of MEIS1 CTD immediately adjacent to the PKNOX1 HD (PKNOX1-SW) abolished this transforming function, indicating that the proximity of heterologous HD interfered with activity of Meis1 CTD. Supporting this possibility, deletion of the HD enhanced affinity of PKNOX1 for PBX-HOX heterodimers and enhanced the transactivation potential of the resulting complex,40 whereas Huang et al36 proposed that, on some enhancers, the HD could affect the ability of MEIS1 to present its CTD in a conformation required for recruitment of transcriptional coactivators. It is conceivable that, in the pro-tumorigenic PKNOX1-MC chimera, the spacer (ie, the PKNOX1 CTD) that separates the PKNOX1 HD and Meis1 CTD was sufficient to relieve the HD-imposed constraints that prevented unmasking of the activation domain of MEIS1 CTD in the context of PKNOX1-SW.
The MEIS1 CTD, in either the context of PKNOX1-MC chimera or the wild-type MEIS1, activated the same functional gene families and exhibited comparable, albeit not entirely equivalent, transformation properties, which could reflect incomplete presentation of CTD, or the requirement for cooperative action of several MEIS1 domains. Favoring the latter possibility, several studies have demonstrated that the N-terminal region and the HD23-25,41 contribute to, but are not essential for, the pro-leukemogenic function of MEIS1. These studies also highlighted a possibility that the DNA binding-dependent and -independent activities of MEIS1 could have complementary roles in acceleration of Hoxa9-induced AML.
To date, several hundred genes have been identified as potential MEIS1 targets.23,25,39,42 However, there is only a minor overlap in differentially expressed genes identified in these transcription profiling studies, indicating that MEIS1 might activate multiple different genes in a genetic and cell context-dependent manner. Our transcriptome analyses thus focused on identification of biological processes, as defined by activities of functionally related gene families, rather than on the identity of individual genes perturbed in response to high levels of MEIS1 or PKNOX1. Results of these analyses suggest that the pro-tumorigenic activity of MEIS1 correlates with enhanced activity of numerous genes implicated in regulation of cellular proliferation and maintenance of genome integrity. These observations thus extend the growing body of evidence identifying MEIS1 as a positive regulator of leukemic cell proliferation,21,43,44 and support the model predicting that pro-tumorigenic activity of MEIS1 reflects its ability to deregulate multiple complementary biological pathways.
Longobardi et al reported that homozygosity for hypomorphic Pknox1i/i allele generates a tumor-prone phenotype and that Pknox1 haploinsufficiency accelerates lymphoma development in the EμMyc mouse model, suggesting a tumor suppressor role for PKNOX1.22 These observations and our results showing that Pknox1 and Hoxa9 do not collaborate in AML development (current study)18 are not mutually exclusive but rather imply that the cellular context might restrict the suppressor function of PKNOX1. Consistent with this possibility, we observed no Pknox1-specific enrichment in GO terms associated with regulation of cellular proliferation and/or survival in our Hoxa9 + Pknox1 bone marrow model.
We show that activation of oncogenic pathways comprising the Meis1 target genes can be attributed to the transactivating function exhibited by the MEIS1 CTD. PKNOX1 was reported to cooperate with the HOX/PBX complex in the in vitro transactivation assays40 and to physically associate with and regulate activity of Bcl2l1 (Bcl-x) and Tp53 (p53) in vivo.45 PKNOX1, however, has no identifiable transactivation domain and shares the target sequences with MEIS1,40 suggesting that PKNOX1/PBX and MEIS1/PBX complexes could be interchangeable at some regulatory sequences. PKNOX1 could thus suppress the oncogenic pathways by preventing the MEIS1-dependent recruitment of transcriptional coactivators and/or corepressors to the target loci. Supporting this possibility, we show that addition of MEIS1 CTD to full-length PKNOX1 is sufficient for activation of Meis1-associated oncogenic pathways and that MEIS1 and PKNOX1 differentially regulate the expression of a subset of genes involved in cellular proliferation, such as Vhr (Dusp3).46
Together, results presented in this report show that elevated levels of MEIS1 perturb expression of numerous genes implicated in several oncogenic pathways, and suggest a direct role for PKNOX1 in attenuation of the pro-tumorigenic activity of MEIS1.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mélanie Fréchette, Andrea Mejia Alfaro, and Angèle Fournier for their assistance with the animal care and transplantation experiments; Nadine Mayotte and Simon Girard for their excellent technical assistance; Jalila Chagraoui for editing the manuscript; Danièle Gagné from the Flow Cytometry Facility of Institute for Research in Immunology and Cancer for assistance in FACS analysis; Christian Charbonneau from the Bio-imaging Facility of Institute for Research in Immunology and Cancer for confocal microscopy assistance; and Simon Drouin for microarray hybridization and scanning.
This work was supported by the Canadian Cancer Society (grant 018478, G.S.). R.B. is a recipient of Canadian Institutes of Health Research and Cole Foundation scholarships. G.S. is a recipient of a Canadian Research Chair in Molecular Genetics of Stem Cells.
Authorship
Contribution: R.B., B.T.W., and G.S. designed the experiments; R.B. performed and analyzed experiments; and R.B., J.K., and G.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Guy Sauvageau, Institute for Research in Immunology and Cancer, University of Montreal, C.P. 6128, Downtown Station, Montreal, QC H3C 3J7; e-mail: guy.sauvageau@umontreal.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal