Cheng and colleagues in this issue of Blood report that implanted donor blood vessel-forming cells inosculate with host blood vessels through a process of “wrapping” and “tapping” to gain access to and divert circulating blood cells away from host vessels and into the newly formed donor-derived vessels.1
Vascular development occurs3 via several well-known paradigms including vasculogenesis, angiogenesis, and arteriogenesis. Vasculogenesis occurs principally during embryogenesis when the primary capillary plexus is established from mesoderm-derived angioblast precursors early in development.2 Angiogenesis is the generation of new blood vessels from pre-existing vessels and is the predominant process through which the systemic circulation responds to changes in tissue demands for enhanced nutrient or oxygen delivery, tissue growth or repair of senescent or damaged tissue, or in response to signals derived from tumor cells.3 Arteriogenesis describes the mechanisms that permit collateral arteries to respond to changes in flow and pressure that generate conductance arteries in a tissue challenged with arterial disruption or occlusion.4
A penultimate element of each mode of vascular development is regulation and promotion of endothelial cell–derived lumen formation.5 Tubular or lumen formation by endothelial cells follows 3 general mechanisms that include budding (a sprout emerges from a pre-existent endothelial tube), cord hollowing (a cord of endothelial cells creates a central luminal space via cell flattening against a cylindrical space), and cell hollowing (individual endothelial cells create intracellular vacuoles that may coalesce between cells to form a lumenized structure). During angiogenic sprouting, endothelial “tip” cells invade the interstitial matrix creating space for the trailing cord of trunk or stalk endothelial cells. The cord of endothelial cells flattens against the outer edges of the matrix space created by the cell membrane proteases of the “tip” cells and the resulting emptied luminal space serves as the tube for the developing vessel. In this mechanism, the growing vessel is fixed at one end by the attachment of the stalk endothelial cells to the vascular site of emergence and is capped by the “tip” cell that must find another growing branch or nearby vessels with which to anastomose to establish a productive functional vasculature.
During vasculogenesis, cell numbers may be more limiting as the endothelial precursors migrate throughout the growing 3-dimensional embryonic tissues to establish the first vascularized structures.5 In this context, greater dependence on cytoplasmic vacuolation within individual endothelial cells with subsequent establishment of cell-to-cell contacts between endothelial cells that permits coalescence of the multicellular vacuoles into tubular structures appears prominent. In this mechanism of vascular growth, the tubular structures are highly branched and interconnected centrally but present many open-ended lumens at the periphery that must eventually anastomose with blood vessels that possess flowing blood to become stable, integrated, and productive vasculature.
Circulating endothelial colony-forming cells (ECFCc) have been isolated from human adult peripheral and umbilical cord blood.6 These cells display clonal proliferative potential in vitro and in vivo vessel forming potential via vasculogenesis on implantation in immunodeficient mice.7 Human umbilical vein endothelial cells (HUVECs) and other human blood vessels have been shown to be composed of similar ECFC clones of varying proliferative potential with in vivo vessel forming potential.6 Coimplantation of mesenchymal stromal cells (MSCs) or other periendothelial supportive cell types enhances the rapidity and stability with which the circulating or resident ECFCs form stable blood vessels within some types of implants in host immunodeficient mice.8 Recent evidence suggests that the presence of host murine myeloid cells is also a critical factor for human blood vessel formation by coimplanted ECFCs with MSCs and may relate to enhanced insoculation or anastomosis of the human vessels with host murine vessels to co-opt blood flow and stabilize the nascent human vessels.9 However, the mechanisms of anastomosis of engineered human vessels to host murine vessels have remained largely unexplored, until now.
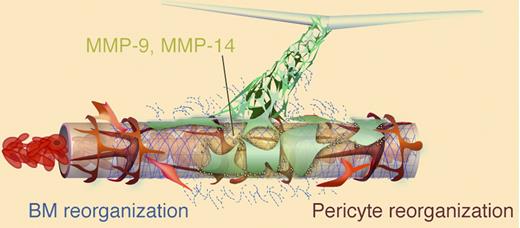
Cheng and colleagues report that human or murine donor vessel-forming endothelial cells suspended in type 1 collagen/fibronectin implants spontaneously formed lumenized vascular structures during the first 2 weeks after implantation.1 At the edges of the gel implant-tissue interface, the open-ended donor vessels “wrapped” around nearby host blood vessels (see figure). The host pericyte cells were displaced in areas of “wrapping” and the donor endothelial cells penetrated into the host basement membrane and via secreted matrix metalloproteinase-9 (MMP-9) and MMP-14 eventually degraded it. Once the donor cells “tapped” into the host vascular endothelial lining, the donor cells essentially diverted the flowing host blood cells into the donor vessels and the original host vessel endothelium regressed. Inhibition of MMP activity with a small molecule inhibitor or monoclonal antibodies significantly inhibited donor vessel attack on the host vascular basement membrane and diminished anastomosis of the donor with host vessels. While host myeloid cells and the coimplanted donor stromal cells were detected in the area of the anastomosing donor-host vessels, neither cell type appeared to be physically involved in the “wrapping and tapping” mechanism of donor vessel inosculation.
Implanted donor (green) endothelial cells (ECs) form vascular tubes with lumens that must anastomose with host vessels to become part of the functional vasculature. The host vessel in this image is composed of endothelial cells (brown), basement membrane (blue), and pericytes (red) and contains flowing red blood cells. The donor ECs wrap around the host vessel to displace the pericytes and proteolytically attack the basement membrane of the host vessel endothelium via matrix metalloproteinases-9 and -14. Subsequently, the donor ECs may now gain access to the host endothelium and displaces some cells to integrate into the endothelial intima and divert blood into the donor vasculature. See Figure 6 in Cheng et al, page 4740.
Implanted donor (green) endothelial cells (ECs) form vascular tubes with lumens that must anastomose with host vessels to become part of the functional vasculature. The host vessel in this image is composed of endothelial cells (brown), basement membrane (blue), and pericytes (red) and contains flowing red blood cells. The donor ECs wrap around the host vessel to displace the pericytes and proteolytically attack the basement membrane of the host vessel endothelium via matrix metalloproteinases-9 and -14. Subsequently, the donor ECs may now gain access to the host endothelium and displaces some cells to integrate into the endothelial intima and divert blood into the donor vasculature. See Figure 6 in Cheng et al, page 4740.
Because developing blood vessels must acquire blood flow as soon as possible to escape regression, understanding the mechanisms through which engineered human blood vessels gain access to host blood flow is a critical step in understanding how to better engineer the vascular constructs to persist in vivo. The results of Cheng and colleagues are intriguing and highlight the important role of donor endothelial MMP activity in the invasive behavior of the donor cells to tap into the host vascular wall.1 Questions remaining include: How do the donor vessels choose which host vessels to “wrap and tap?” What are the specific mechanisms that allow the donor endothelial cells to directly attach and wrap around the host vessel wall? Are there potential tissue-specific properties of host blood vessels that either resist or are more susceptible to the donor vascular inosculating activity? Given the known role of macrophages in enhancing10 or inhibiting11 the process of anastomosing vessel branches, it is surprising that host macrophages did not appear to be directly involved in the “wrapping and tapping” process described by Cheng and colleagues.1 Only further study may reveal the role played by host myeloid cells in the inosculation process. Nonetheless, this is an exciting first step in dissecting the events that need to be clearly delineated to permit greater application of a variety of vascular precursors, including ECFCs, to replace or repair dysfunctional endothelial cells in patients with cardiovascular disease.
Conflict-of-interest disclosure: The author declares a financial interest in EndGenitor Technologies Inc as a consultant. ■