Abstract
Increased hemoglobin A2 (HbA2; ie, levels > 3.9%) is the most important feature of β-thalassemia carriers. However, it is not uncommon to find persons with borderline HbA2 (levels, 3.3%-3.8%), who pose a relevant screening problem. Several genotypes have been associated with borderline HbA2, but sometimes the reasons for this unusual phenotype are unknown. In this paper, we report, for the first time, that mutations of KLF1 result in HbA2 levels in the borderline range. Six different KLF1 mutations were identified in 52 of 145 subjects with borderline HbA2 and normal mean corpuscular volume and mean corpuscular hemoglobin. Two mutations (T327S and T280_H283del) are here reported for the first time. The prevalent mutation in Sardinians is S270X, which accounts for 80.8% of the total. The frequent discovery of KLF1 mutations in these atypical carriers may contribute significantly to the thalassemia screening programs aimed at identification of at risk couples.
Introduction
Accurate determination of the β-thalassemia carrier phenotype is essential for detecting couples at risk for having offspring with thalassemia major. Increased hemoglobin A2 (HbA2) level is considered the most reliable hematologic finding for the identification of β-thalassemia carriers. However, some carriers are difficult to identify because the level of HbA2 is not in the typical carrier range (ie, HbA2 = 3.8%-6.0%). These atypical carriers have borderline HbA2 values (ie, HbA2 levels between normal and β-thalassemia carrier levels, 3.3%-3.8%).1-3 The prevalence of borderline A2 carriers in populations with high frequency of β-thalassemia has been reported in 2.2% to 3.0% in one study and up to 16.7% in another study.2,3 Borderline HbA2 levels associated with reduced mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) are generally the consequence of mild β+-thalassemia mutations (ie, HBB c.92 + 6 T → C), coinherited δ and β-thalassemia, β-promoter mutations −92 (HBB c.-142 C → T), or coexisting iron deficiency anemia.1-5 Borderline HbA2 with normal MCV and MCH may be an outlier value of the normal HbA2 distribution in the noncarrier population or the effect of genetic determinants able to increase HbA2 levels. The genetic determinants so far identified are the triplication of the α-globin genes, β-promoter mutations (HBB c.-151 C → T), and some HBD and HBB gene variants.1-3 However, altogether, these determinants explain only a limited proportion of the borderline HbA2 levels.
Although subjects with borderline HbA2 and reduced MCV and MCH are easily identified with appropriate screening methods, most of the subjects with normal MCV and MCH remain undefined or need a cumbersome laboratory workup (family studies, globin chain synthesis analysis, HBB sequencing) to exclude the presence of globin gene variants, which may interact with β-thalassemia eventually present in the partner.
KLF1 gene mutations have been associated with many different phenotypes both in humans and mice, including hereditary persistence of fetal hemoglobin (HPFH), congenital dyserythropoietic anemia, In (Lu) blood group phenotype.6-11 The increased HBG expression determined by mutations of KLF1 prompted us to explore the possibility that the HBD expression and the consequent HbA2 output could also be increased by defects of KLF1. To test this hypothesis, we sequenced KLF1 in a large group of subjects with borderline HbA2 and normal MCV and MCH, and we identified several KLF1 mutations in a consistent proportion of these subjects.
Methods
We have studied 145 subjects with borderline HbA2, defined as HbA2 values between 3.3% and 4.1%, and normal or slightly reduced MCV and MCH. Borderline HbA2 values have been confirmed in all subjects in 3 repeated determinations (twice in the same blood sample at first examination, the third in a second sample after 1-3 years). In these subjects, we had previously excluded the presence of mutations known to be associated with borderline HbA2, including HBB promoter mutations [−101 (HBB c.-151 C → T); −92 (HBB c.-142 C → T)], triplicated α-globin genes, and hemoglobin variants.1 The aforementioned hemoglobin gene variants were excluded by appropriate methods (ie, HBB sequence from c.-720 to +137, HBA1 and HBA2 genotyping, and hemoglobin high performance liquid chromatography). We also sequenced the HBD gene, which was found normal from c.-580 to +70 in all but one subject (“DNA analysis”). Eighty normal subjects were used as controls.
The study has been approved by the University of Cagliari Institutional Review Board, and the patients signed the informed consent in accordance with the Declaration of Helsinki.
Venous peripheral blood was used for hematologic, hemoglobin, DNA, and expression studies
Complete whole blood cell count was obtained in all subjects, by electronic cell counters (Gen-S and LH750 Hematology Analyzer, Beckman Coulter). Types and amounts of hemoglobin were determined by high performance liquid chromatography (Bio-Rad Variant II analyzer, Bio-Rad). Two-level calibration of the instrument and sample analysis were carried out according to the manufacturer's recommendations. In some subjects, globin chain synthesis analysis was carried out as previously reported.12
Genomic DNA was obtained with standard methods. The KLF1 gene was sequenced using previously described primers.6 The common single nucleotide polymorphism C → T at position −158 of the HBG2 promoter (XmnI site; rs7482144) was detected by direct digestion of polymerase chain reaction amplified DNA with XmnI restriction enzyme.13 Genotyping of individual single nucleotide polymorphisms in the HBS1L-MYB (rs9399137) and BCL11A (rs 11886868)14,15 loci was performed using TaqMan genotyping assay (Applied Biosystems).
The 2-step liquid erythroid cultures were obtained from peripheral blood with the procedure developed by Fibach et al.16 Real-time RT-PCR quantification of mRNA expression was carried out using TaqMan RNA Assay kits according to the manufacturer's protocol (Applied Biosystems).
The amounts of mRNA relative to the endogenous 18S RNA were calculated on day 9 and day 11 of the second phase of liquid culture growth using the comparative cycle threshold (Ct) method (2−ΔΔCt).17 The experiments were carried out in triplicate.
Results
DNA analysis
Fifty-two of 145 (35.9%) subjects with borderline HbA2 had heterozygous mutations in the KLF1 gene. Among the KLF1 mutations, the most common was the non-sense KLF1 p.Ser270X mutation, found in 42 subjects (80.8%). Two subjects had the p.Thr280_His283del mutation, 4 the p.Arg319GlufsX34 frameshift mutation, 1 the p.Leu326Arg, 2 the Thr327Ser, and 1 the p.Lys332Gln missense mutations. Two mutations (p.Ser270X and Thr280_His283del) lie in exon 2 and 4 (p.Arg319GlufsX34, p.Leu326Arg, p.Thr327Ser, and p.Lys332Gln) in exon 3. All mutations, except Thr280_His283del, which has been found in 2 unrelated Filipino subjects, have been identified in Sardinian persons. Two mutations (Thr280_His283del and Thr327Ser) have never been described before. Complete sequencing of the KLF1 gene did not reveal mutations in the remaining 93 subjects with borderline HbA2.
In all families of subjects with mutated KLF1 available for the study, we confirmed the presence of the proband's mutation (20 families with p.Ser270X, 1 with p.Arg319GlufsX34, 1 with p. T280_H283del, 1 with p.Leu326Arg, and 1 with p.Thr327Ser) in 1 of the parents and/or in siblings with the same phenotype (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In one family, both parents had the KLF1 p.Ser270X mutation. It is interesting to note that in this family 2 spontaneous and otherwise unexplained early pregnancy interruptions had occurred. None of the 80 normal HbA2 controls had the KLF1 mutations found in the subjects with borderline HbA2.
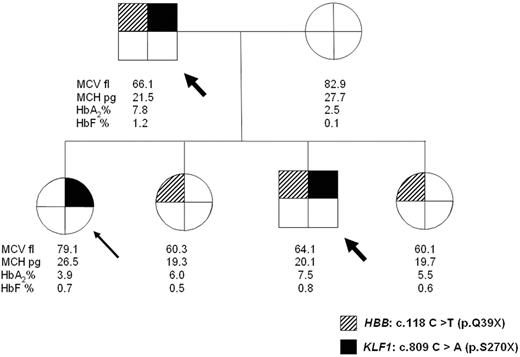
In 3 families (1 is reported in Figure 1 and 2 and in supplemental Table 1), we also detected the presence of the codon 39 β-thalassemia non-sense mutation (HBB c.118 C → T p.Gln39X). Overall, 5 subjects were double heterozygotes for β° 39 non-sense mutation and KLF1 mutations. One of the parents of a subject with KLF1 p.Ser270X mutation was also a δ-thalassemia carrier (HBD c.82 G → T p.Ala28ser).18
Pedigree of a family showing independent segregation of heterozygous β-thalassemia and KLF1 mutation. The arrow indicates the proband: head-arrows indicate double heterozygotes for HBB and KLF1 mutations who have outlier levels of HbA2.
Pedigree of a family showing independent segregation of heterozygous β-thalassemia and KLF1 mutation. The arrow indicates the proband: head-arrows indicate double heterozygotes for HBB and KLF1 mutations who have outlier levels of HbA2.
Hematologic analysis
HbA2 and hemoglobin F (HbF) of the probands with KLF1 mutations are reported in Table 1. Other hematologic data and globin chain synthesis analysis are available in supplemental Table 1. Mean HbA2 value was 3.6% ± 0.2% in subjects with the KLF1 p.Ser270X mutation and 3.5% ± 0.2% in those with the frameshift mutation. In our population, mean HbA2 in normal subjects is 2.8% ± 0.2% (range, 2.1%-3.1%) and in β-thalassemia carriers 5.4% ± 0.4% (range, 4.5%-6.2%). HbF was quite variable, ranging from normal (0.2%) to moderately increased levels (5.8%). No correlation was found in these subjects between HbF levels and the known HbF-associated polymorphism XmnI in the HBG1 gene, rs9399137 in the HBS1L-MYB intergenic region, and rs11886868 in the BCL11A gene.14,15 MCV and MCH were normal in all subjects, except those who coinherited the −3.7-kb α+ thalassemia deletion (supplemental Table 1).
HbA2 and HbF in the probands with different KLF1 mutations
| Mutation . | p.Ser270X . | p.Thr280_His283del . | p.Arg319GlufsX34 . | p.Leu326Arg . | p.Thr327Ser . | p.Lys332Gln . |
|---|---|---|---|---|---|---|
| No. of subjects | 42 | 2 | 4 | 1 | 2 | 1 |
| HbA2, % | 3.6 ± 0.2 (3.3-4.1) | 3.7-3.7 | 3.5 ± 0.2 (3.3-3.7) | 4.0 | 3.4-3.4 | 3.8 |
| HbF, % | 2.1 ± 1.2 (0.2-5.8) | 1.2-1.3 | 2.2 ± 0.8 (1.2-3.2) | 0.70 | 1.3-2.3 | 0.90 |
| Mutation . | p.Ser270X . | p.Thr280_His283del . | p.Arg319GlufsX34 . | p.Leu326Arg . | p.Thr327Ser . | p.Lys332Gln . |
|---|---|---|---|---|---|---|
| No. of subjects | 42 | 2 | 4 | 1 | 2 | 1 |
| HbA2, % | 3.6 ± 0.2 (3.3-4.1) | 3.7-3.7 | 3.5 ± 0.2 (3.3-3.7) | 4.0 | 3.4-3.4 | 3.8 |
| HbF, % | 2.1 ± 1.2 (0.2-5.8) | 1.2-1.3 | 2.2 ± 0.8 (1.2-3.2) | 0.70 | 1.3-2.3 | 0.90 |
Data are mean ± SD (range).
α/β-globin chain synthesis ratios were in the normal range (0.96 ± 0.04) in 11 evaluated subjects with normal α-globin genotype and in the α-thalassemia carrier range (< 0.8) in 5 subjects with coinherited α-thalassemia (supplemental Table 1).
Similar hematologic data were found in the parents or relatives (belonging to 24 available families) with different KLF1 mutations, except 2 who had HbA2 levels in the low normal range, because of associated iron deficiency anemia (HbA2 = 2.1%) or coinherited δ-thalassemia (HbA2 = 2.2%; supplemental Figure 1). Five subjects, double heterozygotes for KLF1 and HBB thalassemia mutations, had quite high HbA2 levels (7.0% to 7.8%), outside of the β-thalassemia carrier range.
Blood group phenotyping randomly performed in 4 subjects with p.Ser270X, one with the p.Arg319GlufsX34, one with the p.Leu326Arg, and one with p.Lys332Gln, showed in all the In(Lu) blood group.
Expression studies
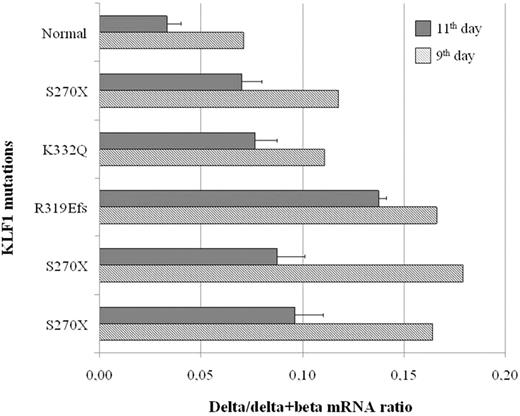
Time point analysis of gene expression by quantitative reverse-transcribed polymerase chain reaction at the 9th day and 11th day of erythroid culture in 5 different carriers with KLF1 gene defects (3 with S270X, one with R319EfsX34, and one with K332Q mutation) showed an overall increase in the expression of the HBD globin gene relative to the HBB globin gene, from the normal mRNA value of 3% to 7% up to the highest value of 18% (Figure 2). The δ/δ + β mRNA globin chain ratios were globally reduced at the 11th day of culture, suggesting that erythroid differentiation is accompanied by a partial δ- to β-globin switch. Hence, with advancing maturation, the ratios of δ/δ + β mRNA expression tend toward the lower values observed at the protein level. The increase of the δ-globin mRNA among the 3 patients carrying the same S270X mutation was variable.
Expression of the δ-globin relative to the expression of the sum of δ- and β-globin genes in erythroid progenitors at 9th and 11th days of liquid culture. All subjects, except the normal control, are heterozygous mutants for the indicated mutations of the KLF1 gene.
Expression of the δ-globin relative to the expression of the sum of δ- and β-globin genes in erythroid progenitors at 9th and 11th days of liquid culture. All subjects, except the normal control, are heterozygous mutants for the indicated mutations of the KLF1 gene.
Discussion
In this study, we report the molecular and hematologic features of a group of persons with borderline HbA2 levels. In 35.9% of the subjects, we found mutations of the KLF1 gene, the most common being the p.Ser270X mutation previously reported associated with increased HbF.7 The non-sense S270X and the frameshift R319EfsX34 mutations will ablate the DNA binding domain and hence result in haploinsufficiency of this key erythroid transcription regulator. Among the remaining mutations, T280_H283del will delete cysteine 281, which is essential for Zn coordination, and it is thus predicted to eliminate the Zn finger structure and binding to DNA L326R, T327S, and K332Q missense mutations affects amino acids adjacent to the residues predicted to directly contact DNA, and they might interfere with the binding of Klf1 to DNA. Alternatively, these mutations could impair the interaction of Klf1 with Brg1 and Baf156, previously mapped to the DNA binding domain,19 thus altering the chromatin remodeling ability of Klf1. Globin gene expression analysis in erythroid cultures supported the increased HbA2 phenotype in absence of β-thalassemia mutations. Our results show that the increase of HbA2, associated with KLF1 mutations, is produced at the transcriptional level. In both normal subjects and patients, the δ/δ + β-globin mRNA ratios are much higher than the corresponding HbA2/HbA ratio found at the protein level in the peripheral blood. However, with the progress of differentiation from the 9th day to the 11th day of erythroid culture, the ratios of δ/δ + β expression decrease, suggesting that in the latest stages of maturation the higher output of β-globin mRNA will correct the δ/β imbalance, leading to the slight final increase of HbA2 observed in the mature red blood cells. It is probable that the borderline HbA2 levels found in peripheral blood cells in KLF1 mutants are caused by a delay in the transcriptional switch from the HBD to the HBB gene. Delayed switch should recognize the same mechanism that leads to HPFH in some KLF1 mutant persons and is indirectly supported by the delayed γ- to β-globin switch experimentally found in crosses between heterozygous Klf1 knockout mice and transgenic mice, carrying the full human β-globin gene cluster.20,21 By analogy with the mice experiments, the delayed switch occurring in humans could be explained by the competition of the different globin genes for the alternative interaction with the LCR. Because the δ-globin promoter has a highly degenerated CACCC box and does not have any other recognizable Klf1 binding site,22,23 it is unlikely that the increased δ-globin transcription results from preferential binding of Klf1 to the δ-globin promoter at reduced Klf1 concentration, as proposed for the increased HbF levels found in the Klf1-related HPFH.24
At the phenotypic level, there are no differences among subjects with different KLF1 mutations. In particular, the absence of anemia in this large series of subjects confirms that one functional KLF1 allele is sufficient to sustain normal human erythropoiesis. This is in agreement with previously reported studies,6,7,9 but in contrast with the family described by Arnaud et al8 in which the presence of KLF1 haploinsufficiency caused a severe congenital dyserythropoietic anemia. The reasons for this discrepancy could be the presence in the congenital dyserythropoietic anemia patients of undetected mutations in other genes or the variable effects of different KLF1 mutations on erythropoiesis.
Two of the parents with KLF1 mutations and associated iron deficiency anemia or coinherited δ-thalassemia have HbA2 in the low normal range. Both conditions are well-known causes of reduced HbA2 levels.4,5 Thus, the normal values of HbA2 are the resultant of factors acting in opposite directions and are only apparent exceptions to the increased HbA2 levels produced by KLF1 mutations. In our cohort of KLF1 heterozygous subjects, known additional mutations previously reported associated with borderline HbA2, such as triplicated α-globin genes and/or HBB promoter deletions, were excluded.
In our subjects with borderline HbA2 and KLF1 mutations, HbF varies from normal to moderately increased levels and does not correlate with the presence of the XmnI polymorphism at HBG1 or SNPss influencing fetal hemoglobin levels at HBS1L-MYB and BCL11A.14,15 Differently from the Maltese HPFH family,7 Sardinian KLF1 heterozygous mutants, even when bearing a comparable non-sense mutation (S270X vs K288X), only rarely cause significant HPFH phenotypes. The discrepancy might be explained by other unknown genetic factors involved in the control of HbF levels.
Interaction of KLF1 mutations with β-thalassemia only results in very high HbA2 levels without any other clinical or hematologic effect. This information is relevant for genetic counseling in couples carrying KLF1 and HBB mutations. The very high levels of HbA2 in these double heterozygotes are probably the result of the cumulative effect of the heterozygous β-thalassemia and KLF1 mutations.
It is interesting to note that the family, in which both parents were heterozygotes for the KLF1 p.Ser270X mutation, had 4 live children and 2 spontaneous early abortions. Hence, the proportion of observed abortions is in good agreement with the mendelian inheritance of a recessive lethal trait, as observed in the Klf1 knockout mice.10 Although this hypothesis is not testable, it is possible that the early pregnancy interruptions were the result of the homozygosity for the KLF1 p.Ser270X mutation, associated with the total absence of KLF1. This is not in contrast with the simple HPFH phenotype of compound heterozygotes for KLF1 S270X non-sense and K332Q missense mutations9 because the K332Q mutant protein has a reduced but not absent expression with a residual activity.
All KLF1 mutations tested in this study were associated with the In(Lu) blood group. The same blood group phenotype has been reported by Singleton et al6 in subjects with 9 different KLF1 mutations. Thus, the In(Lu) phenotype appears to be a constant feature of KLF1 mutations, suggesting that the amount of KLF1 necessary to regulate Lutheran expression is highly limiting.
The “gray zone” of borderline HbA2 is not an uncommon problem in populations with high frequency of β-thalassemia.1,2,25 Although subjects with borderline HbA2 and reduced MCV/MCH are easily identified because of the presence of well-known HBB and HBD mutations, the presence of HbA2 borderline without hematologic changes (ie, normal MCV/MCH) requires a complex laboratory workup, including the cumbersome and not easily available in vitro globin chain synthesis analysis, to exclude the presence of β-thalassemia mutations. Exclusion of all β-thalassemia carrier states, including carriers of silent β-thalassemia mutations, is essential despite the fact that the interaction of silent HBB mutations with classic β-thalassemia usually results in a mild clinical phenotype.
This is the first report showing that mutations of KLF1 cause activation of the HBD gene and result in increased HbA2 levels, adding a new function to the KLF1 gene. The stimulation of HBD is probably an indirect effect mediated by the impaired looping of the LCR with the HBB gene in favor of the competing HBD gene. A model can be envisaged by which in some subjects, for at present unknown mechanisms, heterozygous KLF1 defects cause preferential interaction of the LCR with the HBD gene, whereas a more pronounced decrease of KLF1 concentration, as observed in compound heterozygotes for KLF1 mutations,9 determines preferential looping with the HBG genes and HPFH. In Sardinians, KLF1 mutations explain more than one-third of the borderline HbA2 phenotype, indicating that this trait is genetically heterogeneous. Moreover, in Sardinia, a common mutation (KLF1 p.Ser270X) accounts for 80.8% of the genetic variability of KLF1 mutations, suggesting the existence of a founder effect similar to that observed in the HBB gene, where the common codon 39 non-sense mutation is responsible for 96% of the HBB mutations.
The identification of KLF1 mutations in subjects with borderline HbA2 and the absence of clinically significant phenotypes in association with the classic HBB mutation should facilitate carrier detection and genetic counseling, significantly contributing to the thalassemia screening programs aimed at identification of at risk couples.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Daniela Loi and M. Franca Desogus for technical assistance, Maria Bonaria Tronci for blood group phenotyping, and Laura Placido for editorial assistance.
The study was supported by the Regione Autonoma della Sardegna (LR 11, 1990).
Authorship
Contribution: L.P., S.S., P.M., F.R.D., and L.M. performed research, analyzed data, and contribute to writing the paper; M.C.S. and S.B. collected clinical data; A.C. analyzed data and reviewed the manuscript; and R.G. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Renzo Galanello, Ospedale Regionale Microcitemie, Via Jenner s/n, 09121 Cagliari, Italy; e-mail: renzo.galanello@mcweb.unica.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal