Abstract
Cell interactions with matrices via specific receptors control many functions, with chemistry, physics, and membrane elasticity as fundamental elements of the processes involved. Little is known about how biochemical and biophysical processes integrate to generate force and, ultimately, to regulate hemopoiesis into the bone marrow-matrix environment. To address this hypothesis, in this work we focus on the regulation of MK development by type I collagen. By atomic force microscopy analysis, we demonstrate that the tensile strength of fibrils in type I collagen structure is a fundamental requirement to regulate cytoskeleton contractility of human MKs through the activation of integrin-α2β1–dependent Rho-ROCK pathway and MLC-2 phosphorylation. Most importantly, this mechanism seemed to mediate MK migration, fibronectin assembly, and platelet formation. On the contrary, a decrease in mechanical tension caused by N-acetylation of lysine side chains in type I collagen completely reverted these processes by preventing fibrillogenesis.
Introduction
Collagen fibrils are the main architectural element in several tissues and bones, and the self-assembly processes involved in collagen fibrillogenesis are of enormous importance to matrix pathology and proper cell development.1 Type I collagen plays a critical role in the bone marrow niche environment, preventing platelet formation by MKs through the activation of integrin-α2β1 and the Rho-ROCK pathway.2-4 Changes in mechanical properties are tightly coupled in vivo to changes in matrix composition, crosslinking, and density.5 Moreover, different signaling pathways, related to the transduction of mechanical signals into biochemical responses, regulate cell behavior.6 However, the question on how biochemical and biophysical processes integrate to generate force and, ultimately, to regulate MK fate, is still open. Therefore, our purpose for the present work was to focus on MK function related to type I collagen organization and biomechanics.
Methods
Type I collagen from bovine tendon was modified as previously described9 and as reported in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Antibodies and solid-phase binding assay are described in supplemental Methods. MKs were differentiated from human cord blood progenitors as previously described as well as proplatelet and spreading assay, electron and immunofluorescence microscopy, second harmonic generation, and Western blotting analysis.4,7,8 Human cord blood was collected on informed consent of the parents, with approval from the ethical committee of the Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo Foundation in accordance with the principles of the Declaration of Helsinki. Binding of cells to different collagen preparations was evaluated with 25 × 103 cells seeded for 1 hour in Stem Span medium (Stem Cell Technologies) in a 96-well plate precoated with 5 μg of each collagen preparation. Cells were preincubated with increasing concentrations of anti-α2 (clone P1E6) and anti-GPVI (Fab 9012.2) antibodies. Adhering cells were washed after 1 hour with PBS and counted by phase-contrast microscopy. Cell adhesion values are expressed relative to control (absence of inhibitor). In some experiments, cells were seeded on 12-mm glass coverslips coated with type I collagen, allowed to adhere for 2 hours in the presence of 20 μg/mL of antibodies, and then stained for actin and CD41. For cell migration experiments, 25 × 103 cells were seeded in Stem Span medium in the upper well of transwell migration chambers (8 μm; Millipore). Migration toward 100 ng/mL of SDF-1 (PeproTech) and through filters coated with different collagen preparations (5 μg/well) or BSA was measured after 16 hours. Migrated cells were cytospun, fixed, stained with anti-CD41 antibody, and counted. Atomic force microscopy (AFM) force measurements were performed on a MFP-3D bio-atomic force microscope (Asylum Research) using triangular silicon nitride cantilever probes with nominal tip radius of 42 nm and a nominal spring constant approximately 20 pN/m, as determined from thermal calibration. Young moduli of collagens and cells were derived from force versus indentation profile and extracted using a Hertz model10 with the following formula:
with a tip opening angle of 36°, a tip Poisson ratio (V) of 0.25, a sample Poisson ratio (V) set to 0.5, and a tip modulus (E) of 290 GPa. E was determined by a least squared fit using Igor Pro Version 6.20 data analysis software (WaveMetrics). Cells were fixed with paraformaldehyde 3%, and force curves were taken in spread cells with similar dimension and height. Cells were indented at a site midway between the nucleus and cell margin, and at least 10 force curves were acquired per cell. t test was used to analyze data, with a significant difference set at P < .05. Data are presented as mean ± SD.
Results and discussion
The relationships between MK and bone marrow components are key factors in platelet formation. Importantly, changes in mechanical properties are pervasive in vivo during pathologic processes, such as fibrosis or tumorigenesis.11 Based on insight from the literature as well as from our prior studies,7 we aimed to interrogate the synergy between biochemical and biophysical processes related to MK development within the bone marrow environment. To pursue this goal, we used a chemically modified type I collagen by N-acetylation of lysine side chains, which blocks the formation of banded fibrils in neutral conditions (Figure 1A).9
Characterization of collagen nano-mechanical properties. (A) Coomassie staining of type I collagen and N-acetylated collagen. A total of 2 μg of proteins was loaded on 6% gel of polyacrylamide; > 85% of lysine residue were modified in the native protein determining a different migration in electrophoresis. (B) In vitro analysis of collagens structure. (i) Atomic force microscopy images of 3 μm2 of dehydrated collagen coating. (ii) Second harmonic generation images of collagen supramolecular structure were acquired through a 63× (1.2 NA) objective on a Leica DMIRE2 microscope with a TCS SP2 scanner. Analysis was performed with the Leica confocal software and ImageJ (NIH). Scale bar represents 10 μm. (C) Atomic force microscopy images of collagens in fluid (PBS). A total of 5 μm2 fields were analyzed in contact mode using an MFP-3D Bio-atomic force microscope (Asylum Research). (D) Roles of α2-integrin and GPVI on MK adhesion and spreading on type I collagen. Cord blood-derived MKs were seeded for 2 hours on type I collagen in the presence of 20 μg/mL of anti-α2 (clone P1E6) and Fab GPVI (clone 9012.2) antibodies, fixed, and then stained for actin (red) and CD41 (green). Images were acquired through an Olympus BX51 using a 20×/0.5 UPlan objective. Scale bar represents 50 μm. (E) Effect of chemical modification of type I collagen on α2 integrin and GPVI engagement. MKs were seeded on type I collagen and N-acetylated type I collagen for 1 hour, and cell adhesion was evaluated in the presence of increasing concentrations of anti-α2 and GPVI antibodies. Cell adhesion values are expressed relative to control (absence of inhibitor).
Characterization of collagen nano-mechanical properties. (A) Coomassie staining of type I collagen and N-acetylated collagen. A total of 2 μg of proteins was loaded on 6% gel of polyacrylamide; > 85% of lysine residue were modified in the native protein determining a different migration in electrophoresis. (B) In vitro analysis of collagens structure. (i) Atomic force microscopy images of 3 μm2 of dehydrated collagen coating. (ii) Second harmonic generation images of collagen supramolecular structure were acquired through a 63× (1.2 NA) objective on a Leica DMIRE2 microscope with a TCS SP2 scanner. Analysis was performed with the Leica confocal software and ImageJ (NIH). Scale bar represents 10 μm. (C) Atomic force microscopy images of collagens in fluid (PBS). A total of 5 μm2 fields were analyzed in contact mode using an MFP-3D Bio-atomic force microscope (Asylum Research). (D) Roles of α2-integrin and GPVI on MK adhesion and spreading on type I collagen. Cord blood-derived MKs were seeded for 2 hours on type I collagen in the presence of 20 μg/mL of anti-α2 (clone P1E6) and Fab GPVI (clone 9012.2) antibodies, fixed, and then stained for actin (red) and CD41 (green). Images were acquired through an Olympus BX51 using a 20×/0.5 UPlan objective. Scale bar represents 50 μm. (E) Effect of chemical modification of type I collagen on α2 integrin and GPVI engagement. MKs were seeded on type I collagen and N-acetylated type I collagen for 1 hour, and cell adhesion was evaluated in the presence of increasing concentrations of anti-α2 and GPVI antibodies. Cell adhesion values are expressed relative to control (absence of inhibitor).
AFM and second harmonic generation imaging, a noninvasive optical method that exploits nonlinear light scattering from fibrillar collagen, showed that dehydrated native type I collagen formed a layer of banded fibrils with D-periodicity, whereas the N-acetylated type I collagen was characterized by microfibrillar structure in the early stage of aggregation (Figure 1B). Consistently, AFM nano-topography in fluids showed that, starting from 2 hours of incubation, coatings of native type I collagen, and not N-acetylated collagen, were characterized by the presence of fibrils, on a layer of unorganized material, homogeneously distributed with a length ranging from 1 μm up to several microns (Figure 1C). Nano-indentation studies with AFM demonstrated that type I collagen had a heterogeneous distribution of elastic moduli that derived from points picked on fibrils and points picked on the underlying material. In particular, fibrils in fluids presented an elasticity > 0.15 GPa, whereas material in early stage of self-aggregation had values in the order of 5-75 MPa. On the contrary, homogeneous values in the order of < 75 MPa were found during the analysis of N-acetylated collagen Young moduli.
MKs express 2 main receptors for collagen: integrin-α2β1 and glycoprotein VI.12 Using function-blocking antibodies, a prominent role for integrin-α2β1, and not GPVI, emerged in the early phase of MK adhesion on collagen, as demonstrated by a significant reduction in cell adhesion and spreading (Figure 1D). Importantly, binding to collagen receptors was not affected by the lack of self-aggregation of N-acetylated type I collagen because no significant differences were found in MK adhesion in the presence of increasing concentrations of anti-α2 or anti-GPVI antibodies (Figure 1E).
To analyze whether alterations in type I collagen nano-mechanical properties could affect MK function, human MKs were allowed to adhere on native and N-acetylated collagens for 2 and 16 hours. After 2 hours, ∼ 20% of cells were spread on both collagens (Figure 2B); however, prolonging incubation to 16 hours, MKs adherent on N-acetylated collagen started to lose cell contractility, returned round and extended proplatelets as control MKs cultured in suspension (Figure 2A-C). On the contrary, on the native type I collagen, MKs maintained cell contractility, remained spread over the entire time of incubation, and did not extend proplatelets (Figure 2A-C). Overall, these data demonstrated, for the first time, that decreasing type I collagen stiffness along with a nonorganized fibrillar structure completely reverted type I collagen-dependent functions of human MKs. Consistently, only stiffer native type I collagen, and not the N-acetylated one, was able to refrain MK migration (Figure 2D). These data demonstrated that type I collagen fibrils provide structural cues for MK adhesion and migration.13 To further demonstrate that MKs anchored more strongly to stiffer type I collagen than to the softer N-acetylated one, we took advantage of our recent data on fibronectin (FN) as a mediator of MK adhesion to type I collagen.8 Binding of FN to native and N-acetylated collagen was preserved as verified in solid-phase assays (supplemental Figure 1). Nevertheless, we demonstrated that only adhesion on fibrillar type I collagen led to the assembly of FITC-labeled FN in insoluble matrix on MK surface (Figure 2E) as well as to stable relocation to the plasma membrane of endogenous FN (Figure 2F). Moreover, to demonstrate the feedback of local matrix stiffness on the MK state, Young moduli values of fixed spread MKs on native and N-acetylated type I collagens were measured. Heterogeneity in native type I collagen stiffness was reflected on membrane of adhering MKs as values of elasticity varied from 10.35-183.62 KPa (mean, 41.86 KPa), whereas more homogeneous distribution of values, from 12.78-59.08 KPa (mean, 24.71 KPa), were detected in MKs adherent on N-acetylated type I collagen (Figure 2G). Different kinases and GTPases regulate MK function in response to collagen, but their activation is not completely unraveled. An α2β1-dependent pathway involving Rho-ROCK-MLC has been demonstrated to negatively regulate proplatelet release,2,3 whereas Src and Syk phosphorylation, downstream GPVI activation, were shown to be involved in α2bβ3-dependent migration of MK on fibronectin.14 In our experiments, levels of guanosine triphosphate-bound RhoA resulted comparable on MK adhesion to either type I collagen preparations for 16 hours demonstrating that engagement of integrin-α2β1had occurred. However, an evident reduction of myosin light chain (MLC-2) phosphorylation was observed on MK adhesion on N-acetylated type I collagen with respect to the native one (Figure 2H). Interestingly, engagement of GPVI was shown by Src and Syk phosphorylation, and no differences were observed on adhesion on either collagen preparations (Figure 2H). Overall, our results demonstrated that engagement of integrin-α2β1 is not sufficient to achieve the level of MLC-2 phosphorylation necessary to the inhibition of proplatelet normally exerted by fibrillar type I collagen. Further, GPVI activation in MKs seemed not to be affected by collagen structure and nano-mechanics. Therefore, other, yet unknown, receptors may cooperate with α2β1 to regulate MK function on type I collagen in a structure-dependent manner.
Effects of collagen nano-mechanical properties on MK behavior. (Ai-ii) Phase-contrast images of MKs seeded for 16 hours in adhesion on collagens. Scale bar represents 100 μm. (iii-iv) Stress fiber formation was analyzed after actin (tetramethylrhodamine isothiocyanate [TRITC]-phalloidin, red) staining in immunofluorescence, whereas proplatelet formation was evaluated using an anti–α-tubulin (green) antibody. Nuclei were counterstained with Hoechst 33288 (blue). Images were acquired using a 63×/1.25 UPlanF1 oil-immersion objective. Scale bar represents 20 μm. (v-vi) Scanning electron micrographs of spread MKs on type I collagen and a platelet-releasing MK on N-acetylated type I collagen. Images were acquired with a Cambridge Stereoscan 440 microscope (Leica Microsystems) at 17.5 kV and a magnification of 2000×. Scale bar represents 4 μm. (B) Evaluation of cell spreading after 2 and 16 hours of adhesion. Cells were fixed and stained with TRITC-phallodin. Cells exhibiting stress fibers were counted and presented as mean ± SD of 3 different experiments. P < .05. SHG indicates second harmonic generation. (C) Proplatelet formation was analyzed after 16-hour adhesion on different collagens or in MKs maintained in suspension (none). P < .05. (D) Migration of MKs in transwell plate after 16 hours of incubation; 8-μm polycarbonate pore filters were coated with native and N-acetylated collagen or with BSA (none), and cells were counted after CD41 staining in immunofluorescence. Results are reported as percentage of migrated cells relative to control of 3 different experiments ± SD. (Ei-ii) Assembly of FN under static conditions. MKs were incubated with 25 μg/mL of FITC-labeled FN and then seeded on different matrices. FN fibrillogenesis was then visualized in immunofluorescence using a 63×/1.25 UPlanF1 oil-immersion objective. Hoechst 33288 was used for nuclei staining (blue). Scale bar represents 10 μm. (eiii-iv) Alternatively, cells were removed using deoxycholic acid (DOC) and the underlying matrix stained with an anti-FN antibody (red) and visualized in immunofluorescence, using a 40×/0.75 oil-immersion objective. Scale bar represents 20 μm. (F) Relocation of endogenous FN in MKs after 2- (left panels) and 16-hour (right panels) adhesion on collagens. Cells were stained for FN (red), α-tubulin (green), and Hoechst for nuclei (blue) using a 63×/1.25 UPlan oil-immersion objective. Scale bar represents 10 μm. (G) Distribution of Young modulus values of MKs in adhesion on different collagen samples. Three different experiments were performed, and at least 5 cells for sample were analyzed. P < .05. (H) Analysis of α2β1 and GPVI-dependent pathways in adhering MKs. Rho guanosine triphosphate pull-down experiments and immunoblot analysis of endogenous MLC2, Syk, and Src phosphorylation levels in MKs adhering to native and N-acetylated type I collagen after 16-hour incubation, representative of 3 different experiments. Actin, tubulin, and nonmuscle myosin IIA were revealed to demonstrate equal protein loading.xxxxx.
Effects of collagen nano-mechanical properties on MK behavior. (Ai-ii) Phase-contrast images of MKs seeded for 16 hours in adhesion on collagens. Scale bar represents 100 μm. (iii-iv) Stress fiber formation was analyzed after actin (tetramethylrhodamine isothiocyanate [TRITC]-phalloidin, red) staining in immunofluorescence, whereas proplatelet formation was evaluated using an anti–α-tubulin (green) antibody. Nuclei were counterstained with Hoechst 33288 (blue). Images were acquired using a 63×/1.25 UPlanF1 oil-immersion objective. Scale bar represents 20 μm. (v-vi) Scanning electron micrographs of spread MKs on type I collagen and a platelet-releasing MK on N-acetylated type I collagen. Images were acquired with a Cambridge Stereoscan 440 microscope (Leica Microsystems) at 17.5 kV and a magnification of 2000×. Scale bar represents 4 μm. (B) Evaluation of cell spreading after 2 and 16 hours of adhesion. Cells were fixed and stained with TRITC-phallodin. Cells exhibiting stress fibers were counted and presented as mean ± SD of 3 different experiments. P < .05. SHG indicates second harmonic generation. (C) Proplatelet formation was analyzed after 16-hour adhesion on different collagens or in MKs maintained in suspension (none). P < .05. (D) Migration of MKs in transwell plate after 16 hours of incubation; 8-μm polycarbonate pore filters were coated with native and N-acetylated collagen or with BSA (none), and cells were counted after CD41 staining in immunofluorescence. Results are reported as percentage of migrated cells relative to control of 3 different experiments ± SD. (Ei-ii) Assembly of FN under static conditions. MKs were incubated with 25 μg/mL of FITC-labeled FN and then seeded on different matrices. FN fibrillogenesis was then visualized in immunofluorescence using a 63×/1.25 UPlanF1 oil-immersion objective. Hoechst 33288 was used for nuclei staining (blue). Scale bar represents 10 μm. (eiii-iv) Alternatively, cells were removed using deoxycholic acid (DOC) and the underlying matrix stained with an anti-FN antibody (red) and visualized in immunofluorescence, using a 40×/0.75 oil-immersion objective. Scale bar represents 20 μm. (F) Relocation of endogenous FN in MKs after 2- (left panels) and 16-hour (right panels) adhesion on collagens. Cells were stained for FN (red), α-tubulin (green), and Hoechst for nuclei (blue) using a 63×/1.25 UPlan oil-immersion objective. Scale bar represents 10 μm. (G) Distribution of Young modulus values of MKs in adhesion on different collagen samples. Three different experiments were performed, and at least 5 cells for sample were analyzed. P < .05. (H) Analysis of α2β1 and GPVI-dependent pathways in adhering MKs. Rho guanosine triphosphate pull-down experiments and immunoblot analysis of endogenous MLC2, Syk, and Src phosphorylation levels in MKs adhering to native and N-acetylated type I collagen after 16-hour incubation, representative of 3 different experiments. Actin, tubulin, and nonmuscle myosin IIA were revealed to demonstrate equal protein loading.xxxxx.
In conclusion, our results provide novel insight into the role of nano-mechanics of a bone marrow matrix-environment related to MK biology, leading to new concepts in understanding how MKs sense the stiffness and spatial patterning of their environment to modulate their function and fate. The identification of mechano-sensing receptors could be a novel approach to study MK behavior that deserves further investigations. Modification of matrix structure within bone marrow in a diseased state, such as myelofibrosis, may represent an important example of aberrant megakaryopoiesis dependent on alteration of bone marrow matrix nano-mechanics.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Martine Jandrot-Perrus (Inserm, U698, Hôpital Bichat, Paris, France) for the anti-GPVI antibody, Luigi De Marco (CRO, Aviano, Italy) for helpful discussion, Livia Visai (University of Pavia, Pavia, Italy) for the human fibronectin, Cesare Perotti (Istituto di Ricovero e Cura a Carattere Scientifico San Matteo Foundation, Pavia, Italy) for human cord blood supply, and Luca Monti (University of Pavia, Pavia, Italy) for helping with Western blotting.
This work was supported by the Cariplo Foundation (2006.0596/10.8485), Regione Lombardia (Project SAL-45 and ASTIL 16783; SAL-13, Almamater Foundation, Pavia; A.B.), National Institutes of Health (P41 EB002520; Tissue Engineering Resource Center, D.K.), and Tufts University (faculty startup funds, C.S.).
National Institutes of Health
Authorship
Contribution: A.M. performed experiments, analyzed data, and wrote the paper; C.G., I.P., E.S., R.T., and M.R. performed experiments; D.K., M.E.T., and C.S. analyzed data; and A.B. designed experiments, analyzed data, wrote the paper, and provided overall direction.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alessandra Balduini, Biotechnology Laboratories, Department of Biochemistry, Istituto di Ricovero e Cura a Carattere Scientifico San Matteo Foundation, University of Pavia, via Bassi 21, 27100 Pavia, Italy; e-mail: alessandra.balduini@unipv.it.

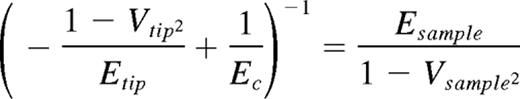

![Figure 2. Effects of collagen nano-mechanical properties on MK behavior. (Ai-ii) Phase-contrast images of MKs seeded for 16 hours in adhesion on collagens. Scale bar represents 100 μm. (iii-iv) Stress fiber formation was analyzed after actin (tetramethylrhodamine isothiocyanate [TRITC]-phalloidin, red) staining in immunofluorescence, whereas proplatelet formation was evaluated using an anti–α-tubulin (green) antibody. Nuclei were counterstained with Hoechst 33288 (blue). Images were acquired using a 63×/1.25 UPlanF1 oil-immersion objective. Scale bar represents 20 μm. (v-vi) Scanning electron micrographs of spread MKs on type I collagen and a platelet-releasing MK on N-acetylated type I collagen. Images were acquired with a Cambridge Stereoscan 440 microscope (Leica Microsystems) at 17.5 kV and a magnification of 2000×. Scale bar represents 4 μm. (B) Evaluation of cell spreading after 2 and 16 hours of adhesion. Cells were fixed and stained with TRITC-phallodin. Cells exhibiting stress fibers were counted and presented as mean ± SD of 3 different experiments. P < .05. SHG indicates second harmonic generation. (C) Proplatelet formation was analyzed after 16-hour adhesion on different collagens or in MKs maintained in suspension (none). P < .05. (D) Migration of MKs in transwell plate after 16 hours of incubation; 8-μm polycarbonate pore filters were coated with native and N-acetylated collagen or with BSA (none), and cells were counted after CD41 staining in immunofluorescence. Results are reported as percentage of migrated cells relative to control of 3 different experiments ± SD. (Ei-ii) Assembly of FN under static conditions. MKs were incubated with 25 μg/mL of FITC-labeled FN and then seeded on different matrices. FN fibrillogenesis was then visualized in immunofluorescence using a 63×/1.25 UPlanF1 oil-immersion objective. Hoechst 33288 was used for nuclei staining (blue). Scale bar represents 10 μm. (eiii-iv) Alternatively, cells were removed using deoxycholic acid (DOC) and the underlying matrix stained with an anti-FN antibody (red) and visualized in immunofluorescence, using a 40×/0.75 oil-immersion objective. Scale bar represents 20 μm. (F) Relocation of endogenous FN in MKs after 2- (left panels) and 16-hour (right panels) adhesion on collagens. Cells were stained for FN (red), α-tubulin (green), and Hoechst for nuclei (blue) using a 63×/1.25 UPlan oil-immersion objective. Scale bar represents 10 μm. (G) Distribution of Young modulus values of MKs in adhesion on different collagen samples. Three different experiments were performed, and at least 5 cells for sample were analyzed. P < .05. (H) Analysis of α2β1 and GPVI-dependent pathways in adhering MKs. Rho guanosine triphosphate pull-down experiments and immunoblot analysis of endogenous MLC2, Syk, and Src phosphorylation levels in MKs adhering to native and N-acetylated type I collagen after 16-hour incubation, representative of 3 different experiments. Actin, tubulin, and nonmuscle myosin IIA were revealed to demonstrate equal protein loading.xxxxx.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/16/10.1182_blood-2011-04-345876/4/m_zh89991178950002.jpeg?Expires=1769081514&Signature=pN639T4JRUlp3P5XtUWKl96JdeEc2FH-JZ3ab8VWSD8hyM51eNB~IFhUSi~YDEXa34B7mq-iKILqHHIE559-4lCm9-sQ45bO7asZexqytxSWEvy4LonJ0qiDnG-Y-qTr~DLAMpJCIKMkblpEhfBa4NMjWU0ZJytHX--8m75zx1JI5omthoEtWBr2cPEHr2Nc9hL2S8pezhm9qw6ou2rRpVWlYmM6nrEpx9nOpdYgMO3qmTSrk5xnej2Vdx7usOLb6F8YzQORXI6NzF~X8ZqeZN7MnH9ZlGF5FV9I83jqOoHY9GPu2Nro8CJciSJrjZqXV2PrusnCGojknh-IoWCd2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal