Abstract
Imatinib has transformed the prognosis and the management of chronic myeloid leukemia (CML) and has probably changed the patterns of mortality rates. We explored this change at each disease severity level (Sokal score) through a flexible statistical modeling of the effect of the year of diagnosis on the excess mortality rate. The study included 691 chronic-phase patients from Nord-Pas-de-Calais French CML registry diagnosed from 1990 to 2007. Imatinib was given to 93% of the patients diagnosed after 2000. Comparing the 1990-1994, 1995-1999, and 2000-2007 periods of diagnosis, the 5-year relative survival improved from 64% to 66% and 88%. The year of diagnosis was associated with a significant reduction of the excess mortality, but only in patients with intermediate to high Sokal scores. In high-risk patients diagnosed in the early 1990s, a peak of excess mortality was observed during the second year of follow-up. That peak decreased progressively over the years of diagnosis until disappearing in patients diagnosed after 2000. This study showed different effects according to Sokal scores of the use of imatinib on mortality in patients with chronic-phase CML and showed that since 2000 the pattern of mortality of high-risk patients became similar to that of intermediate-risk ones.
Introduction
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disease characterized by a t(9;22) translocation that produces the BCR-ABL fusion gene. Allogeneic stem cell transplantation and IFN-α have long been the only therapies that induce cytogenetic responses and improve survival.1 In November 2001, imatinib was approved in Europe as a treatment of chronic-phase CML (CP-CML) resistant or intolerant to IFN. It was later approved as the standard front-line treatment in response to the results of the pivotal International Randomized Interferon versus STI571 (IRIS) clinical trial, in which the authors demonstrated the greater efficacy of imatinib over the combination of IFN-α and cytarabine. However, the IRIS trial did not directly assess a gain in survival, partly because of the high rate of crossing over between the 2 arms of treatment.2
Today, Sokal score, which is determined by clinical and cytologic findings, is one of the most commonly used prognostic factors in CP-CML. Although it was developed > 25 years ago,3 its prognostic value is still reliable. The IRIS study confirmed that patients with a high Sokal score have an enhanced risk of developing accelerated-phase or blastic crisis (AP/BC), even when receiving imatinib.4
The aim of the present article was to investigate the effect of imatinib on the excess mortality from CML, ie, the mortality attributable solely to CML. By using data from a French CML registry, we conducted a retrospective cohort study to examine the change in the dynamics of the excess mortality rate (EMR) according to the year of diagnosis, first in the whole cohort of patients then separately at each level of Sokal score.
Methods
Patients
All the data used for the present study were provided by the Registry of CML of the Nord-Pas de Calais Region of France (an area of 4 million inhabitants subdivided into 2 Départements). From this registry, we first sought the cohort of CML patients diagnosed between January 1, 1990 and December 31, 2007 (794 patients). Each diagnosis was confirmed by the presence of a chromosomal reciprocal t(9;22) translocation or the presence of the BCR-ABL fusion gene through conventional karyotype, PCR, or FISH. Only the following were included in the analyses presented in this paper: patients older than 15 years meeting all the hematologic criteria for diagnosis of CP (< 15% blasts, < 20% basophils, and < 30% blasts plus promyelocytes in the peripheral blood and marrow).2 Thus, 691 patients were included.
The main data sources of this registry were cytogenetic and molecular genetic laboratories because all the cases had to be confirmed by such tests. To improve the completeness of the cohort, we cross-examined other sources of information, including hospital information systems (or claim databases). Most of these patients were monitored in the 8 hematology departments of the Region (referral university hospital of Lille and 7 community hospitals). The geographic bounds (Belgium, the English Channel, and the North Sea) limited the search for care in neighboring French Départements. We assessed the reliability of our data by calculating the age-standardized incidence of CML.
All the medical records were checked in search for the following: patient medical history, hematology test results (including cytogenetic and molecular analyses), Sokal score, treatments (focusing on stem cell transplantation, IFN, and tyrosine kinase inhibitors, including imatinib, dasatinib, and nilotinib), and patient follow-up. The administrative censoring date was January 1, 2008. The study was approved by the national Comité Consultatif sur le Traitement de l'Information en Matière de Recherche dans le Domaine de la Santé. All patients alive at this date were censored; patients only known to be alive at some dates before January 1, 2008, were considered “lost to follow-up” and censored at those dates.
Until 2002, IFN-α was the standard first-line treatment for CP-CML, and allogeneic stem cell transplantation was proposed for patients with low Gratwohl scores5 (ie, in young patients in CP during the first year of the disease), often after a short period of IFN-α therapy. In Nord-Pas de Calais Region, imatinib has been in use since February 2000. Several patients have been included in clinical trials in which imatinib was tested (STI113 trial after resistance or intolerance to IFN-α; IRIS and SPIRIT trials as front-line treatment).
The initial dose of imatinib in CP-CML patients was 400 mg/d, except in patients in the high-dose imatinib arm of SPIRIT trial, that is, STI571 Prospective Randomized Trial, who were given 600 mg/d.6 Within the study period, the second-generation tyrosine kinase inhibitors, nilotinib and dasatinib, were used only after 2005 in clinical trials in patients who did not respond to imatinib.
Statistical modeling of the excess mortality
An excess mortality model7,8 was used to study mortality in CP-CML patients. In this approach, the observed mortality rate of each subject at time t was considered to be the sum of 2 rates: one because of CP-CML, hereafter referred to as λc, the EMR, and another because of other causes, referred to as λE, the expected mortality rate. Thus, λc is the parameter of interest that can be interpreted as the mortality related solely to CP-CML; the survival corresponding to λc is the relative survival. Rate λE can be viewed as the mortality rate of a subject if he or she had no CP-CML. It was obtained from published vital statistics and corresponds to the all-cause mortality of a subject of same age, year of birth, sex, and Département of residency.
To help interpret the results, we remind that, under a low-rate assumption (say < 0.2 deaths per person-year), an annual rate of 0.1 means that patients alive at the beginning of a year have a 10% chance of dying during that year.
Univariate analysis
The age at diagnosis was categorized into 5 classes (15-44, 45-54, 55-64, 65-74, and 75 years and older) and year of diagnosis into 3 classes (1990-1994, 1995-1999, 2000-2007). For each of these modalities and for each Sokal score, the relative survival estimates were calculated by modeling λc, the excess rate. More precisely, the logarithm of λc was modeled as a smoothed parametric function of time chosen among 6 candidate functions according to the Akaike Information Criteria: a cubic regression spline with 2 knots at 1 and 5 years, a cubic regression spline with one knot at 1 year, a cubic polynomial, a quadratic polynomial, a linear polynomial, and a constant function. This strategy yielded smooth and reliable estimates of mortality.8 The crude survival estimates were obtained by the use of the same strategy but setting the expected rate to zero.
Multivariate analysis
In these multivariate analyses, the age at diagnosis and the year of diagnosis were considered as continuous covariates to avoid the loss in information linked with the use of discrete covariates. Because the Sokal score depends on age, we first confirmed, by using the likelihood ratio test (LRT), that no residual age-effect remained after we adjusted on the Sokal score then fitted the following models for each Sokal score:
where λc corresponds to the EMR, t to the time since diagnosis, year to the year of diagnosis, and finally f, g, and h to the regression splines, which allowed a flexible modeling of the EMR.8 These 3 models characterized, respectively, the effect of the year of diagnosis as (1) “no effect,” (2) “proportional (ie, constant over time since diagnosis) and nonlinear,” and (3) “nonproportional and nonlinear.” The choice of the best model was made with the use of the LRT.
Taking advantage of the similarity between the likelihood of our survival model and a well-chosen Poisson likelihood, we obtained the estimates of the model parameters by the use of standard algorithms for Poisson model. We have written our own function in S-Plus language (also available in R language). The whole procedure has been detailed and validated elsewhere.8 Nonproportionality is an important feature of our approach, ie, the effect of the year of diagnosis could differ according to the time since diagnosis. This would be the case if, for example, imatinib reduced mortality only at the beginning of patient follow-up.
Results
Description of the cohort
The main population characteristics are shown in Table 1. The median age at diagnosis was 56 years. The median follow-up duration of the whole cohort was 4 years. At administrative censoring, 389 (56%) patients were alive, 277 (40%) deceased, and 25 (3.6%) lost to follow up. During follow-up, 434 patients (62.8%) received imatinib.
Characteristics of the study cohort of 691 chronic phase-chronic myeloid leukemia patients
| . | Period of diagnosis . | |||
|---|---|---|---|---|
| 1990-1994, n = 153 . | 1995-1999, n = 191 . | 2000-2007, n = 347 . | 1990-2007, n = 691 . | |
| Male sex (n,%) | 83 (54) | 112 (59) | 175 (50) | 370 (54) |
| Median age at diagnosis, y (range) | 55 (17-88) | 56 (16-89) | 57 (16-95) | 56 (16-95) |
| Age classes, y (n, %) | ||||
| 15-44 | 48 (31) | 58 (30) | 85 (24) | 191 (28) |
| 45-54 | 29 (19) | 30 (16) | 75 (22) | 134 (19) |
| 55-64 | 35 (23) | 43 (23) | 67 (19) | 145 (21) |
| 65-74 | 24 (16) | 42 (22) | 53 (15) | 119 (17) |
| 75 and older | 17 (11) | 18 (9) | 67 (19) | 102 (15) |
| Sokal score (n, %) | ||||
| Low | 53 (35) | 73 (38) | 120 (35) | 246 (36) |
| Intermediate | 47 (31) | 61 (32) | 135 (39) | 243 (35) |
| High | 34 (22) | 40 (21) | 72 (21) | 146 (21) |
| Unknown | 19 (12) | 17 (9) | 20 (6) | 56 (8) |
| Patients with imatinib, n, % | 28 (18) | 83 (43) | 323 (93) | 434 (63) |
| Patients with IFN, n, % | 91 (59) | 135 (71) | 98 (28) | 324 (47) |
| Patients treated by allogeneic stem cell transplantation | 29 (19) | 49 (26) | 22 (6) | 100 (14) |
| Life status at January 1, 2008 (n, %) | ||||
| Alive | 32 (21) | 80 (42) | 277 (80) | 389 (56) |
| Dead | 115 (75) | 103 (54) | 59 (17) | 277 (40) |
| Lost to follow-up | 6 (4) | 8 (4) | 11 (3) | 25 (4) |
| . | Period of diagnosis . | |||
|---|---|---|---|---|
| 1990-1994, n = 153 . | 1995-1999, n = 191 . | 2000-2007, n = 347 . | 1990-2007, n = 691 . | |
| Male sex (n,%) | 83 (54) | 112 (59) | 175 (50) | 370 (54) |
| Median age at diagnosis, y (range) | 55 (17-88) | 56 (16-89) | 57 (16-95) | 56 (16-95) |
| Age classes, y (n, %) | ||||
| 15-44 | 48 (31) | 58 (30) | 85 (24) | 191 (28) |
| 45-54 | 29 (19) | 30 (16) | 75 (22) | 134 (19) |
| 55-64 | 35 (23) | 43 (23) | 67 (19) | 145 (21) |
| 65-74 | 24 (16) | 42 (22) | 53 (15) | 119 (17) |
| 75 and older | 17 (11) | 18 (9) | 67 (19) | 102 (15) |
| Sokal score (n, %) | ||||
| Low | 53 (35) | 73 (38) | 120 (35) | 246 (36) |
| Intermediate | 47 (31) | 61 (32) | 135 (39) | 243 (35) |
| High | 34 (22) | 40 (21) | 72 (21) | 146 (21) |
| Unknown | 19 (12) | 17 (9) | 20 (6) | 56 (8) |
| Patients with imatinib, n, % | 28 (18) | 83 (43) | 323 (93) | 434 (63) |
| Patients with IFN, n, % | 91 (59) | 135 (71) | 98 (28) | 324 (47) |
| Patients treated by allogeneic stem cell transplantation | 29 (19) | 49 (26) | 22 (6) | 100 (14) |
| Life status at January 1, 2008 (n, %) | ||||
| Alive | 32 (21) | 80 (42) | 277 (80) | 389 (56) |
| Dead | 115 (75) | 103 (54) | 59 (17) | 277 (40) |
| Lost to follow-up | 6 (4) | 8 (4) | 11 (3) | 25 (4) |
The introduction of imatinib in 2000 has led to almost exclusive use of this drug thereafter: between the 3 periods of diagnosis (1990-1994, 1995-1999, and 2000-2007), important changes in the proportions of patients on imatinib were observed (18%, 43%, and 93%, respectively), with a very short median interval (1 month) between diagnosis and initiation of imatinib treatment for the more recent group. Conversely, the proportions of patients on IFN-α were 59%, 71%, and 28%, respectively. The same trend was observed for patients undergoing allogeneic stem cell transplantation: 19%, 26%, and 6%, respectively. Overall, only a small minority of patients received nilotinib (4%) or dasatinib (7%).
Considering only the patients diagnosed after 2000, the mean duration of imatinib treatment was 35 months. In addition, 80% of patients alive at administrative censoring (221/277) were still receiving only imatinib.
Univariate analyses: estimated crude and relative survival
The crude and relative 5-year survivals according to sex, age class, and period of diagnosis for each Sokal score are shown in Table 2. These survival probabilities appeared to be greater in women than in men. When the patient was younger than 75 years of age at diagnosis, the estimated relative survivals were of the same order of magnitude among age classes (around 75%) whereas, when the patient was older than 75 years, the estimated relative survival was lower; however, the confidence interval of this estimate was wide.
Crude and relative 5-year survival by sex, age at diagnosis, and year of diagnosis by Sokal score (95% confidence interval)
| Strata . | Number of patients . | Crude 5-year survival . | Relative 5-year survival . |
|---|---|---|---|
| Sex | |||
| Male | 370 | 60 (55-65) | 68 (62-73) |
| Female | 321 | 72 (66-76) | 78 (60-88) |
| Age at diagnosis, y | |||
| 15-44 | 191 | 70 (56-80) | 71 (40-88) |
| 45-54 | 134 | 74 (66-80) | 77 (68-83) |
| 55-64 | 145 | 76 (68-82) | 80 (72-86) |
| 65-74 | 119 | 60 (51-68) | 71 (60-79) |
| 75 and older | 102 | 39 (29-48) | 57 (39-72) |
| Year of diagnosis and Sokal score | |||
| 1990-1994 | |||
| Low | 53 | 76 (64-85) | 80 (67-89) |
| Intermediate | 47 | 45 (31-58) | 55 (38-69) |
| High | 34 | 38 (25-51) | 42 (27-56) |
| Unknown | 19 | 51 (30-69) | 58 (34-76) |
| Overall | 153 | 57 (50-63) | 64 (56-71) |
| 1995-1999 | |||
| Low | 73 | 69 (58-78) | 75 (62-84) |
| Intermediate | 61 | 59 (48-68) | 65 (53-75) |
| High | 40 | 47 (34-60) | 54 (39-67) |
| Unknown | 17 | 60 (38-76) | 72 (44-88) |
| Overall | 191 | 59 (52-65) | 66 (57-73) |
| 2000-2007 | |||
| Low | 120 | 87 (79-93) | 91 (81-96) |
| Intermediate | 135 | 78 (69-85) | 95 (82-99) |
| High | 72 | 72 (59-82) | 80 (64-89) |
| Unknown | 20 | 33 (11-57) | 46 (16-73) |
| Overall | 347 | 78 (73-82) | 88 (80-93) |
| 1990-2007 | |||
| Low | 246 | 78 (72-83) | 82 (64-92) |
| Intermediate | 243 | 62 (55-69) | 72 (64-79) |
| High | 146 | 51 (42-60) | 58 (48-67) |
| Unknown | 56 | 47 (34-58) | 55 (41-68) |
| Overall | 691 | 65 (61-69) | 73 (69-77) |
| Strata . | Number of patients . | Crude 5-year survival . | Relative 5-year survival . |
|---|---|---|---|
| Sex | |||
| Male | 370 | 60 (55-65) | 68 (62-73) |
| Female | 321 | 72 (66-76) | 78 (60-88) |
| Age at diagnosis, y | |||
| 15-44 | 191 | 70 (56-80) | 71 (40-88) |
| 45-54 | 134 | 74 (66-80) | 77 (68-83) |
| 55-64 | 145 | 76 (68-82) | 80 (72-86) |
| 65-74 | 119 | 60 (51-68) | 71 (60-79) |
| 75 and older | 102 | 39 (29-48) | 57 (39-72) |
| Year of diagnosis and Sokal score | |||
| 1990-1994 | |||
| Low | 53 | 76 (64-85) | 80 (67-89) |
| Intermediate | 47 | 45 (31-58) | 55 (38-69) |
| High | 34 | 38 (25-51) | 42 (27-56) |
| Unknown | 19 | 51 (30-69) | 58 (34-76) |
| Overall | 153 | 57 (50-63) | 64 (56-71) |
| 1995-1999 | |||
| Low | 73 | 69 (58-78) | 75 (62-84) |
| Intermediate | 61 | 59 (48-68) | 65 (53-75) |
| High | 40 | 47 (34-60) | 54 (39-67) |
| Unknown | 17 | 60 (38-76) | 72 (44-88) |
| Overall | 191 | 59 (52-65) | 66 (57-73) |
| 2000-2007 | |||
| Low | 120 | 87 (79-93) | 91 (81-96) |
| Intermediate | 135 | 78 (69-85) | 95 (82-99) |
| High | 72 | 72 (59-82) | 80 (64-89) |
| Unknown | 20 | 33 (11-57) | 46 (16-73) |
| Overall | 347 | 78 (73-82) | 88 (80-93) |
| 1990-2007 | |||
| Low | 246 | 78 (72-83) | 82 (64-92) |
| Intermediate | 243 | 62 (55-69) | 72 (64-79) |
| High | 146 | 51 (42-60) | 58 (48-67) |
| Unknown | 56 | 47 (34-58) | 55 (41-68) |
| Overall | 691 | 65 (61-69) | 73 (69-77) |
Table 2 shows an overall improvement of survival over the years of diagnosis except between 1990-1994 and 1995-1999 in low-Sokal score patients; the estimated relative survival decreased from 80 (67-89) to 75 (62-84). According to our results, the main improvement was observed between periods 1995-1999 and 2000-2007, especially in patients with intermediate and high Sokal scores in whom the relative survival increased from 65 (53-75) to 95 (82-99) and from 54 (39-67) to 80 (64-89), respectively.
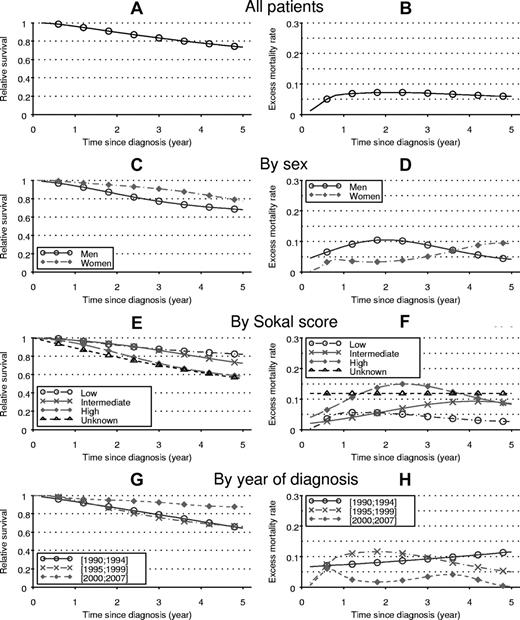
Figure 1 shows the relative survivals and the corresponding EMRs according to the sex, the Sokal score, and the year of diagnosis. Figure 1C shows that, whatever the delay since diagnosis, the relative survivals of women is better than those of men. However, Figure 1D reveals another important aspect of the difference between men and women's mortalities: in men, the EMR during the 5th year (ie, the probability of dying from the disease during that year) is approximately 0.05 deaths per person-years (dppy) or 5% if expressed in term of probability. This probability is ∼ 10% in women. This means that whereas the number of women alive at 5 years is greater that the number of men alive at the same time, the mortality in women is greater than that of men during the 5th year. Figure 1F shows that the dynamics of mortality varied according to the Sokal score. In high-risk patients, the EMR was maximum at approximately 0.15 dppy between the 2nd and 3rd year of follow-up. In low-risk patients, the EMR decreased regularly after an early peak (0.05 dppy or 5%) reached during the first year of diagnosis. In intermediate-risk patients, the EMR increased until the 4th year of follow-up (0.1 dppy) then stabilized. Figure 1H shows that, in the patients diagnosed after 2000, the EMR (all Sokal scores combined) remained under 0.05 dppy and showed 2 peaks reached during the 1st and the 4th years of follow-up.
Relative survival (left) and EMR (right) estimated from the univariate analyses, by stratum. These estimates were calculated for all the patients (A and B; 1st row), by sex (C and D; 2nd row), by Sokal score (E and F; 3rd row), and by year of diagnosis (G and H; 4th row).
Relative survival (left) and EMR (right) estimated from the univariate analyses, by stratum. These estimates were calculated for all the patients (A and B; 1st row), by sex (C and D; 2nd row), by Sokal score (E and F; 3rd row), and by year of diagnosis (G and H; 4th row).
Multivariate analyses: effect of the year of diagnosis on the EMRs according to the Sokal score
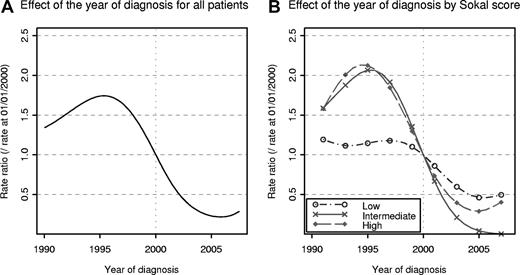
Figure 2 shows the changes in the rate ratio of the excess mortality according to the year of diagnosis, first with all patients (Figure 2A) then by Sokal-score categories (Figure 2B). These rate ratios were estimated from models assuming a “proportional and nonlinear effect of the year of diagnosis” (the reference year being 2000). During years 1990-2007, considering all the patients (Figure 2A), this effect was significant (P < 10−5). Figure 2A indicates that the mortality rates decreased constantly with the year of diagnosis since 1995. The maximum rate ratio was estimated at ∼ 1.7 in patients diagnosed around 1995, that is, in patients diagnosed in 1995 the EMR was 1.7 times than that of patients diagnosed in 2000.
EMR ratio (reference January 1, 2000) according to the year of diagnosis. The excess rate ratio was estimated by the use of a proportional model for all Sokal scores (A) and for each Sokal score separately (B).
EMR ratio (reference January 1, 2000) according to the year of diagnosis. The excess rate ratio was estimated by the use of a proportional model for all Sokal scores (A) and for each Sokal score separately (B).
When the model was adjusted for each Sokal score separately, the effect of the year of diagnosis was significant only in patients with intermediate and high Sokal scores (Figure 2B). Indeed, the P values relative to the LRT in the comparison between a model with and a model without effect of year of diagnosis were .0002, .008, and .16 for intermediate, high, and low scores, respectively. The shapes of the curves indicate that the improvement in survival began in patients with intermediate and high Sokal score diagnosed after 1995. Figure 2B shows that even if the effect of the year of diagnosis was not statistically significant, the EMR in low-Sokal score patients decreased slowly from 1990 to 2007.
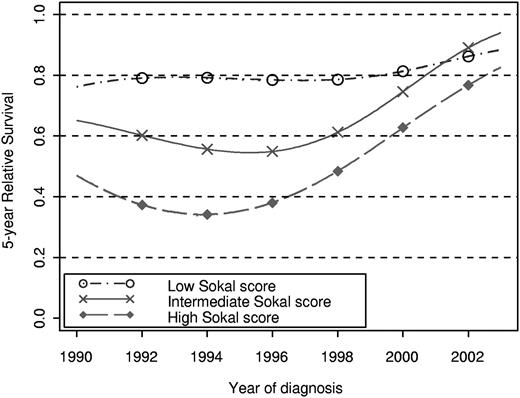
A proportional hazard model was fitted for low and intermediate Sokal scores (proportional hazards null hypothesis tests: P = .15 and P = .44, respectively), whereas a nonproportional hazard model was required for high Sokal scores (P = .003). Figure 3 shows the trends in the 5-year relative survival when these models are used and illustrates the significant improvement of survival in intermediate- and high-Sokal score patients. When the latter models are used, Figure 4 shows the dynamic of the EMR for different years of diagnosis and Sokal scores. In low-Sokal score patients (Figure 4A), the EMR was very low (< 0.1) whatever the year of diagnosis. A slight but constant decrease of the EMR was observed with the increase of the year of diagnosis, although the effect of the year of diagnosis was not significant.
Five-year relative survival according to year of diagnosis estimated from multivariate models for each Sokal score separately. The model assumed a proportional and nonlinear effect of year of diagnosis for low and intermediate Sokal scores, and nonproportional and nonlinear effect of year of diagnosis for high Sokal score.
Five-year relative survival according to year of diagnosis estimated from multivariate models for each Sokal score separately. The model assumed a proportional and nonlinear effect of year of diagnosis for low and intermediate Sokal scores, and nonproportional and nonlinear effect of year of diagnosis for high Sokal score.
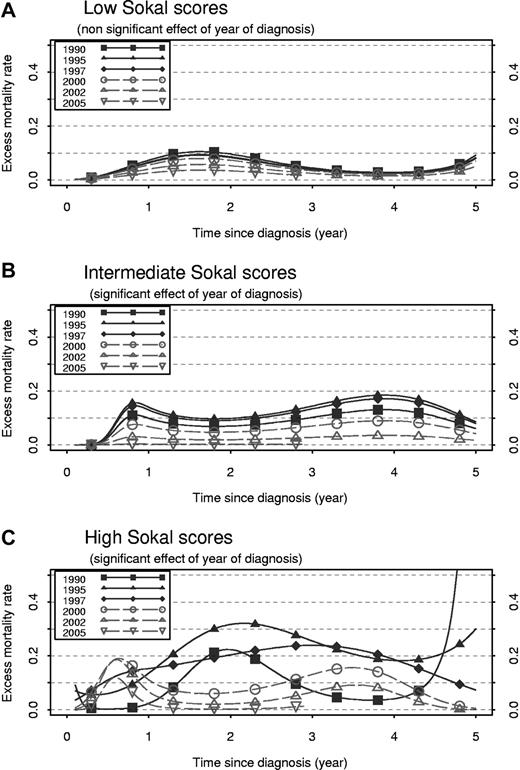
Excess mortality rate from 0 to 5 years after diagnosis, at different years of diagnosis (1990, 1995, 1997, 2000, 2002, and 2005). Excess mortality rate was estimated separately for each Sokal score by the use of a proportional model for low score (A), a PH model for intermediate score (B), and a nonproportional model for high score (C).
Excess mortality rate from 0 to 5 years after diagnosis, at different years of diagnosis (1990, 1995, 1997, 2000, 2002, and 2005). Excess mortality rate was estimated separately for each Sokal score by the use of a proportional model for low score (A), a PH model for intermediate score (B), and a nonproportional model for high score (C).
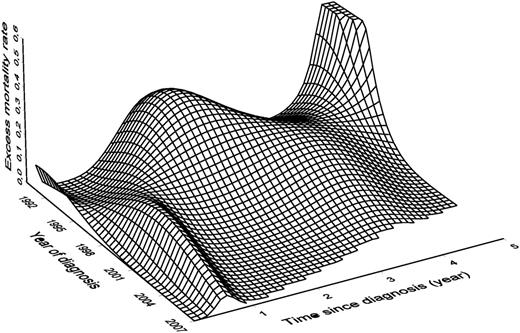
In intermediate-Sokal score patients (Figure 4B), the EMR ranged mainly between 0.1 and 0.2 in the patients diagnosed before 2000. The EMR decreased sharply with the increase of the year of diagnosis and became very small in recently diagnosed patients. In high-Sokal score patients, a strong nonproportional effect is illustrated in Figure 4C: the effect of the year of diagnosis is not the same according to the delay since diagnosis. For example, 2 years after diagnosis, the mortality of patients diagnosed in 1995 was greater than that of patients diagnosed in 2002. If we consider mortality 0.5 years after the diagnosis, we observe the opposite. This makes the shapes of the EMR curves vary greatly according to the year of diagnosis and explains the nonparallelism of these curves (contrary to Figure 4B). Figure 5 is a 3-dimensional plot that shows the EMR in high-Sokal score patients according to the delay since diagnosis and to the year of diagnosis (same information as Figure 4C but for all the years of diagnosis). In patients diagnosed in the early 1990s, the EMRs were the greatest by the second year of follow-up; it was > 0.3 in patients diagnosed in 1995. This peak of mortality disappeared progressively, whereas another smaller peak increased in patients diagnosed between 1995 and 2000 during the first year of follow-up. After 2000, the shape of the curves for high Sokal scores changed and looked almost like those of intermediate scores, ie, a slight increase of mortality during the first year then between the third and the fourth year.
Excess mortality rate according to time since diagnosis (in years) and date of diagnosis for high Sokal score.
Excess mortality rate according to time since diagnosis (in years) and date of diagnosis for high Sokal score.
Discussion
Excess mortality models (and the corresponding relative survival) are useful tools in prognostic studies on cancer. This approach allowed us to quantify precisely the effects of covariates such as the year of diagnosis on the mortality related to cancer, which is not possible with the use of a conventional Cox model.8,9 We used a flexible version of the excess mortality model by using regression splines to model the baseline rate and the nonlinear and nonproportional effects of the covariates. To examine the change in the dynamics of mortality, we decided to focus on the mortality rates rather than on the probability of survival. At a given time t, the probability of survival “cumulates” all mortalities from t0 to t, whereas the mortality rate provides an instantaneous image of what happens at time t. Thus, compared with survival probabilities, mortality rates offer a more relevant representation of the dynamics of mortality and these dynamics provide useful clinical information.
To assess the validity of the model used, the model-based estimates were compared with those of another model that assumes step functions (supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This comparison confirmed that the goodness-of-fit was correct when the mortality rate or the effect of the year of diagnosis were modeled with cubic regression splines.
From a general point of view, when the excess rate model is used, a risk of bias exists when cancer patients have a higher (or lower) risk of dying from other causes than the general population. In such a case, the use of the averaged general population mortality rates becomes unsuitable and leads to bias. For instance, in the study on lung cancer, the excess mortality would be overestimated because lung cancer patients would likely include a greater proportion of smokers than the general population and thus a greater risk of comorbidities (such as cardiovascular diseases). This bias occurs mainly in long-term analyses because comorbidities need time to exert noticeable effects. In our context, this is unlikely because CP-CML is not strongly associated with other factors that can influence survival and because the study was restricted to a short-term analysis (eg, 5 years).
Given the aim of our study, allowing a nonproportional effect of the year of diagnosis was crucial. Indeed, considering that the impact of this factor on mortality remains constant during the entire follow-up would not be justified: if the proportional hazard assumption was true, the curves of the EMR according to the time since diagnosis in high-Sokal-score patients (Figure 4C) would be parallel (as in Figure 4B), which is certainly not the case here. Thus, only a nonproportional hazard model is able to provide a true picture of the changes in the dynamics of mortality.
The IRIS trial, which demonstrated the superiority of imatinib over IFN-α combined with cytarabine in CP-CML, did not assess a gain in overall survival. Only a few published studies based on retrospective analyses have shown a survival advantage for CML patients treated with imatinib over IFN-α-based regimens: Kantarjian et al10 reported their experience at the M. D. Anderson Cancer Center, and Roy et al11 published a historical comparison between two phase 3 clinical trials.
The main advantage of a population study over a randomized trial is the nonrestriction of the survival description to a selected group of patients (ie, young patients without significant comorbidities). Furthermore, the use of relative survival methodology allows us to interpret the survival results integrating the reference population expected mortality knowledge.
In a previous population-based study from the American Surveillance Epidemiology and End Results (SEER) registry data, Brenner et al12 have already shown an improvement of the 5-year relative survival in CML patients diagnosed in 1990-1992 versus those diagnosed in 2002-2004 (27.1 and 48.7, respectively). Nevertheless, these authors did not analyze directly the dynamics of excess mortality or presented results from multivariate models fitted at each level of the prognostic score.
In our study, the median age of the Philadelphia chromosome-positive CP-CML cohort was 56 years, whereas the other CML registries generally indicate a 55-65 year median age.13,14 We found a rather low proportion of patients aged 65 or older (32%), especially in comparison with the data of SEER (53%)12 or FRANCIM (Network of French Cancer Registries; 49%, data not shown). The reason for this low rate is unclear, but it is probably partly because of the youth of the general population in this region that counts one of the lowest rates of people aged 65 and older in France (< 16%, Institut National de la Statistique et des Etudes Economiques). However, the incidence of CML in our cohort was similar to other registry data, which validated the completeness of our case recording13,14 : the world and Europe age-standardized incidence were, 1 and 1.3 in men and 0.7 and 0.9 in women, respectively (number of cases per 100 000 person-years).
In the present work, we focused on the year of diagnosis to evaluate the impact of imatinib on survival. Indeed, almost all patients diagnosed after 2000 received imatinib, whereas the use of IFN-α and allogeneic SCT experienced an important decrease; this allowed us to attribute the gain of relative survival to the use of imatinib. Furthermore, only 8% of the patients of the present study cohort received a second-generation tyrosine kinase inhibitor (all of them were already treated with imatinib), which allowed us to neglect the potential effect of these inhibitors on survival. A particular improvement of survival was observed in high- versus low- or intermediate-Sokal score patients; this corresponds to the disappearance of the peak of mortality during the second year in patients diagnosed after 2000. Interestingly, we found that the shapes of the excess mortality curves in high-Sokal score patients became similar to those observed in intermediate-score patients since 2000, which shows clearly a change in the course of the disease in high-risk patients. Analyzing all 691 patients, we found that the EMR after 2000 was < 5 per 100 person-years, with a small peak between the 3rd and the 4th year. This excess of mortality could be induced by the small peak of progression to AP/BC observed in the IRIS trial during the second year (in 2.8% of the patients with imatinib).15 Furthermore, the IRIS trial has shown that the annual rate of transformation was < 1 per 100 person-years after the 4th year of treatment. This low rate is very encouraging and suggests that the patients would not experience an increased risk of death even with a long imatinib treatment when they are alive without AP/BC after the 2nd year.
The trials of second-generation tyrosine kinase inhibitors (nilotinib and dasatinib) used as front-line treatment of CP-CML have shown an additional reduction of this risk of progression to AP/BC.16,17 This finding suggests that, with these drugs, the EMR in CP-CML may come close to zero. However, the best strategy in the use of the different tyrosine kinase inhibitors in the course of the disease still needs to be clarified. Our results on CML mortality suggest that only a small fraction of patients (ie, high-Sokal score patients with other baseline risk factors, as the detection of additional chromosome abnormalities in Philadelphia chromosome-positive cells18 ) would really benefit from front-line prescription of the more potent second-generation tyrosine kinase inhibitors, given the very low EMR observed in the rest of the cohort diagnosed after 2000, allowing reserving these drugs for imatinib failure situations.
In conclusion, our study demonstrates the interest of combining the results of clinical trials with those of population-based studies to assess the impact of new drugs on survival. The improvement in the relative survival of CP-CML patients seen since the introduction of imatinib was confirmed here only in intermediate- and high-Sokal score patients. We cannot exclude that the slight improvement of survival observed in low-Sokal score patients will become significant with longer follow-up (ie, extended to 10 or 15 years); this is worth being explored. The excess mortality curves in high Sokal scores became similar to those of intermediate scores since 2000, providing evidence for the deep changes in survival induced by the use of imatinib in CP-CML patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Delphine Rea, Hématologie Clinique, Hôpital Saint Louis, Paris, for helpful comments on the manuscript.
This work was supported by Bristol-Myers Squibb.
Authorship
Contribution: S.C., L. Roche, N.B., and L. Remontet designed the research and analyzed data; S.C., J.-B.M., V.C., C.P., C.R.-L., and T.F. collected the data; and S.C., L. Roche, N.B., L. Remontet, F.-E.N., and J.I. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Selim Corm, Hôpital Claude Huriez, Service des Maladies du Sang, Centre Hospitalier Régional et Universitaire, Rue Polonovski, 59000 Lille, France; e-mail: selim.corm@gmail.com.


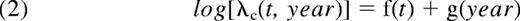
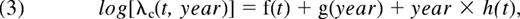
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal