Abstract
In vitro studies have documented β2 glycoprotein I (β2GPI) binding to endothelial cells (ECs) and trophoblast using antiphospholipid antibodies. The in vivo binding of β2GPI to these cells and the conditions that favor their interaction have not been investigated. We analyzed the in vivo distribution of cyanine 5.5-labeled β2GPI in mice and evaluated the effect of pregnancy and circulating antibodies on its tissue localization. The signal was detected in the liver by whole body scan and ex vivo analysis. The β2GPI failed to bind to the vascular endothelium and reacted only with the ECs of uterine vessels. In pregnant mice the protein was localized on ECs and trophoblast at the embryo implantation sites. Immunized mice showed a similar β2GPI biodistribution to naive mice but the immunized pregnant animals exhibited a significant increase in fetal loss associated with C3 and C9 deposition at the implantation sites. Treatment of mice with LPS after β2GPI-Cy5.5 injection promoted protein localization on gut and brain ECs associated with IgG, C1q, and C9 deposition in immunized mice. These findings indicate that β2GPI binding to EC requires priming with pro-inflammatory factors which is not needed for uterine and placental localization probably dependent on hormonal changes.
Introduction
Antiphospholipid syndrome (APS) is characterized by vascular thrombosis and adverse pregnancy outcome associated with circulating antiphospholipid antibodies (aPL) which are believed to play an important pathogenic role in the development of the clinical manifestations of the syndrome.1,2 Human β2-glycoprotein I (β2GPI) has been recognized as the major antigenic target for antiphospholipid antibodies and in vivo models have shown that antibodies directed against this molecule are able to mediate thrombus formation.3,4 Beta2GPI is a heavily glycosylated glycoprotein that circulates in blood at a concentration of 150-300 μg/mL5 and is synthesized mainly in the liver, although expression of β2GPI mRNA has also been detected in endothelial cells (ECs), central nervous system, astrocytes, and placenta. The physiologic function of this protein is still unclear, but the apparently healthy life of humans and mice deficient in β2GPI suggests that its role is not all that critical.6-9 Most of the information now available on β2GPI has been collected following the observation that this protein is the main target of antiphospholipid (aPL) antibodies.10,11 Patients with circulating antibodies to β2GPI are at increased risk of venous and arterial thrombosis as well as of pregnancy complications including miscarriage, preeclampsia and retarded fetal growth.1,12 For this reason antibodies with this specificity have been included among the criteria for the diagnosis of aPL syndrome (APS).13,14 The induction of fetal loss and promotion of thrombosis in animal models as a result of immunization with β2GPI or passive transfer of antibodies further support the pathogenic role of these antibodies.15-18
Endothelial cells,19 platelets,20 monocytes21 and trophoblast22 are among the cell types that have been recognized as targets of β2GPI. TLR4 and annexin II were identified as β2GPI receptor on ECs and monocytes,21,23-26 while ApoER2 serves as receptor on ECs27,28 and platelets.29 Glycoprotein 1ba was suggested to be an additional receptor for β2GPI on platelets.30
Interaction of antibodies with surface-bound β2GPI results in activation of the cell targets that in turn leads to changes in their functional activity. Expression of adhesion molecules by ECs is an example of antibody-mediated cell activation compatible with a pro-inflammatory state, as are the release of cytokines, such as IL-1 and IL-6, and the up-regulation of arachidonic acid metabolism.31
Antibody to β2GPI may cause thrombosis, a frequent feature of APS, in several ways inhibiting prothrombinase activity on platelets, activating factor X and XII, interfering with the activation of protein C and annexin V binding, and impairing fibrinolysis.2 However, the more direct and probably more efficient way for β2GPI-antibody complexes to promote coagulation is to induce expression of tissue factor on ECs, platelets and monocytes thereby activating the extrinsic pathway of coagulation that leads to the development of vascular thrombosis associated with APS.32 Trophoblast is another target of β2GPI that undergoes distinct functional changes on binding of specific antibodies including inhibition of intercytotrophoblast fusion process and reduced human chorionic gonadotropin secretion and trophoblast invasiveness.33,34 These changes are responsible for the defective placentation and contribute to fetal loss.
The molecular organization of β2GPI into 5 domains with repeating stretches of ∼ 60 amino acid residues shared with the complement (C) control proteins, as revealed by the crystal structure, has helped to understand the mechanism of interaction of this molecule with the cell targets and the antibodies.35 It is now well established that the protein binds to the cell surface through the fifth domain and exposes domains I and II away from the cell membrane where they become accessible to antibodies while domains III and IV are shielded by the presence of sugars (glycosylation sites).35-37 Accordingly, antibodies obtained from APS patients were shown by some groups to recognize domain I and were reported to better correlate with the development of thrombosis.38,39 The critical role of domain I as a preferential target of pathogenetic antibodies to β2GPI is highlighted by the observation that the antibody-mediated increase in thrombus size in mice is inhibited by the intraperitoneal injection of recombinant domain I.40
The majority of data on the binding of β2GPI to various cell targets has been obtained from in vitro studies with the obvious caveat that the cells in culture may undergo some changes compared with the native counterpart. Information on in vivo cell binding of β2GPI is lacking with the only exception of the deposition on villous trophoblast documented ex vivo on term placentae by 2 groups.41,42 However, these results should be interpreted with some caution because antibodies were used to reveal cell-bound β2GPI and non specific binding to the Fc receptors present on villous trophoblast cannot be excluded.
The aim of the present investigation was to assess the distribution of labeled β2GPI in pregnant and non pregnant mice and to evaluate the contribution of antibodies to its tissue localization mimicking the situation encountered in patients with APS.
Methods
Purification and characterization of β2GPI
Human β2GPI was purified by perchloric acid treatment of pooled normal human sera obtained from blood donors followed by affinity purification on Heparin column (HiTrap Heparin HP; GE Healthcare) and by ion-exchange chromatography (Resource-S; GE Healthcare). Radial immunodiffusion was used to identify β2GPI, that was further analyzed for purity by SDS-PAGE and Comassie Blue staining and tested for its reactivity with several antibodies from patients with APS.43
Labeling of β2GPI
For the in vivo experiments purified β2GPI was labeled with N-hydroxysuccinimmide ester of the cyanine 5.5 (Amersham Biosciences; FluorolinkCy5.5 Monofunctional Dye 5-pack) to form β2GPI-Cy5.5. A freshly prepared solution of dye (0.05 mg/mL) in 0.1M sodium carbonate buffer pH 9.3 was added to β2GPI (1 mg/mL) in phosphate buffer (vol/vol 1:1). The reagents were incubated by gently shaking for 1 hour at room temperature. Excess unconjugated dye was removed by overnight dialysis against phosphate buffer pH 7.4. The final dye/protein ratio was estimated measuring the absorbance at 678 nm and 280 nm, respectively. The molar concentrations of dye and protein were calculated based on the molar extinction coefficients of 250 000 M−1cm−1 at 678 nm for the Cy5.5 dye and 42 727 M−1 cm−1 at 280 nm for the protein. The ratio of these values that represents the average number of dye molecules coupled to each protein molecule was found to be equal to 0.8 in our preparation. All solvents and chemicals were purchased from Sigma-Aldrich. A similar procedure was followed to label human serum albumin (HSA) purchased from Farma-Biagini S.p.A and used as a control protein labeled with Cy5.5 (HSA-Cy5.5).
Immunization protocol
Eleven Balb/c female mice (6-8 weeks old), purchased from Harlan Laboratories Srl, received an intradermal injection of sterile saline (50 μL) containing 10 μg of human β2GPI, mixed with an equal volume of Freund's complete adjuvant (Sigma-Aldrich), followed 21 days later by a second intradermal injection of the same amount of human β2GPI mixed with incomplete Freund's adjuvant (Sigma-Aldrich). The antibody response to the immunizing antigen was measured using γ-irradiated polystyrene plates (Maxi-Sorp Nunc-Immunoplates; VWR International) coated with purified human β2GPI (10 μg/mL) in bicarbonate buffer overnight, blocked with 1% BSA (Sigma-Aldrich), and incubated with serial dilutions of test serum for 90 minutes at room temperature. The bound antibodies were revealed by alkaline phosphatase–conjugated anti–mouse IgG (Sigma-Aldrich) diluted 1:4000 and the reaction was developed using p-nitrophenyl phosphate (PNPP) as substrate (Sigma-Aldrich) and read at 405 nm.
The different reactivity of the antibodies against the human and murine proteins and the ability of labeled β2GPI to bind to cardiolipin was evaluated by ELISA as previously described with some modifications3 using 96 well polyvinyl ELISA plates (Costar). Each well was coated with 25 μL of cardiolipin (Sigma-Aldrich) at the concentration of 50 μg/mL in ethanol corresponding to 1.25 μg/well, dried overnight at 4°C and then blocked with 10% (vol/vol) human or mouse serum for 2 hours. The mouse serum was IgG depleted by protein G affinity chromatography, following the instructions of the manufacturer (GE Healthcare). After incubation with increasing dilutions of human or mouse sera in blocking solution for 90 minutes at room temperature, the level of bound antibodies was evaluated as specified in the preceding paragraph.
In vivo time-domain optical imaging
Mice were anesthetized using a gaseous anesthesia system (Biologic Instruments), based on isoflurane mixed to 0.5% oxygen and 1% nitrogen protoxide (Siad) and placed inside the eXplore Optix (GE Healthcare) containing 1% of isoflurane. The abdomen was shaved to avoid laser scattering caused by hair. A blank image was acquired before intravenous administration of labeled β2GPI (50 μg) corresponding to 1 nmol of Cy5.5 into the tail vein of mice. The animals were monitored at multiple time points using the small-animal time-domain eXplore Optix pre-clinical imager. In all imaging experiments, a 670 nm pulsed laser diode with a repetition frequency of 80 MHz and a time resolution of 12 ps light pulse was used for excitation. Fluorescence emission was collected at 700 nm and detected through a fast photomultiplier tube and a highly sensitive time-correlated single-photon counting system. Laser power, integration time and scan step were optimized according to the emitted signal. The data were recorded as temporal point-spread functions, and the images were reconstructed as fluorescence intensity and fluorescence lifetime.
The in vivo experiments were performed in accordance with institutional guidelines and in compliance with national and international law and policies. All efforts were made to minimize the number of animals used and their suffering.
Ex vivo tissues analysis
For ex vivo imaging, the mice were killed by cervical dislocation 1 week after the intravenous infusion of β2GPI-Cy5.5 and the organs of interest such as liver, kidney, spleen, heart, lung, brain and uterus were collected, washed in PBS and then analyzed with eXplore Optix with a lifetime gating of 1.3-1.8 ns to detect a specific signal and to eliminate background.
Evaluation of pregnancy outcome
For breeding experiments, 4 β2GPI immunized and 4 naive adult females BALB/c mice (8 ± 12 weeks of age) were housed at the ratio 2:1 with adult stud males and allowed to mate naturally. The day on which a copulation plug was evident was considered day 1 of pregnancy. The labeled protein was injected at day 10 of pregnancy and its distribution was followed for one week by optical imaging. The animals were then killed and the number of live pups per pregnancy as well as the weight of embryos and placentae was evaluated. Resorbed fetuses were identified by their small size and necrotic or hemorrhagic appearance compared with normal embryos. The results are presented as percentage of fetal loss.
Lipopolysaccharide (LPS) treatment
Three immunized and 3 naive mice received an intraperitoneal injection of bacterial lipopolysaccharide (LPS) from Escherichia coli O55:B5 (0.5 mg/kg body weight; Sigma-Aldrich) 20 minutes after intravenous administration of labeled β2GPI. The protein distribution was followed for 4 hours and at the end the animals were killed for ex vivo analysis of the organs mentioned in “Ex vivo tissues analysis.”
Data processing
Immunofluorescence analysis
Sections (7 μm) of snap-frozen samples embedded in OCT medium (Diagnostic Division; Miles Inc) were incubated with rabbit IgG anti-cytokeratin (CK) or anti–von Willebrand Factor (VWF), both purchased from Dako, followed by FITC-conjugated swine anti–rabbit IgG (Dako). Before mounting on slides, samples were incubated with DAPI (Sigma-Aldrich) for 10 minutes to counterstain cell nuclei. The same sections were also examined for the in vivo bound β2 GPI-Cy5.5. The fluorescence images of dissections were acquired using an inverted microscope (Axiovert 200; Carl Zeiss GmbH) with 10× objective lens using FITC, DAPI (4′,6-diamidino-2-phenylindole) and Cy5.5 fluorescence filter sets (Carl Zeiss GmbH). The fluorescence images were captured with a 12-bit near-IR sensitive TE cooled monochrome CCD camera with 1392 × 1040 pixel resolution (DVC-1412AM, DVC Company Inc). The FITC and DAPI images were acquired for 1 second and Cy5.5 images were acquired for 60 seconds.
Tissue deposition of mouse IgG and C3 was analyzed by indirect immunofluorescence using FITC-conjugated goat F(ab′)2 to mouse IgG (Dako) or C3 (MP Cappel), while the binding of mouse C1q and C9 was revealed using rat monoclonal antibody (clone 7H8) to mouse C1q (Hycult Biotech) and rabbit anti–mouse C9 antibody (kindly provided by Prof M. Daha, Leiden, The Netherlands) followed by the relevant FITC-conjugated secondary antibodies (Dako). The sections were examined under a fluorescence microscope Leica DM2000 (Leica).
Results
Biodistribution of β2GPI in naive mice
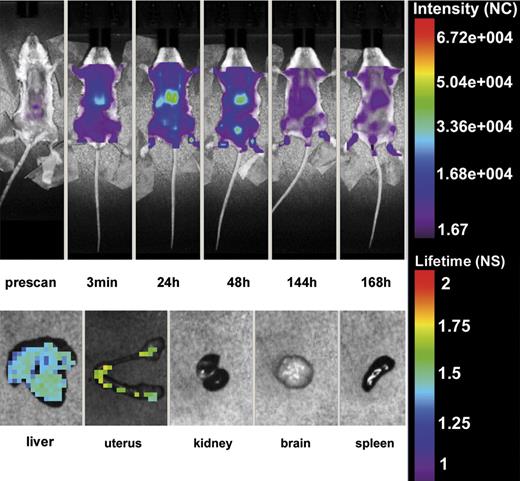
The first series of experiments were performed to assess the tissue localization of β2GPI-Cy5.5 in 4 female mice that were selected for this study to compare the distribution of this protein in pregnant and non pregnant animals. To exclude non specific binding of the labeled protein 3 mice received the fluorescent label followed by in vivo whole body scan. As previously shown,46 the label was rapidly cleared in ∼ 20 minutes through the urine as revealed by the detection of the signal in the bladder (data not shown). After the injection of β2GPI-Cy5.5 into the tail vein, the in vivo distribution of the labeled protein was monitored using the small-animal time-domain eXplore Optix pre-clinical imager. Imaging was performed every 60 minutes for the first 5 hours and then every 24 hours for 7 days. As shown in Figure 1, the compound was rapidly taken up by the liver after injection, and gradually washed out in particular by urinary excretion. A more detailed evaluation of the tissue distribution of β2GPI-Cy5.5 was performed on organs isolated from animals killed one week after injection and analyzed by eXplore Optix. The protein was widely distributed in the liver confirming the in vivo finding, but a distinct signal was observed also in the uterus while undetectable in all the other organs examined (Figure 1). A control group of 3 female mice received HSA-Cy5.5 that showed a wide distribution in different organs and, unlike β2GPI-Cy5.5, was still detected in the circulation one week after injection at the time of sacrifice. Ex vivo analysis revealed the presence of HSA-Cy5.5 in all organs examined except brain, but the intensity of staining in the uterus was substantially lower than that of the other organs (data not shown).
In vivo distribution of β2GPI-Cy5.5. Top panel: Whole body scan of one representative mouse of 4 that received β2GPI-Cy5.5 (50 μg) into the tail vein by Time-Domain Optical Imaging. The distribution of the labeled protein was followed at various time intervals for one week. The signal was rapidly detected in the liver after injection where persisted for 48 hours and was progressively seen in the bladder. The distribution pattern was similar in all treated animals. Bottom panel: Ex vivo imaging of organs isolated from a representative mouse killed one week after injection of β2GPI-Cy5.5 and analyzed by eXplore Optix. Note the presence of the signal in the liver and the uterus. All animals showed a similar organ distribution.
In vivo distribution of β2GPI-Cy5.5. Top panel: Whole body scan of one representative mouse of 4 that received β2GPI-Cy5.5 (50 μg) into the tail vein by Time-Domain Optical Imaging. The distribution of the labeled protein was followed at various time intervals for one week. The signal was rapidly detected in the liver after injection where persisted for 48 hours and was progressively seen in the bladder. The distribution pattern was similar in all treated animals. Bottom panel: Ex vivo imaging of organs isolated from a representative mouse killed one week after injection of β2GPI-Cy5.5 and analyzed by eXplore Optix. Note the presence of the signal in the liver and the uterus. All animals showed a similar organ distribution.
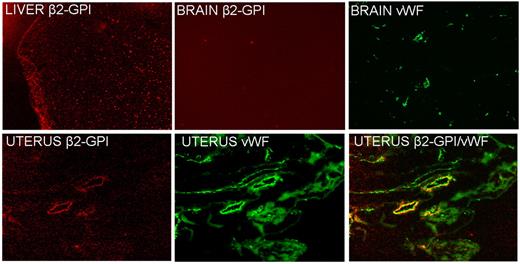
Analysis of tissue sections by the fluorescence microscope confirmed the presence of β2GPI-Cy5.5 in the liver and uterus though with a different distribution pattern (Figure 2). The labeled protein localized in the liver exhibited an intracellular localization with a granular appearance in contrast with the deposition on the vascular endothelium of the uterus that stained for VWF, a marker of ECs. Sections of the brain examined as a control tissue were found to be negative for β2GPI-Cy5.5 on vascular endothelium. We failed to detect deposition of HSA-Cy5.5 on the section obtained from the uterus and other organs examined except for the liver where the distribution pattern was compatible with the clearance of the labeled protein (data not shown).
Detection of β2GPI-Cy5.5 in sections of different tissues by atomic force microscope. Note the absence of the signal in the brain and the different distribution patterns in the liver (granular) and in the uterus, where is colocalized on the vascular endothelium with VWF, a marker of ECs. Original magnification 100×.
Detection of β2GPI-Cy5.5 in sections of different tissues by atomic force microscope. Note the absence of the signal in the brain and the different distribution patterns in the liver (granular) and in the uterus, where is colocalized on the vascular endothelium with VWF, a marker of ECs. Original magnification 100×.
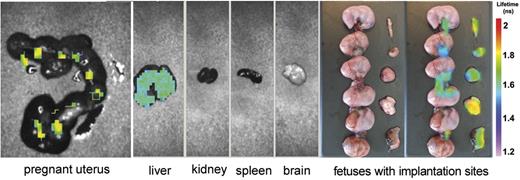
Whole body scan of 4 pregnant mice that received β2GPI-Cy5.5 did not show major difference compared with non pregnant animals. Analysis of the various organs revealed specific β2GPI signal both in the liver and in the uterus. Interestingly, the uterine signal was localized at the implantation sites, but was undetectable on the fetuses (Figure 3). To identify the cell target of β2GPI, sections of the implantation sites were stained for CK, a marker of trophoblast, and for VWF, already used to recognize ECs. Comparing the distribution pattern of the 3 proteins in the labyrinth, we found that β2GPI was deposited both on trophoblast and on ECs (Figure 4).
Analysis of the uterus and other organs isolated from 1 representative pregnant mouse of 4 one week after injection of β2GPI-Cy5.5 by eXplore Optix. The signal is visible in the pregnant uterus and in the liver. Note that the signal in the uterus is detected at the implantation sites and umbilical cords while absent in the fetuses. The distribution pattern was similar in all treated animals.
Analysis of the uterus and other organs isolated from 1 representative pregnant mouse of 4 one week after injection of β2GPI-Cy5.5 by eXplore Optix. The signal is visible in the pregnant uterus and in the liver. Note that the signal in the uterus is detected at the implantation sites and umbilical cords while absent in the fetuses. The distribution pattern was similar in all treated animals.
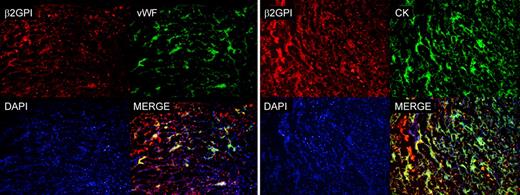
Analysis of sections of implantation sites isolated from pregnant mice by atomic force microscope for the presence of β2GPI-Cy5.5 and by fluorescence microscope for the presence of CK and VWF. Note the colocalization of β2GPI-Cy5.5 and VWF on ECs and β2GPI-Cy5.5 and CK. The cell nuclei were stained with DAPI. Original magnification 100×.
Analysis of sections of implantation sites isolated from pregnant mice by atomic force microscope for the presence of β2GPI-Cy5.5 and by fluorescence microscope for the presence of CK and VWF. Note the colocalization of β2GPI-Cy5.5 and VWF on ECs and β2GPI-Cy5.5 and CK. The cell nuclei were stained with DAPI. Original magnification 100×.
Biodistibution of β2GPI in immunized mice
To evaluate the contribution of the antibodies to the tissue localization of β2GPI, a group of 8 mice was immunized with purified human β2GPI. The animals developed antibodies against the immunizing antigen that reached titres ranging from 1:10 000 to 1:30 000 at the time of the in vivo experiment and had a negligible reactivity against murine proteins. Four immunized animals were mated to evaluate the effect of the antibodies in pregnant mice that received labeled β2GPI.
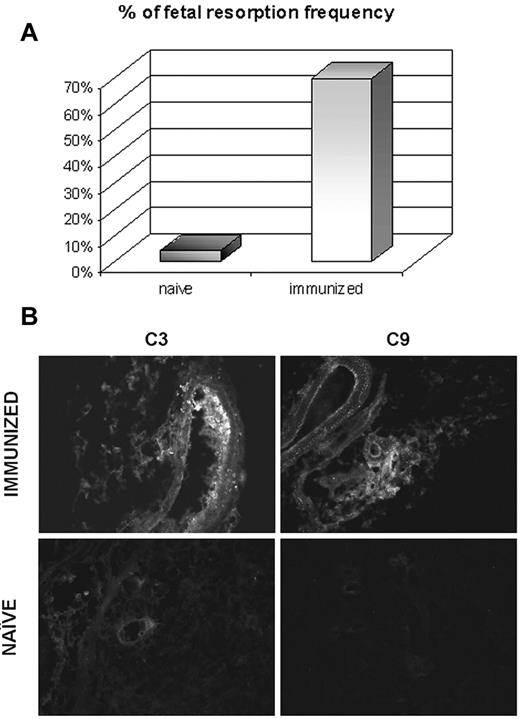
The distribution pattern of β2GPI-Cy5.5 in immunized mice was essentially similar to that observed in naive animals including the localization at the implantation sites in pregnant mice. The only striking difference was the substantial increase in fetal loss observed in the immunized pregnant mice (Figure 5) associated with marked deposits of C3 and C9 at the implantation sites that were not seen in pregnant naive animals (Figure 5) nor in the uterus excised from immunized mice (data not shown).
Effect of antibodies to β2GPI on the resorption rate and C deposition at the implantation sites in pregnant mice. The animals killed on day 17 of gestation 1 week after injection of β2GPI-Cy5.5. The percentage of fetal resorption was evaluated in 4 naive and 4 immunized animals (A) and the deposition of C3 and C9 was analyzed on the implantation sites of both groups of mice (B). Original magnification 200×.
Effect of antibodies to β2GPI on the resorption rate and C deposition at the implantation sites in pregnant mice. The animals killed on day 17 of gestation 1 week after injection of β2GPI-Cy5.5. The percentage of fetal resorption was evaluated in 4 naive and 4 immunized animals (A) and the deposition of C3 and C9 was analyzed on the implantation sites of both groups of mice (B). Original magnification 200×.
Biodistribution of β2GPI in LPS-primed mice
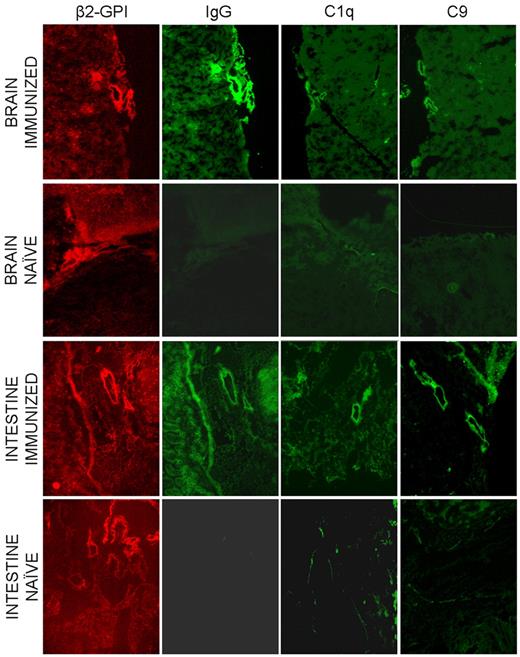
The finding that the endothelium of all the organs examined except for the uterus did not show β2GPI signal in both naive and immunized mice led us to consider the possibility that the ECs may require prior activation to bind the protein. To address this issue we followed the distribution of human β2GPI-Cy5.5 in 3 naive and 3 immunized nonpregnant mice that were treated with LPS 20 minutes after injection of the labeled protein. The animals were monitored for the protein distribution for 4 hours and finally killed. Analysis of the isolated organs by eXplore Optix did not provide reliable information because of the high background signal in all organs examined at the time of the peak level of circulating β2GPI-Cy5.5. To avoid this problem, we examined the tissue sections from various organs and found clear localization of β2GPI-Cy5.5 on the vascular endothelium of gut and brain (Figure 6). The distribution of the protein in immunized mice was similar to that observed in naive animals with the only difference that in this case tissue-bound β2GPI was associated with the deposition of IgG, C1q, and C9 suggesting local C activation (Figure 6).
Distribution of β2GPI-Cy5.5, IgG and the C components C1q and C9 on various tissues collected from LPS treated mice 4 hours after injection of the labeled protein. The same sections were analyzed for the presence of β2GPI-Cy5.5 and for the deposition of IgG and C components by the atomic force microscope. Original magnification 100×.
Distribution of β2GPI-Cy5.5, IgG and the C components C1q and C9 on various tissues collected from LPS treated mice 4 hours after injection of the labeled protein. The same sections were analyzed for the presence of β2GPI-Cy5.5 and for the deposition of IgG and C components by the atomic force microscope. Original magnification 100×.
Discussion
The serum protein β2GPI has attracted a special attention over the last few years because the discovery that it is the main target of antibodies associated with the development of vascular thrombosis and adverse pregnancy outcome. Information on the protein interaction with cell targets involved in the onset of the clinical symptoms has mainly been obtained from in vitro studies. The findings of the present investigation help to clarify the tissue distribution of β2GPI under physiologic conditions and in situations that mimic those encountered in patients with APS. Our data clearly indicate that β2GPI binds in vivo to trophoblast and to uterine and decidual ECs in the absence of antibodies and other stimuli while LPS treatment is required for the protein to bind to ECs from other districts of the vascular tree suggesting that vascular thrombosis and obstetric complications in APS may represent 2 separate clinical entities.1
The β2GPI used in this study was a highly purified protein that kept its functional activity at the end of the purification procedure and after labeling with Cy5.5 as tested for aPL co-factor activity by ELISA. Given the high degree of homology of > 85% with the mouse counterpart, we felt that human β2GPI was suitable for the in vivo analysis of tissue distribution in mice. The detection of the protein signal in the liver shortly after its intravenous injection was a regular finding in all the mice examined under the various experimental conditions. The granular fluorescence pattern with the intracytoplasmic localization of the labeled protein is compatible with its clearance by the liver cells. Removal of a substantial portion of β2GPI may be justified by the heavy glycosylation of the molecule that accounts for ∼ 20% of the molecular weight and favors its aggregation.
Failure to detect protein deposition on the endothelium was a surprise in the light of the wealth of data published on the interaction of β2GPI with ECs. Several receptors for this molecule have been reported to be expressed on ECs that are activated by specific antibodies to express adhesion molecules and tissue factor.2,47 It is not easy to reconcile the in vitro and in vivo findings, though it is worth mentioning that most of the in vitro findings have been obtained using HUVECs that do not necessarily resemble the ECs from big vessels and microcirculation in adult life. It is also possible that ECs maintained in culture undergo functional changes distinct from those of the native cells and that ECs from the various districts of the vascular tree bind differently β2GPI.48 This may explain the unexpected finding that the uterine ECs were the only one to show a clear signal for this protein. The reason for the selective binding of β2GPI to the endothelium of uterine vessels is not apparent but it may be related to local hormonal environment. On the other hand, the signal found in the umbilical cord seems to suggest a special tropism of β2GPI for the umbilical vessel endothelium in line with the in vitro data.19
The embryo implantation sites were found to be an elective target of β2GPI in pregnant mice. The signal detected on placental ECs extends the finding of β2GPI on the uterine endothelium and further supports the hypothesis that changes related to pregnancy may be responsible for the special behavior of these cells. This is not surprising because we have previously reported that decidual ECs in pregnant women are unique in synthesizing C1q used by these cells to promote the adhesion of endovascular trophoblast.49
The finding of β2GPI deposition on trophoblast at the implantation sites in pregnant mice provides a clear and direct evidence in favor of the ability of this protein to bind to trophoblast in vivo. Ex vivo observations by McIntire41 and La Rosa et al42 in the 1990s documented the presence of this protein on villous trophoblast of term placentae. Antibodies were used in both studies to reveal cell-bound β2GPI and this precluded the possibility to evaluate the strength of protein interaction with the cell surface. The current view holds that β2GPI binds to anionic phospholipids exposed on the cell surface and undergoes a conformational change that permits binding of the antibodies to a cryptic epitope resulting in dimerization of the antigen and stabilization of the complex.50,51 Alternatively the adhesion of β2GPI to the cell surface may increase the antigen density thereby favoring the binding of autoimmune aPL that display low avidity.52 The implication of this view is that the affinity of β2GPI for surface anionic phospholipids is relatively low in the absence of antibodies. This does not seem to be the case of cell-associated β2GPI in our experimental conditions that appears to be stably bound to trophoblast despite the fact that the cells were allowed to interact with the protein in circulating blood.
The lack of signal in the fetuses fits well with the clinical observation that aPL affect the neonates only occasionally in spite of the transplacental passage of maternal IgG and the higher thrombophilic state of neonate in comparison with the adults.53
Immunization of mice allowed us to assess the contribution of antibodies to the biodistribution of β2GPI and to the clinical manifestations that may mimic those observed in patients with APS. To this end, we made sure that the antibodies recognized exclusively the human molecule and not the mouse counterpart. This was the case because at the time of the in vivo experiments all immunized mice developed high titers anti–human β2GPI antibodies that cross-reacted with murine proteins bound to CL-coated plates only at very low titers not exceeding dilution 1/10. The tissue distribution of β2GPI did not change in the presence of circulating antibodies and the molecule again failed to bind to the endothelium of most organs examined while still able to deposit on the endothelium of the uterus and at the implantation sites. However, the antibodies definitively contributed to mediate tissue damage that resulted in high rate of fetal resorption thus reproducing similar effects obtained by passive transfer of human antibodies in pregnant mice.17,54 Local activation of the C system seem to be involved in this model as suggested by the deposition of C3 and C9 particularly on the decidual vessels. These data confirm previous findings by other groups on the role played by the C system in a mouse model of antibody-mediated fetal loss.16,55,56 It is interesting to note that binding of β2GPI to the uterine endothelium was not associated with deposition of C components in immunized animals. Failure of the immune complex to activate C in the uterus may be related to a low density of β2GPI and hence to a reduced number of the bound antibody molecules that need to reach a critical level to activate C.
Arterial and venous thrombosis is a typical feature of APS and animal models have clearly shown that deposition of β2GPI–anti-β2GPI complexes on the endothelium is strictly required for its development.2 The inability of β2GPI to bind to the endothelium in vascularized organs seen in our model prevents the formation of thrombi suggesting the requirement of a second hit such as traumata or inflammatory stimuli to set in motion the process. We have previously shown that LPS primes the endothelium of rat mesenteric vessels for anti-β2GPI–mediated thrombosis3 thus representing a good option as a second hit in the light of the well known association of bacterial infections with APS.57 Our present findings extend these observations showing deposition of IgG, C1q, and C9 on the vascular endothelium after intraperitoneal injection of LPS. Despite our efforts, we failed to observe thrombus formation in several organs analyzed, but we believe that the time of observation after LPS injection is probably too short to allow formation of visible thrombi. Of note is the finding that deposition of IgG and C components occurred not only in the intestine but also in the brain and in the heart, but not in the kidney which is compatible with the infrequent renal involvement in APS.
In conclusion, we have provided data indicating that β2GPI injected into mice localize preferentially on the endothelium of the uterine vessels and at the implantation sites both on endothelial cells and trophoblast in pregnant mice. In addition, we have shown that the distribution pattern of this molecule is not modified by the presence of circulating antibodies that are only associated with the occurrence of an increased rate of fetal loss. This finding supports the placental tropism of β2GPI and the local role of anti-β2GPI antibodies in the APS obstetrical manifestations. Finally, evidence have been obtained that LPS is required for the localization of β2GPI on brain, heart, and intestine. The characteristic in vivo expression of β2GPI in the vessel tree further strengthen the view that an inflammatory second hit is pivotal in triggering the aPL effects on the endothelium.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Alessandra Debeus and Mara Saccomano for their collaboration on this research.
This study has been made possible by a research grant of the Ministry of Health (Ricerca Finalizzata RC41/08 e RC 01/09), Italian Association of Cancer Research (AIRC), Fondazione Casali-Trieste and Italian Ministry of University and Research (PRIN MFXE7L_004).
Authorship
Contribution: C.A. performed ELISA assay, designed the experiment, analyzed results, prepared the figures, and contributed to the writing of the paper; S.B. performed the optical imaging experiments, prepared the figures, analyzed the data, and contributed to the writing of the paper; C. Garrovo performed optical imaging experiments; P.D. performed the immunofluorescence analysis and part of the in vivo experiments; A.L. performed the in vivo experiments related to pregnant mice; A.B. performed the immunofluorescence experiments; R.B. critically evaluated β2GPI, Ig, and C deposition at the implantation sites; C. Grossi purified and performed the immunochemical characterization of human β2GPI; M.O.B. performed the functional characterization of human β2GPI; P.L.M. contributed to design the experiments and to write the manuscript; and F.T. the group leader, contributed to design the project, analyzed the results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Francesco Tedesco, Department of Life Sciences, University of Trieste, via Valerio, 28-34127, Trieste, Italy; e-mail tedesco@units.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal