Abstract
Alternatively activated macrophages, generated in a T-helper 2 environment, have demonstrated roles in wound repair and tissue remodeling in addition to being charged with immune tasks. Because the hydrolytic chemistries of the phagosomal lumen are central to many of these functions, we investigated their modification after alternative activation with IL-4 and IL-13. Most significantly, we found striking up-regulation of the proteolytic levels within the phagosome of IL-4–activated macrophages. Two synergistic mechanisms were determined to underlie this up-regulation. First, IL-4–activated macrophages displayed increased expression of cathepsin S and L, providing greater proteolytic machinery to the phagosome despite unchanged rates of lysosomal contribution. Secondly, decreased phagosomal NADPH oxidase (NOX2) activity, at least partially resulting from decreased expression of the NOX2 subunit gp91phox, resulted in a more reductive lumenal microenvironment, which in turn, enhanced activities of local cysteine cathepsins. Decreased NOX2 activity additionally increased the phagosome's ability to reduce disulfides, further enhancing the efficiency of the macrophage to degrade proteins containing disulfide bonds. Together, these changes initiated by IL-4 act synergistically to rapidly and dramatically enhance the macrophage's ability to degrade phagocytosed protein, which, we reason, better equips this cell for its roles in wound repair and tissue remodeling.
Introduction
The ability of the macrophage to dramatically remodel its cell biology in response to environmental cues and immune signals permits this ubiquitous cell lineage to function in a wide range of homeostatic and immune physiologies. Classic activation of the macrophage in response to microbial products and T-helper 1 cytokines has been long known to reprogram the macrophage's biology to enhance microbial killing and its ability to present antigen.1-3 Modulation of the macrophage's key organelle, the phagosome, contributes to this functional reprogramming.4-6 During the past 2 decades, alternative activation of macrophages by the T-helper 2 cytokines IL-4 and IL-13 has been shown to result in a unique phenotype that enables the macrophage to perform distinct physiologic functions. In addition to their demonstrated roles in helminthic infection, alternatively activated macrophages (AAMØs) are gaining considerable attention for their roles in tissue remodeling, wound repair, and control of inflammation.7,8 Concomitant with these roles, several features of AAMØs have been identified that are consistent with the clearance of apoptotic cells and debris, such as up-regulation of certain phagocytic receptors and modification of endolysosomal dynamics.7,9-12 Hitherto changes to the functional chemistries within the phagosome that facilitate the AAMØ's function have not been elucidated.
The lumenal chemistries of phagosomes play a central role in many macrophage functions. In addition to microbial killing and antigen processing, the phagosomal lumen is the site of general macromolecular degradation.13 This degradation enables the removal and recycling of phagocytosed quiescent host cells and debris. It stands to reason that this feature of the phagosomes, like the antimicrobial chemistries, is actively regulated to best facilitate the macrophage's immediate physiologic role. Recent advances in fluorometric methodologies that measure chemistries within the phagosomal lumen of live cells now enable the dissection of the organelle's function in context.13-16 Hence, in addition to recording the recruitment of enzymes to phagosomes (eg, lysosomal hydrolases), local factors and interfering chemistries that influence the activity of the recruited enzymes are also considered. By using this integrative approach, we have recently shown that the antimicrobial effector NADPH oxidase (NOX2) activity decreases phagosomal proteolysis through reversible oxidative modification of local cysteine cathepsins.17 NOX2 activity also profoundly decreases the phagosome's ability to reduce disulfides, an important step in the efficient degradation of proteins containing disulfide bonds.17,18
In this study, we investigated the changes to functional phagosomal parameters after alternative activation of the macrophage with IL-4 and IL-13. We found that AAMØs, particularly in response to IL-4, possess significantly enhanced phagosomal proteolysis, consistent with their roles in tissue repair and remodeling.7 In addition to induced expression of certain lysosomal proteases, we found the up-regulation of proteolysis to be mediated by the down-regulation of phagosomal NOX2 activity. This resulted in a more reductive lumenal microenvironment of the phagosome, which in turn, increased the activities of lumenal cysteine cathepsins. Increased abundance, as well as enhanced activities of cysteine cathepsins, enables IL-4 to profoundly induce the level of proteolysis within the phagosomes of AAMØs.
Methods
Mice and cells
C57BL/6 (WT) mice were purchased from Charles River Laboratories. The congenic mouse strain B6.129S6-Cybb−/− (Cybb−/−) was purchased from The Jackson Laboratory.19 All animal experiments were conducted according to protocols approved by the University of Calgary Animal Care and Use Committee. Murine BM-derived macrophages (BMMØs) were derived from 8- to 12-week-old mice as previously described14 and used for most of the experiments. Murine peritoneal macrophages (PMØs) were isolated by peritoneal lavage with cold PBS, pH 7.2, of euthanized 8- to 12-week-old mice without elicitation. For fluorometric phagosomal analysis, BMMØs or PMØs were seeded in μ-clear 96-well plates (Greiner Bio-One) and allowed to establish a confluent monolayer. Alternative activation was achieved as described.20,21 In brief, fully differentiated BMMØs or PMØs were cultured in the presence of recombinant murine IL-4 and/or IL-13 at 10 ng/mL (Peprotech) for 48 hours before assessment in all experiments unless otherwise stated. Pharmacologic inhibition of NOX2 was achieved with treatment of BMMØs with 0.5μM diphenyleneiodonium (DPI; EMD Chemicals) for 10 minutes preceding phagocytosis of experimental particles. DMSO was used as a vehicle control for untreated samples in experiments in which DPI was used.
Fluorometric assessment of phagosomal pH and hydrolytic activities
Fluorescently labeled, IgG-coupled 3-μm silica particles were prepared and used for phagosomal lumenal characterization in live BMMØs and PMØs as previously detailed.14-16 Measurements were performed in microplate format with the use of a FLUOstar Optima fluorescent plate reader (BMG Labtech) or Safire microplate reader (Tecan) at 37°C, at an MOI of 2-3 particles/macrophage, in an assay buffer consisting of PBS supplemented with 1mM CaCl2, 2.7mM KCl, 0.5mM MgCl2, 5mM dextrose, and 0.25% gelatin.15,16 At the conclusion of each fluorometric assay, cells were microscopically inspected to ensure complete and equal phagocytosis of experimental particles at the correct MOI. For enumeration of phagocytic uptake, extracellular particles were identified through quenching of bound fluorophore by 0.01% trypan blue in PBS. Phagosomal pH was calculated by recording the ratio of the fluorescent emission at 520 nm of carboxyfluorescein succinimidyl ester (SE) conjugated to experimental particles excited at 490 nm and 450 nm followed by polynomial regression to a standard curve as previously detailed.14,16
The hydrolytic activities of phagosomal β-galactosidase, total protease, cathepsin B/S/L, and cathepsin D/E were measured by recording the rate of substrate-liberated fluorescence relative to a calibration fluor with use of the particle-bound fluorogenic substrates 5-dodecanoylaminofluorescein di-β-D-galactopyranoside (Molecular Probes), DQ green Bodipy albumin (DQ-albumin; Molecular Probes), (biotin-LC-Phe-Arg)2-rhodamine 110 ([Phe-Arg]2-Rhodamine110; kindly provided by David Russell, Cornell University), and Mca-GKPILFFRLK (Dnp)-r-NH2 (Anaspec), respectively.14-16 Relative fluorescent units (RFUs), defined by the equation RFU = SFRT/CF (where SFRT indicates substrate fluorescence in real time and CF indicates calibration fluorescence), were plotted against time.
For comparison of hydrolytic capacities across experiments, the slopes (as described by the equation y = mx + c, where y = RFU, m = slope, and x = time) of the linear portion of the relative substrate fluorescence plotted against time were calculated relative to the internal control indicated. Experimental groups were compared by 1-way ANOVA with Bonferroni multiple comparison posthoc test with GraphPad Prism software. Assessment of cysteine cathepsin activities of whole-cell lysates were performed as detailed.17 Further details outlining these methods may be found in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Assessment of rates of phagosome-lysosome fusion
Comparison of rates of phagosome-lysosome fusion was achieved through Immunofluorescent detection of early endosomal antigen 1 (EEA1) and lysosomal-associated membrane protein 1 (LAMP1) on phagosomes according to standard practices.22 In brief, untreated and IL-4–treated BMMØs were allowed to phagocytose IgG-coupled 3-μm silica particles. Cells were fixed at 4°C with 4% paraformaldehyde in PBS at 10-, 20-, and 30-minute periods after phagocytosis. Detection of EEA1 and LAMP1 was achieved with the use of anti-EEA1 rabbit polyclonal IgG (Sigma-Aldrich) and anti-LAMP1 rat monoclonal IgG clone ID4B (Developmental Studies Hybridoma Bank) with Alexa Fluor 488 and Alexa Fluor 594 secondary detection, respectively (Molecular Probes). One hundred phagosomes from each experimental group were microscopically assessed for EEA1 and LAMP1 association with the phagosomal membrane by a blinded reader for 3 individual experiments and expressed as a percentage. Representative images were captured with the Leica SP5 scanning confocal microscope equipped with a 63 × (oil), NA = 1.4 objective at room temperature (Leica). Relative amount of LAMP1 recruitment to phagosomes also was assessed through semiquantitative Western blot analysis of temporally isolated phagosomes.
Fluorometric assessment of oxidative and reductive capacities
We evaluated the generation of reactive oxygen species (ROS) by NOX2 within the phagosomal lumen by measuring the fluorescence after oxidation of the experimental particle-restricted H2HFF-OxyBURST substrate (Molecular Probes), relative to the calibration fluor Alexa Fluor 594 SE.17,23 Total cellular NOX2 activity in response to phorbol-12-myristate-13-acetate (PMA; 1μM; Sigma-Aldrich) stimulation was measured by recording luminescence generated more than 10 minutes in a medium containing 100μM luminol (Sigma-Aldrich).24 The ability of the phagosome to reduce disulfides was evaluated by measuring the rates of fluorescent liberation from the self-quenched cystine-based fluorogenic substrate Bodipy FL L-cystine (Molecular Probes) conjugated to experimental particles, relative to the calibration fluor Alexa Fluor 594 SE, as described previously.17
RFUs were plotted against time. For comparison of oxidative and reductive capacities across experiments, the slopes of the linear portion of the relative substrate fluorescence plotted against time were calculated relative to the internal control indicated. Experimental groups were compared by 1-way ANOVA with Bonferroni multiple comparison post-hoc test with the use of GraphPad Prism software.
Expression and recruitment of phagosomal proteases and NOX2 subunits
Real-time PCR and semiquantitative Western blotting of whole cell lysates and of isolated phagosomes were performed to assess relative expression and recruitment of individual cathepsins and NOX2 subunits after standard procedures. Further details outlining these methods may be found in the supplemental Methods.
Results
Alternatively activated macrophages display increased levels of phagosomal proteolysis
Tight control of phagosomal proteolysis in the dendritic cell is thought to limit the level of antigen proteolysis, that in turn, enhances the generation and preservation of antigenic peptides.25,26 The macrophage, having numerous roles in addition to antigen presentation, many of which require efficient macromolecular degradation, must be equipped to rapidly and significantly modulate levels of proteolysis in response to changes in immune and environmental cues. Because the effect of alternative activation on this essential macrophage process had yet to be examined, we used a fluorometric methodology that allows measurement of general phagosomal proteolysis in real time. This assay follows the hydrolysis of a fluorogenic albumin-based substrate conjugated to experimental particles in phagosomes during their maturation.16 BM-derived macrophages (BMMØs) from C57BL/6 (wild-type; WT) mice were stimulated with 10 ng/mL IL-4, IL-13, or IL-4 and IL-13 for 48 hours to attain an alternatively activated phenotype.20,21 As described, this treatment induced expression of the AAMØ markers arginase 1 and Ym1 (supplemental Figure 1).21,27,28 Because levels of phagocytosis and phagocytic receptor profiles are modulated with alternative activation,10,11,29 for these studies we used 3-μm IgG-conjugated silica experimental particles that induced equal and complete (> 95%) phagocytosis in unactivated (untreated) and alternatively activated BMMØs in our experimental system (supplemental Figure 2). We reasoned that these particles emulate postinflammatory phagocytic targets in damaged tissue after a proinflammatory response with humoral involvement.
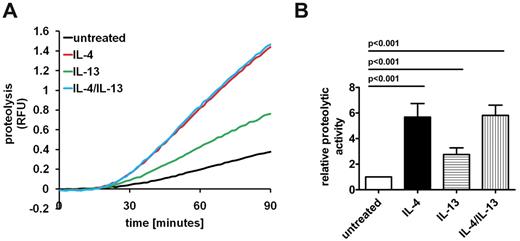
After phagocytosis of these particles bearing the general protein substrate, the rates of phagosomal proteolysis were fluorometrically recorded. The assay revealed that alternative activation by IL-4 led to a profound increase (5.68 ± 1.06-fold) in phagosomal proteolytic activity (Figure 1A-B). Activation with IL-13 alone also showed an increase but to a lesser extent (2.76 ± 0.52). The combination of IL-4 and IL-13 did not show an additive effect because it displayed comparable levels of proteolysis to BMMØs activated with IL-4 alone (5.82 ± 0.8-fold). These results indicate that alternative activation of BMMØs dramatically increases levels of proteolysis in the phagosome, resulting in increased degradative rates of phagocytosed proteins. This finding was consistent in alternatively activated peritoneal macrophages (PMØs; supplemental Figure 3A-B). Because IL-4 activation induced the largest increase in proteolysis, we focused on this form of alternative activation to investigate the mechanisms underlying the observed proteolytic up-regulation in AAMØs.
Alternative activation of BMMØs significantly increases levels of phagosomal proteolysis. Alternative activation was achieved by incubating fully differentiated BMMØs with 10 ng/mL IL-4, IL-13, or IL-4 and IL-13 for 48 hours. After uptake of IgG-coupled 3-μm experimental particles, general proteolytic efficiencies of the resulting phagosomes were assessed in real time by measurement of fluorescence liberated from hydrolysis of particle-associated DQ-albumin substrate relative to calibration fluorescence. (A) Real-time representative traces of phagosomal proteolysis. RFU values are proportional to the degree of substrate hydrolysis. (B) Averaged activities relative to untreated controls from 4 independent experiments. Activities were determined by calculation of the slope of the linear portion of the real-time trace (as described by y = mx + c, where y = relative fluorescence, m = slope, and x = time) and expressed relative to untreated controls. Error bars denote SEM. P values were determined by 1-way ANOVA.
Alternative activation of BMMØs significantly increases levels of phagosomal proteolysis. Alternative activation was achieved by incubating fully differentiated BMMØs with 10 ng/mL IL-4, IL-13, or IL-4 and IL-13 for 48 hours. After uptake of IgG-coupled 3-μm experimental particles, general proteolytic efficiencies of the resulting phagosomes were assessed in real time by measurement of fluorescence liberated from hydrolysis of particle-associated DQ-albumin substrate relative to calibration fluorescence. (A) Real-time representative traces of phagosomal proteolysis. RFU values are proportional to the degree of substrate hydrolysis. (B) Averaged activities relative to untreated controls from 4 independent experiments. Activities were determined by calculation of the slope of the linear portion of the real-time trace (as described by y = mx + c, where y = relative fluorescence, m = slope, and x = time) and expressed relative to untreated controls. Error bars denote SEM. P values were determined by 1-way ANOVA.
Increased proteolytic activity in IL-4–activated macrophages is not due to modified rates of phagosome-lysosome fusion
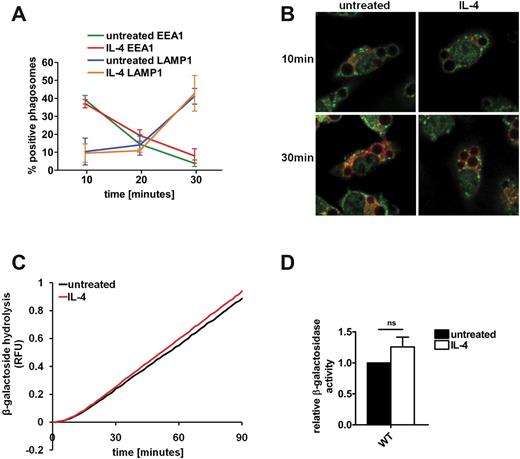
Levels of phagosomal proteolysis can be regulated at several levels, including protease expression, trafficking of lysosomal proteases to the phagosome, and lumenal parameters such as pH and redox potential.17,30,31 Because Montaner et al have demonstrated that IL-4 modifies endolysosomal membrane dynamics in monocyte-derived macrophages,29 we reasoned that an increased delivery of proteases to the phagosome through accelerated phagosome-lysosome fusion could account for an enhanced phagosomal proteolysis in AAMØs. To determine changes to the rates of lysosomal fusion with phagosomes induced by IL-4, we studied the loss of the early endosomal marker EEA1 and the acquisition of lysosomal marker LAMP1 in phagosomal membranes by using Immunofluorescent microscopy. Phagosomes in untreated and IL-4–activated BMMØs showed comparable rates of EEA1 loss and LAMP1 acquisition, indicating a similar rate of phagosomal membrane maturation (Figure 2A-B). Levels of LAMP1 on isolated 30- and 60-minute phagosomes were also found to be unchanged by IL-4 activation when determined by semiquantitative immunoblotting (supplemental Figure 4A-B). Furthermore, the acquisition of the lysosomally derived β-galactosidase activity by phagosomes was unaffected by IL-4 activation (Figure 2C-D). These results indicate that alternative activation by IL-4 does not modify rates of lysosomal contribution to the maturing phagosome in macrophages and is inconsistent with a mechanism that enhances phagosomal proteolysis in AAMØs.
IL-4 activation does not modify rate or extent of lysosomal contribution to the phagosome. (A-B) Rates of modification of the phagosomal membrane by fusion with lysosomes were assessed by following loss of the early endosomal marker EEA1 and gain of the lysosomal marker LAMP1 on maturing phagosomes in untreated and IL-4–activated BMMØs. After the phagocytosis of IgG-coupled 3-μm experimental particles, BMMØs were fixed with formalin at the times indicated. Phagosomes were assessed for association with the maturation markers EEA1 and LAMP1 by Immunofluorescent microscopy in a blinded fashion. (A) Averaged percentage of phagosomes deemed positive for EEA1 and LAMP1 from 3 independent experiments. Error bars denote SEM. (B) Representative images of untreated and IL-4–activated BMMØs stained for EEA1 (green) and LAMP1 (red) at 10 and 30 minutes. (C) Real-time averaged traces of the phagosomal activity of the lysosomally derived β-galactosidase in untreated and IL-4–activated BMMØs. Phagosomal β-galactosidase activity was measured through the hydrolysis of the β-galactosidase substrate 5-dodecanoylaminofluorescein di-β-d-galactopyranoside conjugated to IgG-opsonized experimental particles relative to calibration fluorescence. RFU values are proportional to the degree of substrate hydrolysis. (D) Averaged phagosomal β-galactosidase activities relative to untreated controls from 4 independent experiments. Activities were determined by calculation of the slope of the linear portion of the real-time trace (as described by y = mx + c, where y = relative fluorescence, m = slope, and x = time) and expressed relative to untreated controls. Error bars denote SEM.
IL-4 activation does not modify rate or extent of lysosomal contribution to the phagosome. (A-B) Rates of modification of the phagosomal membrane by fusion with lysosomes were assessed by following loss of the early endosomal marker EEA1 and gain of the lysosomal marker LAMP1 on maturing phagosomes in untreated and IL-4–activated BMMØs. After the phagocytosis of IgG-coupled 3-μm experimental particles, BMMØs were fixed with formalin at the times indicated. Phagosomes were assessed for association with the maturation markers EEA1 and LAMP1 by Immunofluorescent microscopy in a blinded fashion. (A) Averaged percentage of phagosomes deemed positive for EEA1 and LAMP1 from 3 independent experiments. Error bars denote SEM. (B) Representative images of untreated and IL-4–activated BMMØs stained for EEA1 (green) and LAMP1 (red) at 10 and 30 minutes. (C) Real-time averaged traces of the phagosomal activity of the lysosomally derived β-galactosidase in untreated and IL-4–activated BMMØs. Phagosomal β-galactosidase activity was measured through the hydrolysis of the β-galactosidase substrate 5-dodecanoylaminofluorescein di-β-d-galactopyranoside conjugated to IgG-opsonized experimental particles relative to calibration fluorescence. RFU values are proportional to the degree of substrate hydrolysis. (D) Averaged phagosomal β-galactosidase activities relative to untreated controls from 4 independent experiments. Activities were determined by calculation of the slope of the linear portion of the real-time trace (as described by y = mx + c, where y = relative fluorescence, m = slope, and x = time) and expressed relative to untreated controls. Error bars denote SEM.
IL-4 activation of macrophages results in a decreased NOX2-mediated respiratory burst in the phagosome
Beyond protease recruitment to the phagosome, a significant point of regulation of phagosomal proteolysis is through the modulation of lumenal microenvironmental parameters (such as pH) that in turn modulates the activities of local proteases.31 Recently, we have shown that the antimicrobial effector NADPH oxidase complex (NOX2) is a critical regulator of phagosomal proteolysis through the redox control of local cysteine cathepsins.17 To determine whether NOX2 activity associated with the phagosome is modified in IL-4–activated macrophages, we monitored the generation of oxidative radicals by phagosomal NOX2 by recording the fluorogenic oxidation of a particle-restricted H2HFF-OxyBURST substrate after its phagocytosis. Consistent with decreased secretion of superoxide by IL-4–activated human monocyte derived macrophages,32 we found that IL-4–activated BMMØs and PMØs generated a markedly decreased intraphagosomal respiratory burst (50.7 ± 4.1% and 78.9 ± 5.3% reduction, respectively; Figure 3A-B; supplemental Figure 3C-D). The changes in phagosomal respiratory burst were solely NOX2 mediated because no significant differences were observed in IL-4–activated and –untreated BMMØs derived from mice lacking the membrane-bound NOX2 subunit gp91phox (Cybb−/−), or those treated with the NOX2 inhibitor DPI.
IL-4 decreases the NOX2-dependent phagosomal respiratory burst and the expression of the NOX2 subunit gp91phox. (A-B) After phagocytosis of experimental particles, the phagosomal respiratory burst was assessed by measurement of fluorescence liberated by oxidation of particle-associated H2HFF-OxyBURST substrate in WT and the NOX2-deficient Cybb−/− BMMØs. Inhibition of NOX2 was achieved with DPI (0.5μM) where indicated. (A) Real-time traces of phagosomal respiratory burst. RFU values are proportional to the degree of substrate oxidation. (B) Averaged rates of substrate oxidation from 4 independent experiments. Rates of oxidation were determined by calculation of the slope of the linear portion of the real-time trace (as described by y = mx + c, where y = relative fluorescence, m = slope, and x = time) and expressed relative to untreated WT controls. (C) Assessment of extracellular ROS released by BMMØs in response to PMA (1μM) was achieved by measurement of luminol-generated luminescence over 10 minutes and expressed as averaged counts per second from 4 independent experiments. Error bars denote SEM. P values were determined by 1-way ANOVA. (D) Total mRNA levels of major NOX2 subunits were determined by quantitative PCR. Averaged relative mRNA expression level from 5 independent QPCR experiments is shown. Relative expression was expressed as mRNA levels relative to 18S and presented relative to untreated BMMØs. Error bars denote SEM. P values were determined by 1-way ANOVA. (E-F) Total cellular abundance of major NOX2 subunits was determined by semiquantitative Western blotting of whole cell lysates. (E) Representative Western blot images. (F) Average of band relative density from 3 independent Western blot experiments. (G-H) Phagosomal recruitment of major NOX2 subunits was determined by semiquantitative Western blotting of phagosomes isolated 30 minutes after particle uptake. (G) Representative Western blot images. (H) Average of band relative density from 4 independent Western blot experiments. Relative density was determined by calculation of pixel volume for treated sample relative to untreated BMMØ sample by the use of Quantity One analysis software (Bio-Rad). Error bars denote SEM, P values were determined by paired t test. (I) Relative recruitment of p67phox and p47phox (from Figure 3H) normalized to the abundance of phagosomal gp91phox in IL-4–activated BMMØs.
IL-4 decreases the NOX2-dependent phagosomal respiratory burst and the expression of the NOX2 subunit gp91phox. (A-B) After phagocytosis of experimental particles, the phagosomal respiratory burst was assessed by measurement of fluorescence liberated by oxidation of particle-associated H2HFF-OxyBURST substrate in WT and the NOX2-deficient Cybb−/− BMMØs. Inhibition of NOX2 was achieved with DPI (0.5μM) where indicated. (A) Real-time traces of phagosomal respiratory burst. RFU values are proportional to the degree of substrate oxidation. (B) Averaged rates of substrate oxidation from 4 independent experiments. Rates of oxidation were determined by calculation of the slope of the linear portion of the real-time trace (as described by y = mx + c, where y = relative fluorescence, m = slope, and x = time) and expressed relative to untreated WT controls. (C) Assessment of extracellular ROS released by BMMØs in response to PMA (1μM) was achieved by measurement of luminol-generated luminescence over 10 minutes and expressed as averaged counts per second from 4 independent experiments. Error bars denote SEM. P values were determined by 1-way ANOVA. (D) Total mRNA levels of major NOX2 subunits were determined by quantitative PCR. Averaged relative mRNA expression level from 5 independent QPCR experiments is shown. Relative expression was expressed as mRNA levels relative to 18S and presented relative to untreated BMMØs. Error bars denote SEM. P values were determined by 1-way ANOVA. (E-F) Total cellular abundance of major NOX2 subunits was determined by semiquantitative Western blotting of whole cell lysates. (E) Representative Western blot images. (F) Average of band relative density from 3 independent Western blot experiments. (G-H) Phagosomal recruitment of major NOX2 subunits was determined by semiquantitative Western blotting of phagosomes isolated 30 minutes after particle uptake. (G) Representative Western blot images. (H) Average of band relative density from 4 independent Western blot experiments. Relative density was determined by calculation of pixel volume for treated sample relative to untreated BMMØ sample by the use of Quantity One analysis software (Bio-Rad). Error bars denote SEM, P values were determined by paired t test. (I) Relative recruitment of p67phox and p47phox (from Figure 3H) normalized to the abundance of phagosomal gp91phox in IL-4–activated BMMØs.
IL-4–activated macrophages also displayed reduced production of extracellular ROS in response to stimulation with PMA, indicating that diminished NOX2 activity in these AAMØs is not restricted to the phagosome, or entirely because of altered phagocytic receptor signaling (Figure 3C). Quantitative PCR and semiquantitative immunoblotting of whole-cell lysates revealed decreased expression of gp91phox in response to IL-4 (48.9% ± 9.8% and 43.1% ± 7.4% reduction, respectively; Figure 3D-F). This resulted in reduced abundance of gp91phox on the phagosome (58.8% ± 14.2% reduction) and a corresponding reduction in the recruitment of p67phox and p47phox (89.3% ± 4.8% and 69.5% ± 12.2% reduction, respectively), despite the unchanged expression of these cytosolic NOX2 subunits (Figure 3G-H). No significant differences were observed in the relative recruitment of p67phox and p47phox when normalized to the abundance of phagosomal gp91phox, suggesting that NOX2 assembly per se (over and above decreased abundance gp91) was unaffected by IL-4 treatment (Figure 3I). Overall, these results indicate that IL-4 activation of macrophages leads to down-regulation of gp91phox expression, which is at least partially responsible for the decrease in phagosomal NOX2 activity. This in turn, is consistent with a possible mechanism for enhancement of phagosomal proteolysis because of the promotion of a more reductive lumenal microenvironment suitable for cysteine cathepsin activities.
Decreased phagosomal NOX2 activity in IL-4–activated macrophages results in increased phagosomal proteolysis
To directly determine whether the attenuation of phagosomal NOX2 activity in IL-4–activated macrophages is responsible for the observed increase in phagosomal proteolysis, we measured rates of general phagosomal proteolysis in untreated and IL-4–activated BMMØs in the presence and absence of NOX2 function. These experiments used BMMØs derived from Cybb−/− mice (deficient in the gp91phox subunit of the NOX2 complex)19 in combination with pharmacologic inhibition of the NOX2 complex with DPI (0.5μM). As we have shown previously, in the complete absence NOX2 activity, phagosomal proteolysis was profoundly more efficient (4.54 ± 0.50-fold increase; Figure 4A-B).17
IL-4 regulates phagosomal cysteine cathepsin activity in a NOX2-dependent and -independent fashion. Total proteolytic efficiencies (A-B), cysteine cathepsin activities (C-D), and aspartic cathepsin activities (E-F) within phagosomes of untreated or IL-4–activated BMMØs were assessed in the presence of absence of functional NOX2. This was achieved by measuring the hydrolysis of particle-restricted fluorogenic substrates in WT and the NOX2-deficient Cybb−/− BMMØs with or without inhibition of NOX2 by DPI (0.5μM). (A-B) General phagosomal proteolysis was assessed by measurement of fluorescence liberated through hydrolysis of particle-associated DQ-albumin. Relative activities of cysteine cathepsin (C-D) and aspartic cathepsins (E-F) were evaluated with the use of cathepsin B/L- and D/E-specific fluorogenic peptides bound to IgG-coupled experimental particles. (A,C,E) Real-time representative traces. RFU values are proportional to the degree of substrate hydrolysis. (B,D,F) Averaged activities relative to untreated WT controls are from 4 independent experiments. Activities were determined by calculation of the slope of the linear portion of the real-time trace (as described by y = mx + c, where y = relative fluorescence, m = slope, and x = time) and expressed relative to untreated WT controls. Error bars denote SEM. P values were determined by 1-way ANOVA.
IL-4 regulates phagosomal cysteine cathepsin activity in a NOX2-dependent and -independent fashion. Total proteolytic efficiencies (A-B), cysteine cathepsin activities (C-D), and aspartic cathepsin activities (E-F) within phagosomes of untreated or IL-4–activated BMMØs were assessed in the presence of absence of functional NOX2. This was achieved by measuring the hydrolysis of particle-restricted fluorogenic substrates in WT and the NOX2-deficient Cybb−/− BMMØs with or without inhibition of NOX2 by DPI (0.5μM). (A-B) General phagosomal proteolysis was assessed by measurement of fluorescence liberated through hydrolysis of particle-associated DQ-albumin. Relative activities of cysteine cathepsin (C-D) and aspartic cathepsins (E-F) were evaluated with the use of cathepsin B/L- and D/E-specific fluorogenic peptides bound to IgG-coupled experimental particles. (A,C,E) Real-time representative traces. RFU values are proportional to the degree of substrate hydrolysis. (B,D,F) Averaged activities relative to untreated WT controls are from 4 independent experiments. Activities were determined by calculation of the slope of the linear portion of the real-time trace (as described by y = mx + c, where y = relative fluorescence, m = slope, and x = time) and expressed relative to untreated WT controls. Error bars denote SEM. P values were determined by 1-way ANOVA.
Of particular pertinence to AAMØs, we found that in the absence of a functional NOX2 complex, IL-4 was significantly less effective in inducing a further increase in phagosomal proteolysis (1.51 ± 0.09-fold increase in Cybb−/− vs 6.88 ± 0.62 in WT). This finding suggests that the mechanism that accounts for a majority of the IL-4–induced enhancement of phagosomal proteolysis is NOX2 dependent. To test this hypothesis further, we substituted the general protease substrate DQ-albumin for a fluorogenic substrate specific for the cysteine cathepsins B, S, and L and one for the aspartic cathepsins D and E. Specificities of these substrates were confirmed by the use of purified cathepsins and protease inhibitors in a reconstituted system (supplemental Figure 5).
Because the activities of the cysteine cathepsins (which require the active-site cysteines in a thiol state) are more sensitive to redox changes than those of aspartic cathepsins, we predicted that IL-4 would differentially impact the activities of these protease classes in a NOX2-dependent fashion. Indeed, intraphagosomal hydrolysis of the cysteine protease–specific substrate ([Phe-Arg]2-Rhodamine110) was significantly accelerated in IL-4–activated BMMØs in the presence of a functional NOX2 complex (3.47 ± 0.34-fold increase) but less so in the absence of functional NOX2 (1.51 ± 0.05-fold increase in Cybb−/− BMMØs; Figure 4C-D). In contrast, no statistically significant changes were observed in aspartic cathepsin activity between untreated and IL-4–activated macrophages in the presence or absence of NOX2 activity (Figure 4E-F).
Together, these data suggest that the IL-4–induced reduction of phagosomal NOX2 activity is the major factor in determining the up-regulation of phagosomal proteolytic activities in these AAMØs. Interestingly, in the absence of NOX2 function, IL-4 was still able to increase the efficiency of phagosomal proteolysis, albeit more modestly (1.51 ± 0.19-fold; Figure 4A-B). This finding indicates that a NOX2-independent mechanism of proteolytic induction acts in addition to the NOX2-dependent regulation of cysteine cathepsins.
Modification of phagosomal acidification does not enhance phagosomal proteolysis in IL-4–activated macrophages
To determine whether changes to phagosomal acidification could account for either a NOX2-dependent or NOX2-independent mechanism of IL-4–induced changes to phagosomal proteolysis, we dynamically recorded phagosomal pH in untreated and IL-4–activated BMMØs in the presence and absence of NOX2 function. As we have previously reported,17 phagosomes of BMMØs derived from WT and Cybb−/− mice displayed similar phagosomal acidification profiles with a small difference in final pH (0.24 ± 0.04 units lower in Cybb−/− BMMØs; Figure 5). Similarly, consistent with the observed reduction in phagosomal NOX2 activity, the final phagosomal pH in IL-4–activated WT BMMØs was 0.14 (± 0.01) pH units lower than untreated WT BMMØs. No IL-4–mediated differences in final pH were observed in BMMØs without NOX2 function. Although a NOX2-dependent change to final phagosomal pH in IL-4–activated BMMØs is present, this small increase in acidification is inconsistent with a significant fold change to phagosomal proteolysis. In addition, as shown previously, any decrease in final pH below the 5.5 optimum of the total lysosomal protease mixture would theoretically decrease, not increase, protease efficiency.17 Hence, IL-4–mediated modification of pH is inconsistent with a mechanism that enhances phagosomal proteolysis in AAMØs.
Effects of IL-4 activation on phagosomal acidification in the presence and absence of functional NOX2. Acidification profiles of phagosomes were generated in untreated and IL-4–activated WT and Cybb−/− BMMØs, with or without NOX2 inhibition with DPI (0.5μM) or vacuolar ATPase inhibition with concanamycin A (100nM). After phagocytosis, pH was calculated from excitation ratios of the pH-sensitive carboxyfluorescein-SE on IgG-coupled beads by regression to a standard curve. (A) Representative real-time phagosomal acidification profiles. (B) Lumenal pH at 30 minutes postinternalization. Graph represents averaged data from 4 independent experiments. Error bars represent SEM. P values were determined by 1-way ANOVA.
Effects of IL-4 activation on phagosomal acidification in the presence and absence of functional NOX2. Acidification profiles of phagosomes were generated in untreated and IL-4–activated WT and Cybb−/− BMMØs, with or without NOX2 inhibition with DPI (0.5μM) or vacuolar ATPase inhibition with concanamycin A (100nM). After phagocytosis, pH was calculated from excitation ratios of the pH-sensitive carboxyfluorescein-SE on IgG-coupled beads by regression to a standard curve. (A) Representative real-time phagosomal acidification profiles. (B) Lumenal pH at 30 minutes postinternalization. Graph represents averaged data from 4 independent experiments. Error bars represent SEM. P values were determined by 1-way ANOVA.
IL-4 induces increased expression of cathepsins S and L
Because the absence of a functional NOX2 complex did not completely abolish the ability of IL-4 to enhance phagosomal proteolysis, IL-4 induction of lysosomal protease expression was investigated as a possible NOX2-independent mechanism that contributes to the enhancement of phagosomal proteolysis. Quantitative PCR of cathepsin RNA transcripts indicated that cathepsin S and L were up-regulated after IL-4 activation (Figure 6A). To assess the contribution of the possible up-regulation of cathepsin S and L to the total cellular cysteine cathepsin activity in the absence of NOX2 influence, the efficiency of whole-cell lysates to hydrolyze the (Phe-Arg)2-Rhodamine110 substrate was assessed in a reconstituted system at pH 5.5. Consistent with increased expression of cathepsins S and L, IL-4 activation resulted in a 2-fold (1.89 ± 0.13) increase in the cysteine protease activity of whole-cell lysates from WT BMMØs (Figure 6B).
IL-4–activated BMMØs have increased phagosomal levels of mature cathepsin S and L. (A) Average mRNA expression level relative to controls from 5 independent QPCR experiments. Relative expression was expressed as mRNA levels normalized to 18S and presented relative to untreated BMMØs. Error bars denote SEM. P values were determined by 1-way ANOVA. (B) Cysteine cathepsin activity of whole-cell lysates from untreated and IL-4–activated WT and Cybb−/− BMMØs was measured in vitro by the use of a fluorogenic substrate of cathepsin B, S, and L. Graphs represent averaged rates relative to untreated WT sample from 4 independent experiments. Error bars represent SEM. P values were determined by 1-way ANOVA. Relative abundance of the aspartic cathepsin D and the cysteine cathepsins B, S, and L were determined in whole cell lysates of untreated and IL-4–activated WT BMMØs by standardized semiquantitative Western blotting. (C) Representative images of western blots. Mature forms of cathepsins B, S, and L and intermediate form of cathepsin D are shown. (D) Average of band relative density from 3 independent Western blot experiments. Relative abundance of the aspartic cathepsin D and the cysteine cathepsins B, S, and L were determined in phagosomes isolated 60 minutes after particle uptake by untreated and IL-4–activated WT BMMØs by standardized semiquantitative Western blotting. (E) Representative images of western blots. Mature forms of cathepsins B, S, and L and intermediate form of cathepsin D are shown. (F) Average of band relative density from 5 independent Western blot experiments. Relative density was determined by calculation of pixel volume for treated sample relative to untreated BMMØ sample using Quantity One analysis software (Bio-Rad). Error bars denote SEM, P values were determined by paired t test.
IL-4–activated BMMØs have increased phagosomal levels of mature cathepsin S and L. (A) Average mRNA expression level relative to controls from 5 independent QPCR experiments. Relative expression was expressed as mRNA levels normalized to 18S and presented relative to untreated BMMØs. Error bars denote SEM. P values were determined by 1-way ANOVA. (B) Cysteine cathepsin activity of whole-cell lysates from untreated and IL-4–activated WT and Cybb−/− BMMØs was measured in vitro by the use of a fluorogenic substrate of cathepsin B, S, and L. Graphs represent averaged rates relative to untreated WT sample from 4 independent experiments. Error bars represent SEM. P values were determined by 1-way ANOVA. Relative abundance of the aspartic cathepsin D and the cysteine cathepsins B, S, and L were determined in whole cell lysates of untreated and IL-4–activated WT BMMØs by standardized semiquantitative Western blotting. (C) Representative images of western blots. Mature forms of cathepsins B, S, and L and intermediate form of cathepsin D are shown. (D) Average of band relative density from 3 independent Western blot experiments. Relative abundance of the aspartic cathepsin D and the cysteine cathepsins B, S, and L were determined in phagosomes isolated 60 minutes after particle uptake by untreated and IL-4–activated WT BMMØs by standardized semiquantitative Western blotting. (E) Representative images of western blots. Mature forms of cathepsins B, S, and L and intermediate form of cathepsin D are shown. (F) Average of band relative density from 5 independent Western blot experiments. Relative density was determined by calculation of pixel volume for treated sample relative to untreated BMMØ sample using Quantity One analysis software (Bio-Rad). Error bars denote SEM, P values were determined by paired t test.
BMMØs derived from Cybb−/− showed similar levels of cathepsin activities in both untreated and IL-4–activated lysates, confirming that NOX2 had no unexpected effects on total cellular levels of cysteine cathepsins. Semiquantitative immunoblotting of untreated and IL-4–activated BMMØ lysates confirmed that the total cellular levels of the mature forms of cathepsins S and L were greater in IL-4–activated BMMØs (3.07 ± 0.27- and 4.62 ± 0.71-fold, respectively; Figure 6C-D). This was also reflected in the increased presence of these proteases in phagosomes of IL-4–activated BMMØs (1.95 ± 0.36- and 2.03 ± 0.47-fold increase, respectively; Figure 6E-F).
Interestingly, the intermediate form of cathepsin D in whole-cell lysates was found to be increased with IL-4 activation. However, no change was found in the levels of cathepsin D mRNA or the intermediate form in phagosomes, consistent with unchanged levels of aspartic cathepsin activity in these compartments (Figure 4E-F). This finding may indicate differential processing, sequestration, trafficking, or secretion of this enzyme in AAMØs.6 Nonetheless, the up-regulation of cathepsin S and L is consistent with a NOX2-independent mechanism of IL-4–induced enhancement of phagosomal proteolysis. In addition, because both cathepsin S and L are cysteine cathepsins, increased expression of these redox-sensitive proteases would act synergistically with NOX2-down-regulation (increasing their local activities) to significantly enhance proteolysis within the phagosome.
IL-13 induces an increase in protease expression but does not alter NOX2 activity
Although IL-4 and IL-13 are known to have overlapping functions, they induce phenotypically distinct AAMØs.8,33 Having elucidated mechanisms that mediate IL-4–induced enhancement of phagosomal proteolysis, we investigated whether the same mechanisms underlie the more modest increase in proteolytic efficiency observed in IL-13–activated BMMØs. Initially, NOX2-mediated production of phagosomal ROS in the phagosome was measured in IL-13–activated BMMØs. In contrast to IL-4, activation of BMMØs with IL-13 did not reduce the activity of phagosomal NOX2, as evidenced by a respiratory burst comparable in magnitude and timing to that of untreated cells (supplemental Figure 6A-B). Thus, down-regulation of NOX2 activity does not play a role in the modest increase of phagosomal proteolysis seen with IL-13–activated BMMØs. IL-13–activated BMMØs did, however, display greater total cellular levels of cathepsin L (supplemental Figure 6C-D). These data suggest that the comparatively modest increase in proteolytic efficiency induced by IL-13 is solely derived from up-regulated expression of proteases, with no enhancement through down-regulation of NOX2 activity.
IL-4 activation increases the rate of disulfide reduction in phagosomes through down-regulation of phagosomal NOX2 activity
In addition to proteolysis, reduction of disulfides is required for efficient denaturation and degradation of phagocytosed proteins containing inter- or intramolecular disulfide bonds.34 Because NOX2 activity has been shown to compromise the efficiency of disulfide reduction within the phagosomes of macrophages,17 we hypothesized that in addition to the enhancement of peptide hydrolysis, IL-4–mediated down-regulation of NOX2 would also enhance the phagosome's ability to reduce protein disulfides. To investigate this hypothesis, we followed the intraphagosomal reduction of a modified cystine-based fluorogenic substrate conjugated to phagocytosed experimental particles. Reduction of the oxidized cystine disulfide releases 2 cysteine residues, dequenching attached BODIPY fluorophores.17
As predicted, we found that IL-4 activation induced a significant increase (4.44 ± 0.74-fold) in the rate of phagosomal disulfide reduction in WT BMMØs (Figure 7A-B). In comparison, IL-4 activation of BMMØs derived from Cybb−/− mice induced only a small change in reductive efficiency (1.38 ± 0.03-fold increase). Consistent with the pattern of reduction of the cystine-based substrate, IL-4 activation enhanced the efficiency of the phagosome to reduce intermolecular disulfides between heavy and light chains of phagocytosed IgG in both a NOX2-dependent and a NOX2-independent manner (Figure 7C-D). Together, these data demonstrate that IL-4 activation significantly enhances the reductive capacity of the phagosome in BMMØs. Moreover, these findings show that IL-4–mediated moderation of phagosomal NOX2 activity constitutes a common mechanism that enhances the efficiencies of both hydrolysis and disulfide reduction of phagocytosed protein. The combined increase of these catalytic efficiencies, would in turn, significantly improve the efficiency of AAMØs to degrade structural proteins containing disulfides, such as those often encountered in the course of tissue remodeling.
IL-4 activation enhances the reductive capacity of the phagosome in a NOX2-dependent fashion. The reductive capacity of the phagosome was assessed by measuring intraphagosomal rates of disulfide reduction in untreated and IL-4–activated, WT, and Cybb−/− BMMØs. (A-B) Measurement of fluorescence liberated through intraphagosomal reduction of a modified fluorogenic cystine-based reagent covalently bound to IgG-coupled experimental particles after phagocytosis, relative to calibration fluorescence. Inhibition of NOX2 was achieved with DPI (0.5μM) where indicated. (A) Real-time representative traces. RFU values are proportional to the degree of substrate reduction. (B) Averaged rates relative to DMSO-treated untreated WT samples from 4 independent experiments, error bars denote SEM, P values were determined by ANOVA. (C-D) Assessment of intraphagosomal efficiency to reduce inter-molecular disulfide bonds of particle-restricted IgG. Biotinylated anti-BSA IgG was used to opsonize BSA-coupled experimental particles and given to BMMØs in the presence of protease inhibitors. After 1 hour at 37°C, cells were lysed in nonreducing sample buffer and protein separated by SDS-PAGE. Biotinylated whole and heavy-chain IgG were identified by Western blot with the use of streptavidin-HRP and standard chemiluminescent detection. Despite the presence of protease inhibitors, increased loss of IgG in both whole and heavy chain forms was noted in IL-4–treated Cybb−/− BMMØs. Hence, the efficiency of IgG dissociation was assessed with the use of heavy chain density normalized to whole IgG band density. (C) Representative Western blot of reduction of whole IgG molecule in untreated and IL-4–activated BMMØs derived from WT and Cybb−/− mice. (D) Efficiency of reduction of IgG as expressed by ratio of heavy chain band density to whole IgG molecule band density. Band density was determined by calculation of pixel volume by the use of Quantity One analysis software (Bio-Rad). Graphs represent averaged data from 6 independent experiments. Error bars represent SEM. P values were determined by paired t test.
IL-4 activation enhances the reductive capacity of the phagosome in a NOX2-dependent fashion. The reductive capacity of the phagosome was assessed by measuring intraphagosomal rates of disulfide reduction in untreated and IL-4–activated, WT, and Cybb−/− BMMØs. (A-B) Measurement of fluorescence liberated through intraphagosomal reduction of a modified fluorogenic cystine-based reagent covalently bound to IgG-coupled experimental particles after phagocytosis, relative to calibration fluorescence. Inhibition of NOX2 was achieved with DPI (0.5μM) where indicated. (A) Real-time representative traces. RFU values are proportional to the degree of substrate reduction. (B) Averaged rates relative to DMSO-treated untreated WT samples from 4 independent experiments, error bars denote SEM, P values were determined by ANOVA. (C-D) Assessment of intraphagosomal efficiency to reduce inter-molecular disulfide bonds of particle-restricted IgG. Biotinylated anti-BSA IgG was used to opsonize BSA-coupled experimental particles and given to BMMØs in the presence of protease inhibitors. After 1 hour at 37°C, cells were lysed in nonreducing sample buffer and protein separated by SDS-PAGE. Biotinylated whole and heavy-chain IgG were identified by Western blot with the use of streptavidin-HRP and standard chemiluminescent detection. Despite the presence of protease inhibitors, increased loss of IgG in both whole and heavy chain forms was noted in IL-4–treated Cybb−/− BMMØs. Hence, the efficiency of IgG dissociation was assessed with the use of heavy chain density normalized to whole IgG band density. (C) Representative Western blot of reduction of whole IgG molecule in untreated and IL-4–activated BMMØs derived from WT and Cybb−/− mice. (D) Efficiency of reduction of IgG as expressed by ratio of heavy chain band density to whole IgG molecule band density. Band density was determined by calculation of pixel volume by the use of Quantity One analysis software (Bio-Rad). Graphs represent averaged data from 6 independent experiments. Error bars represent SEM. P values were determined by paired t test.
Discussion
Here we show that AAMØs activated by IL-4 possess a profoundly enhanced ability to hydrolyze proteins and reduce disulfide bonds within their phagosomes. Two synergistic mechanisms were found to underlie the increase in proteolytic capacity of the AAMØ phagosome. First, IL-4–induced expression of cathepsins S and L effectively increased the abundance of cysteine proteases delivered to the phagosome. Second, IL-4–mediated down-regulation of the NOX2 respiratory burst, caused at least in part by decreased cellular levels of the gp91phox subunit, enhanced the local activity of phagosomal cysteine cathepsins through the promotion of a more reductive lumenal microenvironment. We propose that together, these mechanisms act in a synergistic fashion in response to IL-4 activation, to dramatically and efficiently up-regulate the proteolytic capacity of the phagosome. Moreover, diminished NOX2 activity was associated with an enhanced ability of the AAMØ phagosome to reduce disulfide bonds. This would additionally increase the phagosome's efficiency to degrade proteins containing disulfides. These findings not only have implications regarding the function of AAMØs but detail a physiologically relevant example of redox control of phagosomal function.17
The findings from this study outline changes to the lumenal chemistries of the phagosome that are consistent with promoting AAMØ function in wound repair and tissue remodeling. The rapid and dramatic up-regulation of proteolytic efficiencies of the phagosome would intuitively enhance the clearance, degradation, and recycling of phagocytosed quiescent cells and postinflammatory debris in an IL-4–dominant environment. Aside from the advantageous role of tissue remodeling in healing, tumor-associated macrophages (TAMØs), which have characteristics in common with AAMØs, have been shown by Gocheva et al to promote tumor growth and invasion of an RT2 model of pancreatic islet cell cancer in mice.35 Interestingly, they attributed this feature of TAMØs to increased secretion of cysteine cathepsins in response to tumor-derived IL-4. Consistent with the IL-4 induction of cathepsin S and L in BMMØs documented in this study, they found that that TAMØs possessed high levels of active, undefined cysteine cathepsins. It would be interesting to determine the role of TAMØ endo-lysosomally located cathepsins in this phenotype and the influence of NOX2. The transcriptional regulation of the cysteine cathepsins by IL-4 pathways and their dependency on STAT6 have yet to be defined. However, given that STAT6-dependent up-regulation of certain cathepsins by IL-13 has been reported in lung tissue, it is probable that the up-regulation of cysteine cathepsins in AAMØs, as with arginase 1 and Ym1, also involves STAT6.21,36,37
Although likely to facilitate roles in tissue remodeling and wound repair, on the basis of current understanding, a high level of phagosomal proteolysis in AAMØs could be to the detriment of antigen processing efficiencies.25,26 Although intralumenal proteolysis is a prerequisite for antigen processing, too much proteolysis is thought to “overdigest” the oligopeptides needed for presentation. The authors of some studies have reported increased expression of MHC II and costimulatory molecules (CD40, CD80, and CD86), which, in addition to increased endocytic rates, points to a specific role of this polarized macrophage in antigen presentation.29,38 Although MHC-guided antigen processing may serve to protect T-cell epitopes from overdigestion,39 high rates of phagosomal proteolysis are generally inconsistent with an antigen presenting role in AAMØs. The relationships between phagosomal proteolysis, MHC-II expression, and antigen processing efficiency in AAMØs, however, have yet to be directly evaluated.
NOX2 is a major antimicrobial effector of the macrophage's phagosome. Conflicting observations regarding the effect of IL-4 treatment on the extracellular secretion of superoxide anions by macrophages have been reported.9,11,32,40-43 Here, we demonstrate that IL-4, but not IL-13, reliably diminishes the NOX2-mediated respiratory burst in the phagosome in both BMMØs and PMØs. This action was achieved through direct measurement of ROS within the lumenal microenvironment, which itself, as outlined, significantly influences other phagosomal chemistries.17 Lowered expression of gp91phox in response to IL-4, found in this study and by others,40,41 may fully account for diminished NOX2 activity on the phagosome.
However, it is possible that alterations to posttranslational modification and trafficking of NOX2 subunits, as well as modified receptor profiles and signaling pathways, may also act to diminish the respiratory burst in phagosomes.12,44,45 Particularly because Fcγ receptors are reportedly down-regulated after alternative activation,11,46 decreased signaling from these receptors in response to IgG-opsonized particles may also act to decrease NOX2 assembly on maturing phagosomes in AAMØs. Nevertheless, an IL-4–mediated decrease in the generation of phagosomal ROS, in addition to its inhibition of reactive nitrogen species generation,47,48 would undoubtedly compromise the phagosome's ability to kill phagocytosed microbes by oxidative means.
In this study, we also show that the IL-4–mediated down-regulation of NOX2 activity on the phagosome creates a more reductive lumenal microenvironment. In this scenario, it appears that the decrease in NOX2 activity is the major mechanism through which IL-4 mediates the enhancement of phagosomal disulfide reduction. However, in the absence of NOX2 function, IL-4 was still able to enhance disulfide reduction of phagocytosed cargo, albeit more modestly. This NOX2-independent mechanism is yet to be defined, but, increased expression of IFN-γ–inducible lysosomal thiolreductase reported in AAMØs may account for this additional enhancement of phagosomal disulfide reduction.21,49 Alternatively, altered expression or activity of the ill-defined machineries responsible for maintenance of the redox potential in the phagosome may be contribute to this phenotype. Nonetheless, by enhancing rates of disulfide reduction within the phagosome, IL-4 would not only promote local cysteine cathepsin activity but also facilitate protein denaturation that in turn would allow increased access of proteases to otherwise obscured stretches of polypeptide.18 The demonstrated importance of redox control of other cellular processes50 suggests that redox modification of this organelle by IL-4 may influence phagosomal chemistries beyond proteolysis and disulfide reduction, of which have yet to be defined.
Overall, this study shows that alternative activation of macrophages by IL-4 profoundly enhances the cell's capacity to degrade phagocytosed protein and outlines the synergistic mechanisms that drive this modification. Further to these findings, this study reiterates the importance of the crosstalk between spatio-temporally intimate phagosomal chemistries to the overall function and plasticity of this organelle.13,17,31 In this scenario, the majority of the enhancement of phagosomal protein degradation is not derived through modified protease expression or vesicular trafficking but through the regulation of a proximal antimicrobial effector.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank David Prescott, Laura Montoya, Dr Derek McKay, and Dr Yan Shi for their assistance during the study.
This work was supported by the Canadian Institutes of Health Research and Alberta Innovates- Health Solutions. B.L. was a visiting scientist at the University of Calgary facilitated in part by the Shandong Provincial Education Association for International Exchanges.
Authorship
Contribution: D.R.B., B.L., and R.M.Y. conceived and designed the experiments; D.R.B., B.L., E.R.O.A., J.M.R., and R.M.K performed experiments; and D.R.B., B.L., and R.M.Y. analyzed results and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robin M. Yates, Department of Comparative Biology and Experimental Medicine, Faculty of Veterinary Medicine, University of Calgary, 3330 Hospital Dr NW, Calgary, AB, T2N 4N1, Canada; e-mail: rmyates@ucalgary.ca.