Case presentations
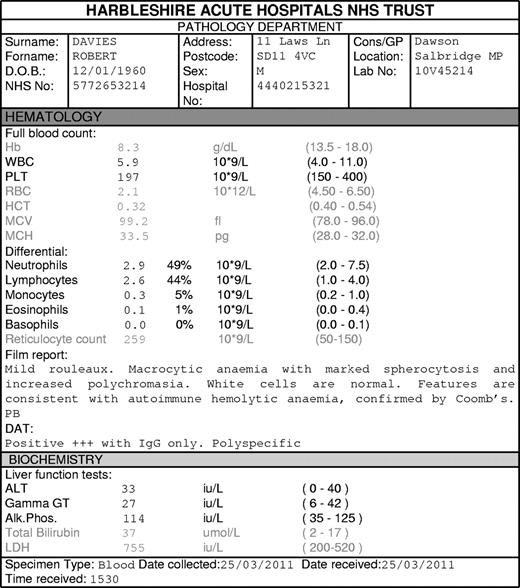
Case 1. Robert Davies, a 50-year-old man, presents with tiredness and lethargy. Examination demonstrates pallor and jaundice. Investigations reveal anemia that is consistent with warm autoimmune hemolytic anemia (AIHA; Figure 1). He has no evidence of any other autoimmune disease or chronic lymphocytic leukemia (CLL) and is not taking any medications; these factors suggest idiopathic AIHA. How should he be treated?*
AIHA is an uncommon but potentially fatal condition. It results from autoantibodies with specificity against RBC Ags leading to the premature removal of red cells from the circulation. Anemia may occur if the rate of red cell removal exceeds the ability of the BM to produce new red cells. AIHA may be secondary to drugs or underlying conditions such as CLL, infections, or autoimmune disorders. This guideline will deal specifically with idiopathic AIHA, and will not discuss secondary AIHA.
Several of the treatments in this article are not licensed for the treatment of AIHA and come with little evidence, so clinicians and patients should be informed of known risks.
A decision was made a priori to limit this review to published data only. Where there is no published evidence, or only expert opinion, we suggest referring to the recent Blood “How I treat” article on AIHA.1
Medline, Embase, and the Cochrane Library were searched for relevant articles, along with reference lists of identified articles. Search strategies are found in supplemental Data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Identified articles were then screened for inclusion. English-language articles were included only if they provided useful extractable data on adult (> 18 years) patients with idiopathic AIHA. Secondary AIHA, cold hemagglutinin disease, and patients with concomitant thrombocytopenia (Evans syndrome) were excluded.
Secondary AIHA was defined as AIHA in the presence of other autoimmune conditions, hematologic malignancies, or drugs known to cause AIHA, or where the authors of the article described the anemia as secondary. Conference abstracts were not searched. To reduce the number of articles, it was decided a priori that if a randomized controlled trial (RCT) was found, then nonrandomized controlled studies and case series would be excluded. If no RCT was found, only nonrandomized controlled studies would be included. If there were no nonrandomized controlled studies then case series with > 10 participants would be included; if there were no case series with > 10 participants then any case series would be included. Case reports were excluded. This is similar to the method used in the recent American Society of Hematology (ASH) Immune Thrombocytopenia Guidelines.2
Articles meeting the inclusion criteria underwent data extraction and quality assessment using pre-prepared forms (example found in supplemental data). Searching, data extraction, and quality assessment were all done in duplicate. The guidelines were given a Grading of Recommendations Assessment Development and Evaluation (GRADE) recommendation3 (summarized in Table 1).
Summary of GRADE recommendations for grading levels of evidence
| Grade . | |||
|---|---|---|---|
| 1 | Evidence strongly suggests that the benefit of the procedure outweighs potential risk or risks of the procedure outweighs potential benefits | A | Consistent evidence from systematic reviews or high quality randomized studies or high quality observational studies |
| B | Evidence from randomized and observational studies with important methodological flaws | ||
| 2 | Evidence suggests the benefit and risk of a procedure is finely balanced or uncertain | C | Evidence from randomized and observational studies with major methodological flaws or other sources of evidence e.g. case series |
| Grade . | |||
|---|---|---|---|
| 1 | Evidence strongly suggests that the benefit of the procedure outweighs potential risk or risks of the procedure outweighs potential benefits | A | Consistent evidence from systematic reviews or high quality randomized studies or high quality observational studies |
| B | Evidence from randomized and observational studies with important methodological flaws | ||
| 2 | Evidence suggests the benefit and risk of a procedure is finely balanced or uncertain | C | Evidence from randomized and observational studies with major methodological flaws or other sources of evidence e.g. case series |
Each recommendation in the guideline is given a numerical score which denotes how likely the patient is to gain benefit from the intervention and a letter which demonstrates the strength of the evidence.3
Search results
The search was last performed on October 18, 2010. The electronic search generated 7745 results; review of the title and occasionally the abstract produced 145 articles on the treatment of AIHA. More detailed review of the abstract provided 45 articles which were reviewed in full leading to the 27 included articles. One article was a nonrandomized controlled study; the remainder were case series. Summaries of the included articles and the quality assessment can be found in supplemental data.
Symptomatic treatment
The majority of articles described RBC transfusions as symptomatic treatment. Appropriate use of red cell transfusion is beyond the scope of this guideline and will not be discussed here.
Initial treatment
Before the introduction of corticosteroids in the 1940s, AIHA was almost exclusively treated with blood transfusion and splenectomy. Articles describing the response to corticosteroids did not provide enough detail to differentiate idiopathic from secondary AIHA and often used drug preparations no longer used.4,5 However, these articles established corticosteroids as the standard first-line treatment for AIHA. Two subsequent studies describe the response to corticosteroids in enough detail to extract information.6,7 Allgood et al followed 43 patients, of whom 32 (74%) responded to corticosteroids but 25 (78%) of the responders relapsed in the 9 months to 11 years of follow-up (definitions of response and relapse were unclear in this study).6 Zupanska et al demonstrated similar results with the majority of patients initially responding, but only 19 (46%) of 41 continuing to respond to treatment after 3 weeks.7
Pignon et al added 600-800 mg of danazol to 1 mg/kg prednisone for the initial treatment of AIHA and was able to demonstrate an excellent response (hemoglobin > 12.5 g/dL and prednisone < 5 mg/d) in 6 of 6 patients after a follow-up of 16-37 months.8 Side effects included cramps, myalgia, and abnormal liver function tests. It is unclear, as this was an uncontrolled study, whether there was benefit from the addition of danazol.
For the initial treatment of AIHA, we suggest:
The use of corticosteroids as a first-line treatment (GRADE 2C) but cannot suggest a specific dosing regimen.
That danazol could be considered in addition to corticosteroids but there is insufficient evidence to provide a recom-mendation.
Case 2: Despite treatment with corticosteroids there is still evidence of hemolysis severe enough to cause symptomatic anemia 4 weeks later. The patient also has side effects of the treatment with proximal myopathy. What treatments can be used if corticosteroids are ineffective or producing unacceptable side effects?
Relapsed and refractory disease
There is no good evidence to guide when AIHA should be described as refractory to treatment. The 2 most commonly described second-line treatments are splenectomy and rituximab. These treatments might also be used if a patient were intolerant of first-line therapies.
Splenectomy
The most commonly described treatment for relapsed or refractory AIHA is splenectomy. Unfortunately, evidence supporting its use is limited by heterogeneous study populations, variable outcome measures, and possible confounding variables. First, studies evaluating splenectomy frequently included patients who never previously received corticosteroids and to which the hemolysis might have been responsive. Second, outcome measures vary between studies making direct comparisons difficult, but we attempted to class responses as substantial (defined as no or minimal hemolysis), partial response (defined as an increase in hemoglobin and reduction in medications) or no response (defined as no improvement in hemoglobin and no reduction in medication). Last, although the efficacy of splenectomy would not be expected to change over time, surgical mortality has probably declined. A total of 8 studies were included.6,7,9-14 Patients demonstrating a substantial response varied from 3 (25%) of 12 to 9 (75%) of 12 with an average of 45% (73 of 162). The rate of partial response was between 1 (4%) of 28 and 10 (71%) of 14 with an average of 22% (35 of 162). Nonresponders to splenectomy varied between 0 (0%) of 12 and 9 (41%) of 22. From these data, it can be concluded that some response is achieved in between 59% and 100% of patients, although whether this response is substantial enough in many patients to justify the procedure is unclear.
Patients may relapse after a successful splenectomy and the reported studies have varying lengths of follow-up. Allgood et al reported initial complete response (CR) in 17 of 28 patients but at 1 year, 6 had relapsed.6 Grant et al also reported temporary remission in 5 of 12 patients relapsing at between 5 and 24 months.13 It is unclear when these patients relapsed whether the condition was as severe as before. Long-term remissions are described with rates of 70%10 and 63%12 reported with a mean follow-up of 40 and 33 months, respectively.
Mortality was reported at between 0 (0%) of 14 and 8 (29%) of 28. However, these studies reported operations performed between 1955 and 2003, during which considerable improvements in surgical techniques and supportive care occurred. Allgood et al reported procedures carried out between 1955 and 1965 and describe 8 (28.6%) deaths, but it is unclear how these patients died.6 Of the 21 reported cases in Cherthow et al, 2 died because of infection.11 Grant et al reported 5 deaths, 1 because of the procedure, 1 because of infection, and 3 because of hemolysis.13 The most recent article is Balague et al which reports 10 cases between 1993 and 2003, with the 1 reported death because of the hemolytic anemia.10 The article contains information on 255 laparoscopic splenectomies, with an overall mortality of 0.8%. Musser et al does not report any deaths in the 14 cases reported. It is unclear whether the infective deaths are because of the splenectomy or to concomitant immunosuppressant medications.14
mAbs
Rituximab.
Three articles were included which reported 42 relapsed or corticosteroid-refractory patients.15-17 Rituximab 375 mg/m2 was given weekly for 4 weeks. Follow-up was variable, but the CR rate (variably defined as normalization of hemoglobin and no ongoing treatment for hemolysis) ranged between 3 (29%) of 13 and 6 (55%) of 11 and with an average of 43% (18 of 42). Partial response (PR; defined as either hemoglobin > 10 g/L with an increase in hemoglobin of 2 g/dL ± ongoing hemolysis or need for corticosteroids at < 10 mg/d; or hemoglobin increase of > 2 g/L and improvement in signs of hemolysis; or transfusion independence or if previously transfusion independent an increase in hemoglobin of 2 g/L) was achieved in 21 (50%) of 42 (reported range 33%-77%).
Relapse was reported in all 3 case series. Bussone et al reported 2 of 13 responders relapsing after a mean follow-up of 20.9 months.16 The other 2 series followed up patients for 2-10317 and 0-6215 months. Some patients took several months to respond to treatment. Repeated doses of rituximab were used but it is unclear whether relapsed patients would benefit from repeat administration.
The safety of rituximab in this setting is described by Bussone et al16 : of the 27 patients receiving rituximab, 2 had infusion reactions, 1 severe sepsis, and 1 Pneumocystis jiroveci pneumonia. Two patients died in the 12 months after rituximab, 1 of limb ischemia and the other of a myocardial infarction. It is harder to determine true complications of rituximab from Dierickx et al as it reports deaths in 4 patients who did not respond to rituximab, 1 of malignancy, and 3 because of infection, and of the 53 responders, 15 either “relapsed, progressed, or died.”15 D'Arena et al does not report deaths or complications.17
Alemtuzumab.
Two patients with AIHA treated by corticosteroids and azathioprine received anti-CD52 mAb (alemtuzumab).18 One achieved CR with red cell independence off corticosteroids, the other a PR (> 50% decrease in transfusions on a small dose of prednisolone).
Immunomodulatory drugs
Azathioprine.
Worlledge et al reported 4 corticosteroid-treated patients who received azathioprine (100-200 mg/d): demonstrated anemia was controlled in 1 patient, no effect in 2 patients, and worsening anemia in 1 patient.19 Zupanska reported 26 patients receiving either azathioprine or cyclophosphamide and reported response rates of 4 of 26 excellent (no hemolysis and no relapse in 1 year), 10 of 26 good (compensated hemolysis on low-dose corticosteroids or immunosuppressive agents) and 12 of 26 fair (compensated hemolysis on higher doses).7 The dosing in these patients is unclear, as are the number of patients who received azathioprine and the number that received cyclophosphamide.
Danazol.
In addition to primary treatment, 3 studies reported on the efficacy of danazol as second-line treatment in patients whom had been previously treated with corticosteroids.8,20,21 Ahn et al21 included patients previously reported20 and demonstrated either a good (hematocrit 30%-40% with ≤ 5 mg of prednisone per day) or excellent (hematocrit > 40% with ≤ 5 mg of prednisone per day) response in 10 (83%) of 12 of the patients. This was in contrast to Pignon et al who reported failure (hemoglobin < 12.5 g/dL or prednisone > 10 mg/d) in 2 of 3 patients.8
Cyclosporine.
Hershko et al reported the administration of cyclosporine to 2 corticosteroid refractory patients.22 Both normalized their hemoglobin levels but required ongoing corticosteroids.
High-dose cyclophosphamide.
The use of high-dose cyclophosphamide was studied in 5 patients,23 all of whom were previously treated with corticosteroids. CR (normal hemoglobin-off transfusions with < 10 mg/d of prednisone) was attained in 4 of 5 patients with PR (hemoglobin > 10 g/dL and no transfusions) in 1 of 5.
Mycophenolate mofetil.
Vincristine.
Two case series describe the use of vincristine-loaded platelets in AIHA.26,27 All patients had previously been treated with corticosteroids. Ahn et al described 4 patients of whom 3 of 4 responded (no clear definition, but patients were able to reduce or stop their corticosteroids) although they required repeated doses.26 Shvidel et al demonstrated responses of hemoglobin > 10 g/dL in 2 of 2 patients.27
Plasma-derived therapies
Intravenous Ig.
Plasma exchange.
One small nonrandomized controlled study investigated the effect of plasma exchange in patients previously treated with corticosteroids and other treatments.31 In 5 patients who underwent plasma exchange compared with 4 controls, no differences in transfusion requirements were observed.
Others
HSCT.
The European Group for Blood and Marrow Transplantation published their registry data on the outcomes of transplantations for AIHA.32 Seven transplantations were performed. Five autologous transplantations produced 1 transient response, 2 no responses, and 2 deaths (1 because of disease and 1 because of the transplantation). Allogeneic transplantation led to 1 CR (normalization of blood counts) and 1 death.
We suggest, for the treatment of relapsed or refractory AIHA, that:
Rituximab (375 mg/m2 weekly for 4 weeks) or splenectomy should be considered over alternate therapies (GRADE 2C). Furthermore, although there are no head-to-head comparisons of rituximab and splenectomy, published evidence supporting the use of rituximab is more extensive than splenectomy (ungraded recommendation). The choice of second-line therapies should depend on patients' values and preferences and local circumstances.
There is some evidence for the use of alemtuzumab, cyclosporine, danazol, high-dose cyclophosphamide, mycophenolate mofetil, and vincristine-labeled platelets, but the small number of reported cases and the potential side effects prevent us from making a recommendation.
We found no evidence to support the use of azathioprine, HSCT, IVIG, or plasma exchange.
Discussion
Unfortunately, the evidence available for the treatment of AIHA is sparse and of low methodologic quality, being predominantly small case series. The recommendations made are therefore all of GRADE 2C level or expert opinion. We would encourage researchers to publish the results of any registry data and ideally perform randomized controlled trials evaluating different treatments for AIHA.
The online version of the article contains a data supplement.
The names and details of the patients used in the case presentations are fictitious and any resemblance to anyone alive or dead is coincidental.
Acknowledgments
The authors thank Gail Campbell for her help sourcing some of the papers.
Authorship
Contribution: M.C. designed the protocol, performed the search, data extraction, and quality assessment, and wrote the manuscript; Y.L.T.C. and I.K.G. performed the search, data extraction, and quality assessment; W.L. and M.A.C. designed the protocol and wrote the manuscript; and M.A.V. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark Crowther, Department of Haematology, Worcestershire Royal Hospital, Charles Hastings Way, Worcester, WR5 1DD, United Kingdom; e-mail: mark.crowther@nhs.net.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal