Abstract
Extramedullary (EM) manifestations of acute leukemia include a wide variety of clinically significant phenomena that often pose therapeutic dilemmas. Myeloid sarcoma (MS) and leukemia cutis (LC) represent 2 well-known EM manifestations with a range of clinical presentations. MS (also known as granulocytic sarcoma or chloroma) is a rare EM tumor of immature myeloid cells. LC specifically refers to the infiltration of the epidermis, dermis, or subcutis by neoplastic leukocytes (leukemia cells), resulting in clinically identifiable cutaneous lesions. The molecular mechanisms underlying EM involvement are not well defined, but recent immunophenotyping, cytogenetic, and molecular analysis are beginning to provide some understanding. Certain cytogenetic abnormalities are associated with increased risk of EM involvement, potentially through altering tissue-homing pathways. The prognostic significance of EM involvement is not fully understood. Therefore, it has been difficult to define the optimal treatment of patients with MS or LC. The timing of EM development at presentation versus relapse, involvement of the marrow, and AML risk classification help to determine our approach to treatment of EM disease.
Introduction
Acute leukemia may present in a variety of extramedullary (EM) tissues with or without bone marrow disease. EM involvement by acute leukemia is a relatively rare, but clinically significant, phenomenon that often poses therapeutic dilemmas. Myeloid sarcoma (MS) and leukemia cutis (LC) represent 2 well-known EM manifestations. MS (also known as granulocytic sarcoma or chloroma) is a rare EM tumor of immature myeloid cells. It was first described in 18111 and later named “chloroma” by King2 in 1853 because of its green color caused by the presence of myeloperoxidase (MPO).3 Five decades later, the relationship of MS to acute leukemia was identified.4 The term “granulocytic sarcoma” was introduced later by Rappaport to describe only tumors of granulocytic origin5 ; however, the term is now often applied to any tumor related to acute leukemia or myelodysplastic syndrome (MDS).
LC is the infiltration of the epidermis, dermis, or subcutis by neoplastic leukocytes (leukemia cells) resulting in clinically identifiable cutaneous lesions. LC commonly results in subcutaneous nodules and can be confusingly referred to as cutaneous granulocytic sarcoma.6,7 For the purposes of this article, LC will refer to cutaneous involvement only. LC has been described mostly in acute myeloid leukemia (AML), but also in the accelerated phase of chronic myeloid leukemia, MDS, and rarely in acute lymphocytic leukemias.7 In this article, we describe an approach and therapeutic strategies for patients with EM manifestations of leukemia by addressing a series of questions commonly raised by the practicing clinician.
How common are MS and LC, respectively?
MS is reported in 2.5%-9.1%8-10 of patients with AML and occurs concomitantly, following, or, rarely, antedating the onset of systemic bone marrow leukemia.11 Isolated MS, defined by the absence of a history of leukemia, MDS, or myeloproliferative neoplasm and a negative bone morrow biopsy, has been described in limited case reports.12 Many of these patients are often misdiagnosed with lymphoma.13,14 Certain known AML cytogenetic abnormalities, in particular t(8,21), have been associated with a higher incidence.15 MS can also develop at relapse with or without marrow involvement. The incidence of patients developing MS after allogeneic hematopoietic cell transplantation (HCT) has been reported to be 0.2%-1.3% with poor overall survival.16,17
LC occurs in ∼ 3% of patients with AML18 and less frequently in chronic leukemias.19 The reported incidence may be overestimated if biopsy is not performed because skin lesions similar to LC have a wide range of inflammatory, neoplastic, and infectious etiologies.19 Certain subtypes of AML are more commonly associated with skin infiltration. The most frequent association occurs with acute myelomonocytic and monocytic differentiation with involvement in up to 50% of patients.18,20,21 The incidence of LC may be higher in children, particularly infants with myeloid leukemia.22
How do MS and LC most often present clinically?
MS most frequently develops in patients with AML but can occur in association with accelerated-phase chronic myeloid leukemia, MDS, and, rarely, in the absence of marrow involvement.9,14,23 The frequency with which certain MS sites are accompanied by marrow involvement has not been adequately studied. The clinical manifestations of MS are diverse given the various sites of occurrence, with signs and symptoms determined by its specific location and size. The most common locations include the soft tissue, bone, periosteum, and lymph nodes; however, numerous sites have been described.14,23 Central nervous system (CNS) involvement is rare24-26 and is a distinct entity from meningeal leukemia, an EM leukemic manifestation not discussed in this article.
LC can develop concomitantly, following, or rarely antedating27 the onset of systemic leukemia. The latter, termed “aleukemic leukemia cutis,” occurs most often in patients who eventually develop AML.27 Aleukemic involvement is usually diffuse and papulonodular in presentation.28 In most patients, LC occurs after a diagnosis of leukemia has been established and most frequently presents with single or multiple erythematous papules and nodules.29 The cutaneous lesions produced by different leukemia subtypes have remarkable uniformity; however, a patient may develop distinctly different morphologies over the course of the disease.29,30 The most commonly involved anatomic location are the lower extremities followed by the upper extremities, back, trunk, and face.31 Interestingly, LC may have a predilection for sites of previous or current inflammation.31-33 In infant leukemia, LC is one of the causes of “blueberry muffin” appearance.22
When should a tissue biopsy be obtained, and which analyses on the tissue should be performed?
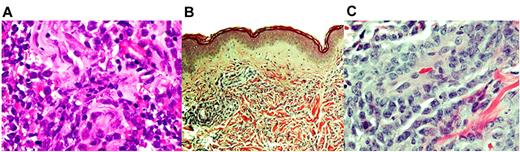
The diagnosis of MS in patients with an established leukemia is relatively straightforward and should always be included in the differential diagnosis of patients with AML who develop a soft tissue mass. Our practice in these patients is to always attempt to obtain a tissue sample to confirm the diagnosis if the risks of biopsy are reasonable. In general, a fine needle aspiration is usually not adequate for diagnosing a hematologic malignancy; however, when obtained, immunohistochemistry of the leukemia cells can be valuable. The morphologic appearance of MS on H&E can vary according to the degree of myeloid differentiation. MS typically consists of a diffuse and infiltrative population of myeloblasts and granulocytic cell components (Figure 1). The malignant cells are typically large with abundant cytoplasm and large nuclei. Importantly, the neoplastic cell lineage should be consistent with the underlying leukemia. In addition, we routinely send the tissue samples for immunohistochemistry (see next paragraph) and, when feasible, for flow cytometry, fluorescence in situ hybridization, and molecular analysis, although these assays are more difficult to perform on cells in tissue than bone marrow.
Histologic appearance of EM leukemia. (A) Hematoxylin and eosin staining of a MS along the right psoas muscle (original magnification ×40), demonstrating blasts in the skeletal muscle. Dense dermal infiltrate of monocytic leukemic cells (B; original magnification ×10; C: original magnification ×40), consistent with LC. Images were captured by the Olympus BX 41 microscope using an Olympus DP20 camera (Olympus) and imported directly as a series of .tif files.
Histologic appearance of EM leukemia. (A) Hematoxylin and eosin staining of a MS along the right psoas muscle (original magnification ×40), demonstrating blasts in the skeletal muscle. Dense dermal infiltrate of monocytic leukemic cells (B; original magnification ×10; C: original magnification ×40), consistent with LC. Images were captured by the Olympus BX 41 microscope using an Olympus DP20 camera (Olympus) and imported directly as a series of .tif files.
Immunohistochemistry is the most practical method for establishing the diagnosis of MS and can be an easier than flow cytometry, which requires cells to be in suspension. Myelocytic differentiation can be confirmed by Leder stain,9 which historically has been helpful in establishing the diagnosis in the absence of bone marrow leukemia. Immunohistochemistry can also discriminate between myeloid and nonmyeloid cells with monoclonal antibodies to MPO and lysozyme helpful in this regard.34 MPO staining is very often positive in the malignant cells of EM, which is a quick way for establishing the diagnosis and ruling out other tumors. A panel of immunohistochemical markers can be added for diagnosis confirmation and further lineage characterization (Figure 2).35,36 CD68-KP1 is the most commonly expressed marker followed by MPO.35
Immunophenotypic profile of EM myeloid leukemia. A panel of immunohistochemical markers may be useful in establishing the diagnosis of MS or LC. Myeloid (A) and monocytic (B) blast cells can express variable immunophenotypes, which may aid in their distinction.
Immunophenotypic profile of EM myeloid leukemia. A panel of immunohistochemical markers may be useful in establishing the diagnosis of MS or LC. Myeloid (A) and monocytic (B) blast cells can express variable immunophenotypes, which may aid in their distinction.
In the absence of a clinical history of leukemia, a diagnosis of MS can be difficult, and we always make every effort to obtain a tissue diagnosis. MS can often be misdiagnosed, most typically as non-Hodgkin lymphoma, in up to 46% of patients.14 This occurs most often with poorly differentiated MS, in which the morphology may resemble large-cell non-Hodgkin lymphoma when the cells are MPO-negative and weakly stained.37 Included in the differential diagnosis of MS are other forms of non-Hodgkin lymphoma, lymphoblastic leukemia, melanoma, Ewing sarcoma, blastic plasmacytoid dendritic cell neoplasm, and EM hematopoiesis.38 Similar to patients with a history of leukemia, we also send the tissue samples for immunohistochemistry, flow cytometry, fluorescence in situ hybridization, and molecular analysis. In addition, once the EM mass is established as leukemia, we perform a bone marrow biopsy, which is sent for identical studies.
Patients with suspected LC should always undergo biopsy unless there is a contraindication because a clinical diagnosis is not sufficient. The infiltrate is typically nodular, consisting of a population of mononuclear cells in a diffuse and infiltrative pattern (Figure 1B-C). Scattered macrophages or granulocytic cells may accompany the leukemic cells. Focal adnexal destruction can be present. Immunophenotypic profiling can be helpful in establishing the specific leukemic diagnosis (Figure 2). Staining for MPO may be absent in monocytic LC.7 Interestingly, the immunohistochemical profile in skin may be discordant with the immunophenotype of the leukemic blasts.39 A number of markers helpful in this regard have been recently reviewed.7,39 If a diagnosis of leukemia is not already established, a bone marrow biopsy should be performed.
Which cytogenetic abnormalities and genetic mutations are associated with MS and LC, respectively?
A variety of chromosomal abnormalities have been reported in patients with AML with EM involvement (Table 1). There have been limited associations between cytogenetic abnormalities and MS sites,40,41 and further study is required before specific cyto-genetic abnormalities can predict the site of involvement. The t(8;21) translocation is the most commonly reported cytogenetic abnormality associated with EM involvement, both at presentation and at relapse.15,42 In children, it has been associated with orbital MS.40,43 The inv(16) is another cytogenetic abnormality with a higher incidence of EM involvement, particularly in the abdomen.41 Molecularly, t(8;21) and inv(16) result in the AML1/ETO44 (RUNX1/RUNX1T1) and CBFβ/MYH1145 fusion genes, respectively, which carry a relatively favorable prognosis.46 EM involvement in infants has been associated with 11q23,47 which has a characteristic MLL rearrangement.48 Other reported abnormalities in MS include t(9;11),49 del(16q),50 t(8;17),51 t(8;16),52 and t(1;11).53 Abnormalities of chromosome 8 have also been associated with the development of LC in patients with AML.18,54 In particular, trisomy of chromosome 8 has been reported in a number of patients.55 Tetrasomy and pentasomy of chromosome 8 have also been reported in limited cases.56,57
Cytogenetic abnormalities in extramedullary leukemia
| Chromosome abnormality . | Associated genetic alterations . | MS/LC . |
|---|---|---|
| t(8;21)15,42 | AML1/ETO fusion44 | MS |
| inv(16)41 | CBFβ/MYH11 fusion45 | MS |
| 11q2347 | MLL rearrangment48 | MS |
| t(9,11)49 | — | MS |
| t(8;17)51 | — | MS |
| t(8;16)52 | — | MS |
| t(1;11)53 | — | MS |
| del(16q)50 | — | MS |
| del(5q)35 | — | MS |
| del(20q)35 | — | MS |
| Monosomy 735 | — | MS |
| Trisomy 435 | — | MS |
| Trisomy 818,35,55 | — | MS/LC |
| Trisomy 1135 | — | MS |
| Tetrasomy 856 | — | LC |
| Pentasomy 857 | — | LC |
| Chromosome abnormality . | Associated genetic alterations . | MS/LC . |
|---|---|---|
| t(8;21)15,42 | AML1/ETO fusion44 | MS |
| inv(16)41 | CBFβ/MYH11 fusion45 | MS |
| 11q2347 | MLL rearrangment48 | MS |
| t(9,11)49 | — | MS |
| t(8;17)51 | — | MS |
| t(8;16)52 | — | MS |
| t(1;11)53 | — | MS |
| del(16q)50 | — | MS |
| del(5q)35 | — | MS |
| del(20q)35 | — | MS |
| Monosomy 735 | — | MS |
| Trisomy 435 | — | MS |
| Trisomy 818,35,55 | — | MS/LC |
| Trisomy 1135 | — | MS |
| Tetrasomy 856 | — | LC |
| Pentasomy 857 | — | LC |
— indicates not applicable.
Recent systematic fluorescence in situ hybridization analysis on MS samples detected several chromosomal aberrations, including monosomy 7, trisomy 4, trisomy 8, trisomy 11, del(5q), and del(20q), among others previously mentioned.35 In this series, chromosomal aberrations were detected in 54% of patients, suggesting an overall high prevalence. The degree of concordance between the marrow and tissue has not been adequately studied. Pileri et al compared fluorescence in situ hybridization in tissue with karyotyping in the marrow or peripheral blood in a subset of 14 patients and found discordance in 4 patients with full concordance in the remainder.35 Given the small number of patients and the speculative etiologies of discordance, the significance of this finding is limited but worthy of further investigation.
EM disease has been increasingly reported in patients with acute promyelocytic leukemia (APL).58-60 The leukemia cells in APL are almost always characterized by the t(15;17) chromosomal translocation associated with the PML/RAR-α gene rearrangement.61 Most of the relapses occur in the CNS in the form of meningeal leukemia, but other EM sites have also been reported.62,63
Are prognostic genetic markers expressed in MS?
Molecular mutations and genetic aberrations have recently become an important tool in evaluating acute leukemia and assessing prognosis. However, there is limited information regarding the role of genetic mutation status in MS. Nucleophosmin gene (NPM1) mutation is commonly found in ∼ 50% of AML patients with a normal karyotype and carries a favorable prognosis.64 NPM1 mutations have been reported in ∼ 14% of MS in one study of 181 MS samples.65 FMS-related tyrosine kinase 3 gene (FLT3) mutations have been reported in ∼ 35% of AML patients with normal karyotype and carry an unfavorable prognosis.64 In a small pathologic series, FLT3 mutations were identified in 15% of MS cases.66 Interestingly, there was discordance between the MS and marrow in 2 of the patients. The rate and biologic significance of discordance between leukemic cells in the marrow and involved tissue of the same patient are unknown and require further study.
Which imaging tests aid in the diagnosis of MS and evaluating treatment response?
Given the wide variety of sites in which MS develops, imaging can facilitate diagnosis and monitor treatment response. MS often appears as a soft-tissue mass best suited to imaging by computed tomography (Figure 3A).67 Positron emission tomography can also be used (Figure 3C) and is particularly helpful for radiation therapy (RT) planning68 and monitoring response to treatment (Figure 4). When MS develops in the CNS, magnetic resonance imaging is useful.69 MS uniformly enhances with gadolinium, which should be administered when there are no contraindications (Figure 3B).67 The radiographic appearance of MS was recently reviewed by Fritz et al with many representative images provided.70 In our practice, computed tomography is routinely performed with consideration of a combined positron emission tomography scan if RT is planned.
Radiographic appearance of MS. MS along with the path of the right femoral nerve on (A) coronal computed tomography imaging, (B) coronal axial T1 postmagnetic resonance imaging, and (C) positron emission tomography.
Radiographic appearance of MS. MS along with the path of the right femoral nerve on (A) coronal computed tomography imaging, (B) coronal axial T1 postmagnetic resonance imaging, and (C) positron emission tomography.
Radiation therapy treatment field design and response. (A) MS in the right pelvis on positron emission tomography. (B) Radiation field treated to 24 Gy. Positron emission tomography scan 2 months after radiation, demonstrating resolution of lesion.
Radiation therapy treatment field design and response. (A) MS in the right pelvis on positron emission tomography. (B) Radiation field treated to 24 Gy. Positron emission tomography scan 2 months after radiation, demonstrating resolution of lesion.
Does the presence of EM involvement confer a worse prognosis than AML without EM disease?
Data on the prognostic significance of MS are limited. Although the presence of EM disease may be associated with a poor prognosis and shorter survival,23 5-year survival rates for patients with MS range between 20% and 30%, which appear similar to AML in general.71,72 The prognostic significance of cytogenetic alterations in the presence of MS is not fully understood. Although the presence of translocation t(8;21) is associated with a relatively good prognosis when treated with standard induction and intensive consolidation chemotherapy, it remains unclear whether this favorable prognosis remains in the presence of EM disease because there are conflicting reports.73,74 Byrd et al analyzed 84 AML patients with t(8;21) and reported that those with EM disease had significantly worse survival, which in part could have been the result of including a high proportion of patients with spinal or meningeal involvement.74 In a later study of 20 patients with isolated MS by Tsimberidou et al, the presence of cytogenetic abnormalities, specifically abnormalities in chromosome 8, appeared to confer a worse outcome, although this was not statistically significant.72 However, a more recent study by the same authors helped clarify this issue to some degree by comparing outcomes in 16 patients with isolated MS with those of a large cohort of AML who underwent standard treatment.75 When matching for age and cytogenetics in 14 patients, isolated MS was associated with improved event-free and overall survival when treated with anti-AML chemotherapy. Until we have more definitive data, we consider MS an additional poor prognostic factor in the overall evaluation of AML.
The presence of skin involvement has been suggested to be a marker of aggressive disease and poor outcome with shortened survival.27,54 However, a more contemporary study of 381 consecutive AML patients did not find that the presence of LC conferred a statistically significant worse response to treatment or shortened survival.18 Notably, there were trends toward shorter disease remission duration in patients with LC, which did not achieve significance probably because of the low number of patients.18 We consider LC a marker of aggressive disease that can be difficult to control and patients prone to EM relapses.
Treatment
Treatment strategies for MS are largely dependent on whether they develop at initial diagnosis or at relapse. LC almost always represents a local manifestation of underlying systemic disease and therefore should be managed as such. A summary of our treatment approach can be found in Table 2.
A summary of our approach to extramedullary disease
| MS development . | Extent of involvement . | Strategies . |
|---|---|---|
| Initial | Isolated | Intensive AML chemotherapy with consideration of RT as consolidation |
| Concurrent MS and marrow | Intensive AML chemotherapy with consideration of HCT; RT if MS persists after induction chemotherapy | |
| Relapse | Isolated | |
| After chemotherapy | Reinduction AML chemotherapy with consideration of HCT | |
| After transplant | Donor lymphocyte infusion, tapering of immunosuppression, RT, and/or clinical trial | |
| MS and marrow | ||
| After chemotherapy | Reinduction AML chemotherapy with consideration of HCT, RT, and/or clinical trial | |
| LC | Marrow status | Strategies |
| Negative | Intensive AML chemotherapy | |
| AML | Intensive AML chemotherapy with consideration of HCT; TSEB after chemotherapy for persistent LC if marrow negative |
| MS development . | Extent of involvement . | Strategies . |
|---|---|---|
| Initial | Isolated | Intensive AML chemotherapy with consideration of RT as consolidation |
| Concurrent MS and marrow | Intensive AML chemotherapy with consideration of HCT; RT if MS persists after induction chemotherapy | |
| Relapse | Isolated | |
| After chemotherapy | Reinduction AML chemotherapy with consideration of HCT | |
| After transplant | Donor lymphocyte infusion, tapering of immunosuppression, RT, and/or clinical trial | |
| MS and marrow | ||
| After chemotherapy | Reinduction AML chemotherapy with consideration of HCT, RT, and/or clinical trial | |
| LC | Marrow status | Strategies |
| Negative | Intensive AML chemotherapy | |
| AML | Intensive AML chemotherapy with consideration of HCT; TSEB after chemotherapy for persistent LC if marrow negative |
TSEB indicates total skin electron beam therapy.
MS
Should chemotherapy be initiated for patients with isolated MS without marrow involvement?
Although the optimal timing and treatment of isolated MS are not clear, delayed or inadequately systemically treated isolated MS will almost always progress to AML.13 The median time to the development of AML in this setting ranges from 5-12 months.9,72,76 Using RT-PCR, gene fusions specific for AML in the bone marrow of patients with isolated MS have been detected, suggesting that marrow involvement might occur early in the process before clinical detection.77
In our practice, we use remission-induction chemotherapy similar to that used for AML.23,72,76,78-80 The postremission chemotherapy has not been adequately studied in isolated MS; and in particular, the role of HCT is not clear. We judge each patient individually and assess multiple prognostic factors, including age, comorbidities, degree of dissemination, and cytogenetic and molecular abnormalities when deciding on a postremission strategy. After chemotherapy, we consider RT a consolidation treatment for isolated MS, particularly because the effective RT dose is low. The radiation site and associated toxicities are important factors in this consideration. In circumstances that require debulking or rapid symptom relief because of compression of a vital structure, we consider RT or even surgery in certain patients upfront, followed by aggressive chemotherapy; however, there is no evidence that this combined approach is superior to aggressive chemotherapy alone.
Is treatment intensification with HCT after chemotherapy warranted for patients with concurrent MS and marrow involvement?
MS presenting concurrently with marrow involvement always warrants systemic treatment directed at the underlying leukemia. There have been no randomized trials addressing the optimal treatment for patients with EM involvement. Two retrospective studies have demonstrated superior outcomes with the use of allogeneic or autologous HCT.35,81 In a study by Chevallier et al, which assessed the outcome of 51 patients with MS who underwent allogeneic HCT, 5-year overall survival was 47%.81 Remission status at the time of HCT is an important prognostic consideration.81 Given the favorable results, we consider treatment intensification with allogeneic HCT for patients with MS and concurrent marrow involvement together with other patient-related factors mentioned in the previous section, including standard age- and cytogenetic- and molecular-based risk profiling. Most often, our approach is to treat with conventional AML-type chemotherapy,23,71 with regimen choice and dosing following standard age- and cytogenetic-based risk profiling.82-84 In our practice, we consider RT in these instances in which less than a complete response is achieved with chemotherapy because incomplete MS response after chemotherapy represents a significant risk for early bone marrow relapse after therapy.14
Should isolated MS relapse be treated as a systemic relapse?
Isolated MS at relapse is rare and often heralds systemic relapse. The median time to marrow relapse in this setting is ∼ 7 months.85 Treatment strategies are dependent on whether the patient relapsed after chemotherapy alone or after HCT. For patients who have relapsed after chemotherapy alone, we often use a strategy of reinduction chemotherapy and RT to the tumor. There is no standard chemotherapy regimen for relapsed AML, and our approach would be to select a regimen that would have applied to relapsed AML. We often recommend HCT, although its potential benefits in this setting are unclear. As the optimal treatment is not defined, we also consider entry into a clinical trial if relapsed MS is included among the eligibility criteria.
MS after HCT has conventionally been considered the first manifestation of relapsed systemic disease; however, rare cases of isolated MS after HCT have been described.86,87 The outcome of these patients is poor, and there is no standard management in this setting. We judge each of these patients individually, and our approach may include donor lymphocyte infusion,87-89 tapering of immunosuppression if tolerated, or investigational agents if patients are eligible. We also consider RT mostly a palliative modality, particularly after HCT in which further chemotherapy may be difficult to deliver. The hypomethylating agent 5-azacitidine, which can be administered after HCT, represents an alternative strategy, although it is not adequately studied in MS.90
What are the salvage treatment options for bone marrow and EM relapse?
Frequently, MS develops concurrently with marrow relapse. Reinduction chemotherapy is always warranted. We consider RT in patients in whom a complete response is not achieved with chemotherapy. The potential benefits of HCT in this setting are not defined and should be carefully considered. When bone marrow and EM relapse occurs synchronously after transplantation, survival is very poor,17 and we consider entry into a clinical trial or palliative measures depending on the context.
When is the addition of RT indicated?
There have been limited studies addressing the role of RT in the management of MS.68,91 In our practice, we consider RT in patients with isolated MS, inadequate response to chemotherapy, recurrence after HCT, and in circumstances that require rapid symptom relief because of vital structure compression. RT results in excellent, durable local control at the targeted site68 ; however, it is not clear that the addition of RT results in a superior overall outcome compared with chemotherapy alone. A low-dose RT regimen of 24 Gy in 12 fractions using conventional treatment can be applied to the majority of patients with excellent disease control and minimal morbidity.68 Notably, RT within these dose ranges does not preclude using total body irradiation as an HCT conditioning regimen or vice versa.
Should patients with APL undergo CNS prophylaxis for EM disease?
The increasingly reported number of APL patients with CNS relapse is of concern because of its poor associated outcome in an otherwise favorable disease. The Gruppo Italiano Malattie Ematologiche dell'Adulto recently reported that the proportion of CNS relapses among all relapses has increased in the more recent AIDA (all-trans retinoic acid + idarubicin) 2000 study compared with there earlier protocol AIDA 0493 study conducted during the 1990s.92 It is not clear whether the administration of all-trans-retinoic acid contributes to the possible increased incidence in the all-trans–retinoic acid era or whether it is attributable to prolonged overall survival. CNS disease appears to occur in patients with high-risk disease; therefore, it is reasonable for those patients to receive intrathecal prophylaxis to prevent EM (CNS) disease once in remission. Although further study is needed to determine how to prevent CNS relapses, we consider prophylactic intrathecal therapy for patients with relapsed APL who present with or develop leukocytosis during the initial phase of treatment. The best prophylaxis approach has not been determined, but we usually administer 5 doses of intrathecal methotrexate during the consolidation cycles without cranial radiation.
LC
Is chemotherapy indicated in patients with aleukemic LC?
The literature on aleukemic LC is very limited.93 In our practice, we manage patients with aleukemic LC similarly to isolated MS using intensive chemotherapy.
Is treatment intensification with HCT warranted in patients with LC?
Curative treatment should be aimed at eradicating the systemic disease using chemotherapy and/or HCT. However, chemotherapy adequate to induce and maintain marrow remission does not always control cutaneous involvement.54 Some investigators have advocated intensified treatment with HCT in the first remission.18 When examined specifically, allogeneic HCT in patients with LC has not resulted in differences in overall relapse rates compared with non-LC patients; however, LC patients have a remarkable tendency to relapse in EM sites, including the skin after HCT.94 In patients with LC that develops after HCT, the graft-versus-leukemia effect may be important for cutaneous disease control, and tapering immunosuppressive medications has anecdotally been shown to be helpful in this regard.87 We consider treatment intensification with allogeneic HCT for patients with LC in conjunction with other patient-related prognostic factors. When HCT is not planned, our approach is to treat with conventional AML-type chemotherapy, with regimen choice and dosing following standard age- and cytogenetic- and molecular-based risk profiling.82-84
When should RT be considered?
Control of cutaneous involvement is essential for long-term disease control because blasts from the skin may reseed the marrow resulting relapse. Therefore, skin-directed therapies, such as RT, can be an important part of treatment.95-99 From a radiation perspective, therapy is definitive only in the setting of marrow remission because leukemic cells in the marrow will continue to reseed the skin if they are not eradicated. In the setting in which there is a negative marrow but persistent LC, our approach is to use total skin electron beam therapy to ensure maximal disease control.99 By contrast, we treat patients with active marrow disease at the time of RT in a palliative manner, with RT directed at symptomatic lesions only. In such patients, RT provides rapid symptom relief of lesion-associated pain and pruritus.99 Total skin electron beam is not appropriate in this setting unless the patient has diffuse, symptomatic disease. In patients in whom there is a complete response in the skin and marrow after chemotherapy, there is no evidence that RT offers any benefit.
Is toxicity a concern when chemotherapy is administered near the time of radiation?
Although generally well tolerated, it is important for both hematologists/oncologists and radiation oncologists to be aware of the development of subacute severe skin toxicity. Previous cases in the literature describe the development of a severe skin reaction with doxorubicin administered near the time of total skin electron beam.54,100 Therefore, it has been suggested that high-dose cytarabine should be used in place of anthracyclines when chemotherapy is to be given in conjunction with RT.54 However, in our recent series, a case of severe radiation recall occurred after administration of clofarabine and cytarabine, raising questions about these recommendations.99 Caution is advised when chemotherapy is being administered shortly after radiation, particularly total skin electron beam.
Should patients with MS or LC be followed differently than other AML patients?
Patients with MS and LC have a predisposition to EM relapses.94 After treatment, we follow patients with MS or LC similar to other AML patients, including detailed physical examinations and routine peripheral blood to confirm continued complete remission. We always biopsy any new or suspicious soft tissue or skin abnormality; and if positive for recurrence, we always reevaluate the marrow. Repeat imaging is reasonable for patients with MS after treatment completion. In our practice, if the imaging studies are negative after treatment, further scans are not routinely performed.
What is our current understanding of the pathogenesis of EM?
The mechanisms for EM involvement are not fully understood. In general, homing to specific tissues is intricately controlled by expression of different chemokine receptors and adhesion molecules. Blast neural cell adhesion molecule (CD56) has long been implicated in EM pathogenesis (Figure 5).14 Supporting this, neural cell adhesion molecule blast expression has been associated with a high incidence of MS101,102 and is common in patients with t(8;21).103 Mechanistically, neural cell adhesion molecule is also highly expressed in breast, testicular, ovarian, and gut tissue, which could account for EM homing to these sites.85,104 In the case of inv(16), it has been speculated that the deregulation of CBF transcription factors involved in cellular adhesion and recognition may play a role in the pathogenesis of MS45 ; however, mechanistic studies are needed to support this.
Leukemic cell surface markers implicated in EM homing. Blast cell CD56 (neural cell adhesion molecule) expression has been associated with both MS and LC. Homing of blast cells into the skin may be mediated through interactions between lymphocyte function-associated antigen-1 (LFA-1) and intercellular adhesion molecule-1 (ICAM-1) and/or cutaneous leukocyte antigen (CLA) and E-selectin.
Leukemic cell surface markers implicated in EM homing. Blast cell CD56 (neural cell adhesion molecule) expression has been associated with both MS and LC. Homing of blast cells into the skin may be mediated through interactions between lymphocyte function-associated antigen-1 (LFA-1) and intercellular adhesion molecule-1 (ICAM-1) and/or cutaneous leukocyte antigen (CLA) and E-selectin.
MS at relapse may occur in EM sites, such as CNS structures and reproductive organs, which have inherent barriers105,106 and are known to escape systemic therapy. Interestingly, among patients developing MS after transplantation, a large percentage (48%) occurred in the CNS and ovaries, suggesting that MS may arise in sanctuary sites where leukemic cells survive treatment with chemoradiotherapy.16,17 The administration of intrathecal chemotherapy and the use of a testis boost for men during total body irradiation can overcome these barriers. We recently reported a case of recurrent MS along peripheral nerves87 and hypothesized that the existence of a blood-nerve barrier could explain how relapse of acute leukemia might originate with leukemic cells that have persisted in peripheral nerves. Therefore, it is surprising that more relapses do not occur in the peripheral nervous system because no such treatment method exists for bypassing the blood-nerve barrier.
The underlying molecular basis responsible for the invasion of leukemic cells into the skin is also not fully understood. Expression of T-cell antigens by blast cells has also been associated with high incidences of EM involvement.107 It is probable that such T-cell antigens result in skin-selective homing of a unique subpopulation of leukemic cells (Figure 5). Similar to MS, expression of CD56 may be associated with cutaneous involvement. One study has demonstrated that CD56+ AML developed more skin manifestations than CD56− AML.108 In a recent review, Cho-Vega et al hypothesized that the presence of similar chemokine receptors and adhesion molecules in leukemic cells with normal memory T cells, which home to the skin, may explain why certain leukemias have a predilection for LC development.7 Supporting this hypothesis, immunophenotypic analysis has demonstrated that blast expression of T-cell antigens is associated with a higher incidence of LC.109 Cutaneous lymphocyte antigen, which is involved in T-cell homing to the skin,110 was found to be elevated in a small series of patients with acute myelomonocytic leukemia.111 Cutaneous lymphocyte antigen's interaction with E-selectin on dermal endothelial cells may potentially explain the tropism of this leukemia to the skin. Endothelial intercellular adhesion molecule-1, which interacts with lymphocyte function-associated antigen-1 on blast cells, may also play a role in skin homing.7 It has been suggested that certain therapies, including all-trans retinoic acid, may alter the expression of these cell adhesion molecules and thereby facilitate cells leaving the marrow and circulating toward EM sites.112 Specifically, up-regulation of intercellular adhesion molecule-1 and lymphocyte function-associated antigen-1 has been hypothesized to be involved.113
Future studies
Establishing the underlying molecular basis for the migration of leukemia cells to specific sites is critical to understanding the specific pathophysiology of EM involvement and developing novel therapies. Cellular homing studies and chemotactic assays further characterizing the specific chemokine and cellular adhesion molecules involved in directing blast cells to a particular site are essential in establishing such mechanisms with clinical correlations required to confirm their relevance. Once properly identified, blast chemokine or adhesion molecule receptor profiling could potentially serve to predict EM sites of involvement as well as be exploited therapeutically with targeted agents.
Our understanding of EM involvement needs to evolve in parallel with our expanding knowledge of AML. The frequency with which EM disease has distinct genetic or cytogenetic profiles from the marrow will require further study. In the case of abnormal cytogenetics, the concordance between marrow and tissue needs to be established, and if discordant, the abnormalities driving prognosis identified. In the case of normal cytogenetics, the mutation status of genes known be prognostically important in AML, such as NPM1 and FLT3, as well as other novel mutations, needs to be more rigorously studied in MS, and their prognostic significance and concordance with the marrow better defined. It might also be of interest to study the molecular and cytogenetic concordance as a function of time from marrow involvement. Conceivably, EM disease that develops concurrent with or after marrow involvement might represent a clonal evolution from the marrow and contain additional abnormalities. Ultimately, the genetic and/or cytogenetic profile of MS and LC should be integrated into the risk classification of AML to more definitively determine whether and when novel treatment strategies are warranted. Given the rarity of MS and LC, such studies will require the collaboration of multiple cooperative groups to create a registry, procure tissue, conduct uniform correlative laboratory studies, develop clinical trials, and generate treatment guidelines.
Acknowledgments
The authors thank Dr Christopher Y. Park for advice on some of the figures.
This work was supported by the Lymphoma Foundation and the Sports Foundation Against Cancer. R.L.B. is a recipient of the Dr Mortimer J. Lacher Clinical Lymphoma Fellowship.
Authorship
Contribution: R.L.B. and J.Y. designed the manuscript; R.L.B. performed the literature review and designed the figures and tables; and all authors wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joachim Yahalom, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: yahalomj@mskcc.org.