Loss-of-function mutations in Janus kinase 3 (JAK3) are an underlying cause of severe combined immunodeficiency (SCID), whereas hyperactive JAK3 mutants have been identified in hematolocigal malignancies.1-3 In this issue of Blood, Elliot et al place JAK3 again under the magnifying lens and describe novel gain-of-function mutations in the FERM (Founding members: band 4.1, Ezrin, Radixin, and Moesin) domain of JAK3 in adult patients with T-cell leukemia/lymphoma (ATLL).4
Human T-cell lymphotropic virus 1 (HTLV-1) is the primary etiologic agent in ATLL. The retrovirus infection leads to the expression of the TAX oncogene that activates the common γ chain (cγc) cytokine networks to promote erratic cell growth. JAKs are nonreceptor tyrosine kinases that mediate cytokine function by activating cell signaling pathways.5 Unlike other JAK family members, the function of JAK3 is restricted: it mediates exclusively the cγc cytokine family signaling in lymphocytes, which makes it essential for the development of a proper adaptive immune system. However, mutated and consequently overactive JAK3 can also cause the dysregulation of myeloid cell growth.2 Structure-function studies have identified 4 functional domains in the JAK structure (see figure).4 The catalytic kinase domain phosphorylates the target proteins, while the pseudokinase domain has an important regulatory function as clearly attested by the JAK2Y617F mutation found in myeloproliferative disorders (MPDs). The function of the SH2 domain is currently incompletely understood, whereas the N-terminal FERM domain is primarily thought to mediate JAK binding to receptor chains. Importantly, previous studies have also shown that, like the pseudokinase domain, the FERM domain also has autoregulative properties and can potentiate JAK3 kinase activity.6
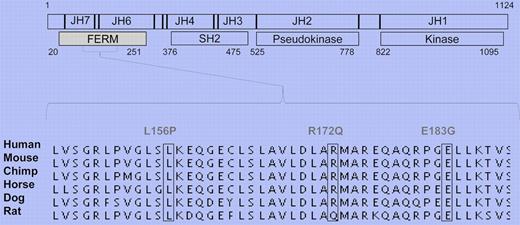
JAK3 FERM domain gain-of-function mutations in ATLL. Schematic presentation of JAK3 structure. Identified mutations occur in highly conserved residues.
JAK3 FERM domain gain-of-function mutations in ATLL. Schematic presentation of JAK3 structure. Identified mutations occur in highly conserved residues.
The previously reported findings that show the importance of cγc cytokines in ATLL pathology prompted Elliot and colleagues to sequence JAK3 in patients. They screened 36 ATLL patients and 24 ethnically matched controls and identified novel somatic JAK3 FERM domain mutations in 4 patients. The mutations occurred in residues that are highly conserved in mammals (see figure).4 They went on to show that the patient mutations promote JAK3 kinase activity and the signaling pathways that regulate downstream cell growth. When mutated JAK3 was introduced to a pro-B cell line, cells lost their dependency on IL-3, which directly implies transforming potential for the identified mutations. The complete crystal structure of JAK3 has not yet been solved; therefore, the authors used the decoded FAK structure to model the interaction that JAK3 FERM might have with its kinase domain. This molecular modeling data suggested that JAK3 FERM can indeed have intramolecular interactions, and the patient mutations may interfere with this mode of autoregulation. This model is in line with previous in vitro experiments that show the direct interaction of these two domains.6 Furthermore, the mutated JAK3 proteins had an elongated half-life in the cell, which can also contribute to the enhanced activation of downstream signaling pathways and dysregulated cell growth.
Interfering with JAK3 activity in autoimmunity has received much attention over the past few years. Because of its critical and restricted function in lymphoid cells, inhibiting JAK3 is considered an efficient and well-tolerated approach to inhibit proinflammatory lymphocytes in autoimmune diseases. Elliot and colleagues investigate whether pro-B cells transduced with ATLL mutant JAK3 respond to a well-established JAK3 inhibitor, Tofacitinib (CP-690,550).7 They found that the cells that contain mutated JAK3 are considerably more sensitive to the inhibitor compared with the cells with wild-type JAK3. Interestingly, the authors also showed that Tofacitinib inhibits the ATLL cell lines where JAK3 FERM mutations are not present. These findings are analogous with a recent study by Ju et al where Tofacitinib was successfully used to restrain the malignant growth of ATLL cells ex vivo and the IL-15–driven mouse leukemia model8
With current treatments ATLL remains a disease with a poor prognosis. Therefore, developing novel therapeutic approaches is critical. The study by Elliot and colleagues shows that some of the ATLL patients have gain-of-function mutations in their JAK3 FERM domain, which offers novel insights into the pathology of ATLL and the regulation of this kinase. At present, there are more than a dozen clinical trials under way testing JAK3 inhibitors in autoimmune diseases. Preliminary data from these first trials demonstrate efficacy with acceptable toxicity.9 Findings by Elliot et al emphasize that it is increasingly important to consider novel JAK inhibitors in the treatment of hematologic malignancies as well. Inhibiting JAK1/2 in myelofibrosis, for example, has recently shown promising results.10
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal