Abstract
In lymphocytes, the phosphoinositide 3′-kinase (PI3K) isoform p110δ (PI3Kδ) transmits signals from surface receptors, including the B-cell receptor (BCR). CAL-101, a selective inhibitor of PI3Kδ, displays clinical activity in CLL, causing rapid lymph node shrinkage and a transient lymphocytosis. Inhibition of pro-survival pathways, the presumed mechanism of CAL-101, does not explain this characteristic pattern of activity. Therefore, we tested CAL-101 in assays that model CLL-microenvironment interactions in vitro. We found that CAL-101 inhibits CLL cell chemotaxis toward CXCL12 and CXCL13 and migration beneath stromal cells (pseudoemperipolesis). CAL-101 also down-regulates secretion of chemokines in stromal cocultures and after BCR triggering. CAL-101 reduces survival signals derived from the BCR or from nurse-like cells, and inhibits BCR- and chemokine-receptor–induced AKT and MAP kinase (ERK) activation. In stromal cocultures, CAL-101 sensitizes CLL cells toward bendamustine, fludarabine, and dexamethasone. These results are corroborated by clinical data showing marked reductions in circulating CCL3, CCL4, and CXCL13 levels, and a surge in lymphocytosis during CAL-101 treatment. Thus, CAL-101 displays a dual mechanism of action, directly decreasing cell survival while reducing interactions that retain CLL cells in protective tissue microenvironments. These data provide an explanation for the clinical activity of CAL-101, and a roadmap for future therapeutic development.
Introduction
Chronic lymphocytic leukemia (CLL), the most common leukemia in Western countries, is characterized by the accumulation of CD5+/CD23+ monoclonal B cells in the blood and tissue compartments (marrow and secondary lymphatic tissues).1 CLL cells are resistant to cell death in vivo. However, they rapidly die from spontaneous apoptosis once removed from the patient unless they are cocultured with accessory stromal cells, such as marrow stromal cells (MSCs)2 or monocyte-derived nurse-like cells (NLCs).3 Cross-talk between CLL cells and these supporting cells in tissue microenvironments comprises a complex signaling network that may be critical for disease progression and drug resistance. Interference with this cross-talk may constitute a new therapeutic target. Several molecular pathways related to leukemia cell migration, B-cell receptor (BCR) signaling, and interactions between CLL cells and T cells have been identified over recent years (reviewed in Burger et al4 ).
The chemokines, CXCL12 and CXCL13, are constitutively secreted by MSCs and NLCs5,6 and attract CLL cells via their respective cognate chemokine receptors, CXCR4, CXCR5, thereby regulating homing and retention of the leukemia cells in the tissue compartments. In addition, BCR signaling in the lymphatic tissue microenvironment promotes the clonal expansion of normal and malignant B cells.1,7,8 CLL cells isolated from lymph nodes8 or high-risk patients9 display gene expression profiles that indicate BCR activation. In response to BCR activation and in NLC cocultures, CLL cells secrete the chemokines CCL3 and CCL4 (also called MIP-1α and β),10 presumably for recruitment of accessory cells, such as regulatory T cells.11,12 We proposed that the secretion of CCL3 and CCL4 by CLL cells correlates with the responsiveness of the BCR, based on higher secretion of CCL3/4 in ZAP-70+ cases,10 and a close correlation between CCL3 plasma levels and ZAP-70, IgHV mutational status, and prognosis.13
Phosphoinositide 3′-kinases (PI3Ks) integrate and transmit signals from diverse surface molecules, such as the BCR,14 chemokine receptors, and adhesion molecules, thereby regulating key cellular functions, including growth, survival, and migration.15 The PI3Ks are divided into 3 classes; I, II, and III. The class I kinases contain 4 isoforms designated PI3Kα, β, γ, and δ. While the PI3Kα and β isoforms are ubiquitously expressed and the PI3Kγ isoform has a particular role in T-cell activation, PI3Kδ expression is largely restricted to hematopoietic cells, where it plays a critical role in B-cell homeostasis and function.16 Mice with inactivating PI3Kδ mutations have reduced numbers of B1 and marginal zone B cells, show reduced levels of immunoglobulins, display poor responses to immunization, manifest defective BCR and CD40 signaling, and can develop inflammatory bowel disease.16-18
CAL-101 is a potent and highly selective PI3Kδ inhibitor19 that promotes apoptosis in B-cell lines and primary cells from patients with different B-cell malignancies, including CLL,20 mantle cell lymphoma and multiple myeloma.19,21 CAL-101 inhibits constitutive and CD40-, TNF-α–, fibronectin-, and BCR-derived PI3K signaling leading to suppression of Akt activation.19-21 These studies suggested that disruption of intrinsic and extrinsic survival signals could be a critical mechanism for the clinical activity of CAL-101.
In CLL patients, CAL-101 induces a redistribution of CLL cells from the tissue compartments into the blood, causing a rapid and sustained lymph node size reduction and a transient lymphocytosis during the first weeks of treatment.22 These findings suggest that survival pathways may not be the only target of CAL-101, at least during early treatment, and that disruption of CLL cell migration, homing, and underlying chemokine networks could be involved. To investigate this hypothesis, we characterized the activity of CAL-101 in assays that model the complex in vivo interactions between CLL cells and their microenvironments.
Methods
CLL cell purification, cell lines, cell viability testing, and reagents
After informed consent, peripheral blood samples were obtained from patients fulfilling diagnostic and immunophenotypic criteria for CLL at the Leukemia Department at M. D. Anderson Cancer Center. Patient consent for samples used in this study was obtained in accordance with the Declaration of Helsinki on protocols that were reviewed and approved by the Institutional Review Board at M. D. Anderson Cancer Center. PBMCs were isolated via density gradient centrifugation over Ficoll-Paque (GE Healthcare) and were used fresh or were placed into FBS (BD Biosciences) plus 10% DMSO (Sigma-Aldrich) for viable frozen storage in liquid nitrogen. CD19/CD5-positive CLL cells accounted for > 90% of analyzed cells (see supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The murine MSC lines, KUSA-H1, and 9-15C, were purchased from the Riken cell bank and maintained in RPMI 1640 medium supplemented with 2.05-mM l-glutamine (HyClone), 10% FBS (SAFC Biosciences), and penicillin-streptomycin (Cellgro). The murine stromal cell line TSt-4 derived from fetal thymus tissue (also from Riken) was maintained in RPMI 1640 medium supplemented with 2.05-mM l-glutamine, 5% FBS, and penicillin-streptomycin. CLL cell viability was determined by analysis of mitochondrial transmembrane potential using 3,3′ dihexyloxacarbocyanine iodide (DiOC6; Molecular Probes, Invitrogen) and by cell membrane permeability to propidium iodide (PI; Sigma-Aldrich).23 CAL-101 was provided by Calistoga Pharmaceuticals and was dissolved in DMSO at 10mM and stored at −20°C until use.
Chemotaxis assay
To determine the effect of CAL-101 on CLL cell chemotaxis, we performed chemotaxis assays across polycarbonate Transwell inserts as described.5 Briefly, CLL cells (107/mL) were incubated at 37°C in 5% CO2 with 10 μg/mL of anti-IgM (MP Biomedicals) in complete RPMI medium with or without 5μM CAL-101. After 1 hour, CLL cells were centrifuged and resuspended in RPMI 1640 with 0.5% BSA to a concentration of 107 cells/mL. One hundred milliliters of cell suspension was added to the top chamber of a Transwell culture insert (Corning) with a diameter of 6.5 mm and a pore size of 5 μm. Filters then were transferred to wells containing medium with or without 200 ng/mL of CXCL12 (Upstate Biotechnology) or 1 μg/mL of CXCL13 (R&D Systems). The chambers were incubated for 3 hours at 37°C in 5% CO2. After this incubation, the cells in the lower chamber were suspended and divided into aliquots for counting with a FACSCalibur (BD Biosciences) for 20 seconds at 60 μL/min in duplicates. A 1/20 dilution of input cells was counted under the same conditions.
Migration assay evaluating CLL pseudoemperipolesis
Pseudoemperipolesis is an in vitro phenomenon in which CLL cells spontaneously migrate beneath marrow stromal cells in a CXCR4-dependent fashion; the effect mimics in vivo migration and homing to stromal cells in the tissues.5,24,25 We quantified CLL cell pseudoemperipolesis, as described.5 Briefly, the murine stromal cells (9-15C and TSt-4) were seeded the day before the assay onto collagen-coated 12-well plates at a concentration of 1.8 × 105 cells/well in RPMI 1640 supplemented with 10% FCS and penicillin-streptomycin-glutamine. CLL cells were suspended to a concentration of 107 cells/mL in medium. They were then incubated at 37°C in 5% CO2 in complete medium supplemented with or without 5μM CAL-101 for 30 minutes before addition of 10 μg/mL of anti-IgM (polyclonal goat F[ab']2 fragments to human IgM; MP Biomedicals); control samples were not exposed to CAL-101 or stimulated with anti-IgM. After another 1-hour incubation, CLL cells were added to the stromal cell layers. The plates were incubated at 37°C in 5% CO2 for 4 hours. Cells that had not migrated into the stromal cell layer were removed by vigorously washing 3 times with RPMI 1640 medium. The complete removal of nonmigrated cells and the integrity of the stromal cell layer containing transmigrated cells were assessed by phase-contrast microscopy and documented photographically. The stromal cell layer containing transmigrated cells was detached by incubation for 1 minute with trypsin/EDTA prewarmed to 37°C (Invitrogen). Cells were then immediately suspended by adding 1 mL of ice-cold RPMI/10% FBS, washed, and suspended in 0.4 mL of cold medium for counting by flow cytometry for 20 seconds at 60 μL/min in duplicates. Based on relative differences in size and granularity (forward scatter and side scatter) between cell types, a lymphocyte gate was set to exclude stromal cells from the counts. The number of migrated cells under each condition was expressed as percentage of the control.
NLC and MSC cocultures and drug sensitivity testing
NLC cocultures were established by suspending PBMC from patients with CLL in complete RPMI 1640 medium with 10% FBS and penicillin-streptomycin-glutamine (HyClone) to a concentration of 107 cells/mL (total 2 mL). Cells were incubated for 14 days in 24-well plates (Corning Life Sciences) as previously described.3 To evaluate whether CAL-101 could sensitize CLL cells toward conventional cytotoxic drugs, CLL cells were cultured under standardized conditions on MSC or in suspension, as previously described.23 Briefly, stromal cells were seeded the day before the experiment onto 48-well plates (Corning Life Sciences) at a concentration of 5 × 104 cells/mL/well and incubated at 37°C in 5% CO2. After confirming the confluence of the stromal layer by phase contrast microscopy, CLL cells were added onto the MSC layer at a concentration of 5 × 106 cells/mL. For comparison, CLL cells were cultured in suspension at the same density. Cultures were then treated with 10μM fludarabine (9-β-D-arabinofuranosyl-2-fluoroadenine; F-ara-A; Sigma-Aldrich), 10μM dexamethasone (Sigma-Aldrich), or 10μM bendamustine (Sigma-Aldrich) in the presence or absence of 5μM CAL-101. At the indicated time points, CLL cells were collected and assayed for cell viability.
Measurement of chemokine concentrations using ELISA
The effect of CAL-101 on CCL3 and CCL4 secretion after stimulation of CLL cells with anti-IgM (10 μg/mL) or with NLCs was measured as previously described.10 After 24 hours, supernatants were harvested and assayed for CCL3 and CCL4, and CXCL13 protein (only in NLC cocultures) by quantitative ELISA according to the manufacturer's instructions (Quantikine; R&D Systems). In cocultures containing NLCs, CXCL13 was similarly assessed.
Multiplex assay (human cytokine assay)
NLC cocultures provide a mode of stimulation to CLL cells that is strikingly similar to that observed in lymph nodes,8,10 and therefore represent the best in vitro model to study the lymph node microenvironment. To better characterize the impact of CAL-101 on the CLL-stromal cross talk, cytokine/chemokine levels were analyzed in supernatants from CLL-NLC cocultures with different concentrations of CAL-101. After 24 hours, supernatants of CAL-101 treated cocultures and controls were harvested, centrifuged, and stored at −20°C until analysis. Before analysis, samples were thawed overnight at 4°C and centrifuged at 1500g to remove debris. Chemokines were analyzed with multiplexed bead suspension arrays (MBAs; Millipore). MBAs were analyzed using a Luminex 200 machine and data were organized and analyzed using 3.1 xPONENT software (Luminex).
Immunoblotting
Freshly isolated CLL cells were cultured in suspension and stimulated with 200 ng/mL CXCL12 (Upstate) or 10 μg/mL anti-IgM for 10 minutes. Cells then were lysed on ice for 30 minutes in lysis buffer containing 25mM HEPES, 300mM NaCl, 1.5mM MgCl2, 0.5% sodium deoxycholate, 20mM glycerophosohate, 1% Triton X-100, 0.1% SDS, 0.2mM EDTA, 0.5mM dithiothreitol, 1mM sodium orthovanadate, and protease inhibitor. Cells were centrifuged at 14 000g for 15 minutes at 4°C, and supernatant was stored at −80°C until use. Protein content was determined using the detergent compatible (DC) protein assay kit, according to manufacturer's instructions (Bio-Rad Laboratories). Aliquots (50 μg) of total cell protein were boiled with Laemmli sample buffer and loaded onto 4%-12% SDS-polyacrylamide gradient gels and transferred to nitrocellulose membranes (GE; Osmonics Labstore). Membranes were blocked for 1 hour in PBS-Tween containing 5% nonfat dried milk and incubated with primary antibodies either overnight or for 3 hours followed by species-specific HRP-conjugated secondary antibody (diluted 1:10 000) for 1 hour. The blots were visualized by enhanced chemiluminescence according to the manufacturer's instructions (Pierce Biotechnology) and normalized to the actin levels in each extract. Membranes were probed at 4°C with the following primary antibodies: anti-total AKT, phospho-AKT (S473 and T308), ERK1/2, phospho-ERK (Cell Signaling), and β-actin (Sigma-Aldrich). Immunoreactive bands were visualized using peroxidase-conjugated secondary antibodies (GE Healthcare) and enhanced chemiluminescence detection system (Pierce Biotechnology). Cell viability was measured by flow cytometry for each time point and condition.
Pharmacodynamic assessments in patients receiving CAL-101
To provide in vivo correlation with the in vitro results, changes in activation of the PI3K pathway were assessed and circulating plasma concentrations of CCL3, CCL4, and CXCL13 were evaluated in the first 12 patients with previously treated CLL enrolled into an ongoing dose-ranging clinical trial (http://clinicaltrials.gov/ct2/show/NCT00710528). Patients were receiving CAL-101, 100 mg (N = 7) or 150 mg (N = 5) twice per day. PBMCs and plasma samples were collected in EDTA at baseline (pretreatment) and during treatment (day 28). Samples were centrifuged at 1100g (relative centrifugal force) for 10 minutes at 4°C for separation of plasma and mononuclear cell layers. Plasma and CLL cells (in 10% DMSO) were stored at −80°C until analyses. Cells were stained with anti-CD5–FITC and either anti–phospho-Akt T308 (Alexa Fluor 488), or an isotype-matched control antibody (mouse IgG1–Alexa Fluor 488 conjugate, Cell Signaling). The gates were set using anti-CD5 (FITC) and isotype-FITC. FITC-CD5+ cells were gated and analyzed by 2-color flow cytometry to quantify intracellular p-AktT308 levels using the Beckman Coulter Cytomics FC 500MPL and analyzed using MXP Version 2.2 software (Beckman Coulter). Thawed plasma samples were subjected to analysis using ELISA (Quantikine; R&D Systems). The absorbance was recorded on a microplate reader (SectraMax; Molecular Devices), and data collection and analysis were performed using dedicated software (SoftMax Pro 5.2. Data Acquisition & Analysis Software, Molecular Devices). Pretreatment and day 28 peripheral blood lymphocytes counts in the patients were obtained from routine clinical complete blood counts performed at study sites.
Data analysis and statistics
Results are shown as mean ± SEM of at least 3 experiments each. For viability assays, mean relative viabilities were determined to account for variability in spontaneous apoptosis rates in different patient samples. The mean relative viability was defined as the mean CLL cell viability of a particular sample (treated with drug in the presence or absence of MSC at a given time point), divided by the mean cell viability of the same sample at the same time point of control CLL cells, cultured in suspension culture. As appropriate for the analysis, Student paired or unpaired t tests were used for statistical comparisons. Analyses were performed using GraphPad Prism 4 software for Macintosh (GraphPad). A P value < .05 was considered statistically significant. Flow cytometry data were analyzed using FlowJo Version 8.8.7 software (TreeStar).
Results
CAL-101 inhibits CLL cell chemotaxis and migration beneath marrow stroma cells (pseudoemperipolesis)
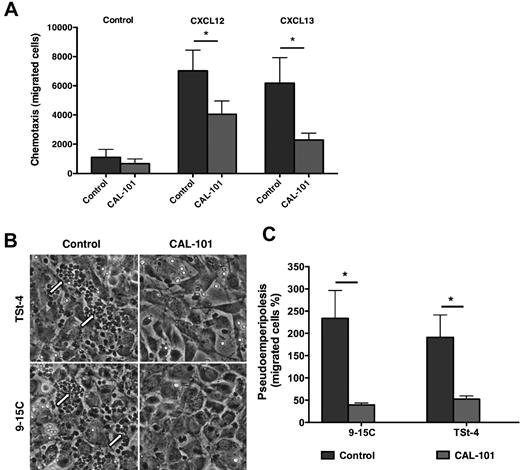
In control cultures, CLL cells displayed chemotaxis toward the chemokines CXCL12 and CXCL13 (Figure 1A). However, in the presence of 5μM of CAL-101, CLL cell chemotaxis was significantly reduced; in samples from 10 patients, the mean (± SEM) number of cells migrating toward CXCL12 decreased from 7022 (± 1420) to 4044 (± 918), while the mean (± SEM) number of cells migrating toward CXCL13 decreased from 6179 (± 1742) to 2276 (± 470; Figure 1A). Additional chemotaxis experiments in which lower concentrations of CAL-101 were used also showed significant inhibition of CLL cell chemotaxis (see supplemental Figure 2). CAL-101 also significantly reduced CLL cell migration beneath marrow stromal cells. Phase-contrast photomicrographs (Figure 1B) depict a representative CLL sample cocultured with TSt-4 and 9-15C. The data demonstrate a marked reduction in CLL cell migration beneath the stromal cells after pre-treatment with 5μM CAL-101; in samples from 9 patients, the mean (± SEM) number of cells migrating declined from 18 553 (± 4683) to 3171 (± 1615) using 9-15C stromal cells, and from 10 900 (± 4260) to 2962 (± 1192) using TSt-4 stromal cells. This corresponded to a relative migration of 234% (± 62.4%) of anti-IgM–stimulated CLL cells compared with unstimulated control CLL cells (100%, not displayed). CAL-101 reduced this increased migration to 39.1% (± 4.6%) on 9-15C stromal cells, and from 191.1% (± 50.3) to 52.3% (± 7.3%) on TSt-4 stromal cells (Figure 1C).
CAL-101 inhibits CLL cell chemotaxis toward CXCL12 and CXCL13 and migration beneath MSCs (pseudoemperipolesis). (A) CLL cells were incubated in medium alone (control) or medium containing 5μM CAL-101, and then allowed to migrate towards 200 ng/mL CXCL12 or 1 μg/mL CXCL13; or control cells without chemokine. The bar diagram represents the mean chemotaxis (± SEM) of CLL cells from 10 different patients in the presence or absence of CAL-101. Chemotaxis toward both CXCL12 and CXCL13 was significantly inhibited by CAL-101, with P < .05, as indicated by the asterisks. (B) Representative phase-contrast photomicrographs displaying CLL cell migration beneath TSt-4 or 9-15C stromal cells when CLL were either untreated (control) or pretreated with 5μM CAL-101 (CAL-101). Pseudoemperipolesis is characterized by the dark appearance of CLL cells that have migrated into the same focal plane as the stromal cells. There are numerous migrated CLL cells in the control wells (on the left), as indicated by the arrows, but only a few such cells in wells containing CLL cells pretreated with CAL-101 (on the right). (C) The bar diagram represents the mean pseudoemperipolesis (± SEM) of CLL cells from 9 different patients beneath of each of the 2 types of stromal cells in the presence or absence of CAL-101. Pseudoemperipolesis beneath TSt-4 or 9-15C stromal cells was significantly inhibited by CAL-101, with P < .05, as indicated by the asterisks.
CAL-101 inhibits CLL cell chemotaxis toward CXCL12 and CXCL13 and migration beneath MSCs (pseudoemperipolesis). (A) CLL cells were incubated in medium alone (control) or medium containing 5μM CAL-101, and then allowed to migrate towards 200 ng/mL CXCL12 or 1 μg/mL CXCL13; or control cells without chemokine. The bar diagram represents the mean chemotaxis (± SEM) of CLL cells from 10 different patients in the presence or absence of CAL-101. Chemotaxis toward both CXCL12 and CXCL13 was significantly inhibited by CAL-101, with P < .05, as indicated by the asterisks. (B) Representative phase-contrast photomicrographs displaying CLL cell migration beneath TSt-4 or 9-15C stromal cells when CLL were either untreated (control) or pretreated with 5μM CAL-101 (CAL-101). Pseudoemperipolesis is characterized by the dark appearance of CLL cells that have migrated into the same focal plane as the stromal cells. There are numerous migrated CLL cells in the control wells (on the left), as indicated by the arrows, but only a few such cells in wells containing CLL cells pretreated with CAL-101 (on the right). (C) The bar diagram represents the mean pseudoemperipolesis (± SEM) of CLL cells from 9 different patients beneath of each of the 2 types of stromal cells in the presence or absence of CAL-101. Pseudoemperipolesis beneath TSt-4 or 9-15C stromal cells was significantly inhibited by CAL-101, with P < .05, as indicated by the asterisks.
CAL-101 abrogates BCR- and NLC-derived survival signals
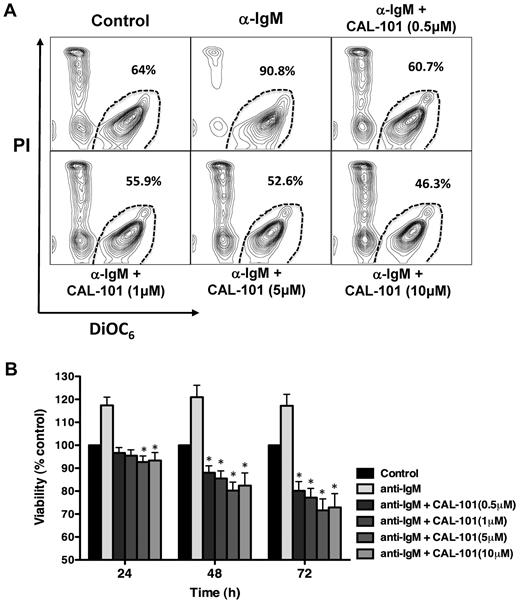
Growing evidence suggests that signals from the tumor microenvironment are essential for the expansion and survival of malignant B cells.2,3 To determine the effects of CAL-101 on CLL cell viability mediated via the BCR, we stimulated cells with anti-IgM in the presence of increasing concentrations of CAL-101. As shown in a representative case (Figure 2A), anti-IgM stimulation increased CLL cell viability, while CAL-101 abrogated the pro-survival effect of anti-IgM in a dose-dependent fashion at lower dose levels (< 5μM), the effects of 5 versus 10μM CAL-101 appeared relatively similar in this assay. As quantified in Figure 2B, anti-IgM stimulation of CLL cells from 15 patients induced a mean (± SEM) increase in viability to 117% (± 3.6%) after 24 hours, 121% (± 5.2%) after 48 hours, and 117% (± 5%) after 72 hours compared with controls in medium alone (100%). CAL-101 treatment at concentrations of ≥ 5μM was maximally effective over the 72-hour time course in reducing CLL cell viability. At the 5μM concentration, CAL-101 significantly decreased the mean (± SEM) pro-survival effect of anti-IgM to 92.7% (± 2.6%) after 24 hours, 80.2% (± 3.6%) after 48 hours, and 71.6% (± 5%) after 72 hours. Subset analyses of IGHV mutated versus unmutated CLL cases did not show any significant differences in terms of anti-IgM and/or CAL-101 responsiveness (see supplemental Figure 3).
Specific PI3Kδ inhibition with CAL-101 induces CLL apoptosis and abrogates BCR-derived survival signals. (A) CLL cells were incubated in medium alone (control), medium containing 10 μg/mL of anti-IgM mAbs, or medium with anti-IgM mAbs and various concentrations of CAL-101. Displayed are representative contour plots that depict CLL cell viability after 48 hours and after post staining with DiCO6 and PI (horizontal and vertical axes, respectively). The viable cell population is characterized by bright DiCO6 staining and PI exclusion, and is gated in the bottom right corner of each contour plot. The percentage of viable cells is displayed above each of these gates. (B) The bar diagram represents the mean relative viabilities of CLL cells cultured in complete medium (control), or medium supplemented with 10 μg/mL of anti-IgM, or anti-IgM and various concentrations of CAL-101. Viabilities in CAL-101–treated samples were normalized to the viabilities of control samples at the respective timepoints (100%) to account for differences in spontaneous apoptosis in samples from different patients. Displayed are the means (± SEM) from 15 different patient samples, assessed after 24, 48, and 72 hours. CLL cell survival in the presence of anti-IgM mAbs was significantly inhibited by CAL-101, with P < .05, as indicated by the asterisks describing the comparison of results from each culture containing CAL-101 to the results from the culture containing anti-IgM alone.
Specific PI3Kδ inhibition with CAL-101 induces CLL apoptosis and abrogates BCR-derived survival signals. (A) CLL cells were incubated in medium alone (control), medium containing 10 μg/mL of anti-IgM mAbs, or medium with anti-IgM mAbs and various concentrations of CAL-101. Displayed are representative contour plots that depict CLL cell viability after 48 hours and after post staining with DiCO6 and PI (horizontal and vertical axes, respectively). The viable cell population is characterized by bright DiCO6 staining and PI exclusion, and is gated in the bottom right corner of each contour plot. The percentage of viable cells is displayed above each of these gates. (B) The bar diagram represents the mean relative viabilities of CLL cells cultured in complete medium (control), or medium supplemented with 10 μg/mL of anti-IgM, or anti-IgM and various concentrations of CAL-101. Viabilities in CAL-101–treated samples were normalized to the viabilities of control samples at the respective timepoints (100%) to account for differences in spontaneous apoptosis in samples from different patients. Displayed are the means (± SEM) from 15 different patient samples, assessed after 24, 48, and 72 hours. CLL cell survival in the presence of anti-IgM mAbs was significantly inhibited by CAL-101, with P < .05, as indicated by the asterisks describing the comparison of results from each culture containing CAL-101 to the results from the culture containing anti-IgM alone.
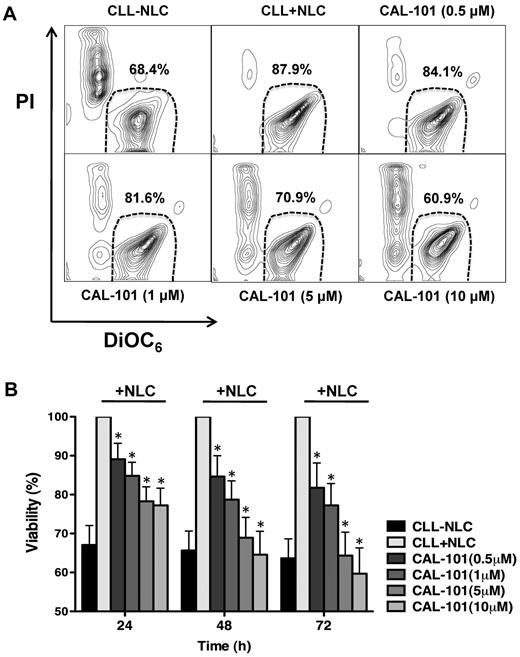
NCLs also support CLL cell viability, acting via several mechanisms, including CXCL12,3,24 B cell–activating factor of the TNF family (BAFF), a proliferation-inducing ligand (APRIL),24 and the BCR.10 CAL-101 decreased CLL cell viability in cocultures with NLCs. As shown in a representative case (Figure 3A), CAL-101 demonstrated a dose-dependent inhibition of CLL viability when cells were cocultured with NLCs. Mean (± SEM) CLL cell viability in 12 cocultures with NLCs was significantly reduced by CAL-101 in a time- and dose-dependent manner to 89.1% (± 4.1%) after 24 hours, 84.6% (± 5.3%) after 48 hours, and 81.7% (± 2.3%) after 72 hours, even at the lowest CAL-101 concentration of 0.5μM (Figure 3B). Subset analyses again did not show significant differences between IGHV mutated or unmutated CLL cases (supplemental Figure 3).
CAL-101 antagonizes NLC-mediated CLL cell survival. (A) CLL cells were cultured alone (control), cocultured with NLCs in medium alone or in medium containing various concentrations of CAL-101. Displayed are representative contour plots that depict CLL cell viability after 48 hours and after staining with DiOC6 and PI (horizontal and vertical axes, respectively). The viable cell population is characterized by bright DiOC6 staining and PI exclusion, and is gated in the bottom right corner of each contour plot. The percentage of viable cells is displayed above each of these gates. (B) The bar diagram represents the mean relative viabilities of CLL cells cocultured with NLCs compared with CLL cells alone (control) and cocultured with NLCs plus various concentrations of CAL-101. Viabilities of CAL-101–treated samples were normalized to the viabilities of control samples at the respective timepoints (100%). Displayed are the means (± SEM) from 12 different patient samples, assessed after 24, 48, and 72 hours. CLL cell survival in the presence of NCLs was significantly inhibited by CAL-101, with P < .05, as indicated by the asterisks describing the comparison of results from each culture containing CAL-101 to the results from the control culture.
CAL-101 antagonizes NLC-mediated CLL cell survival. (A) CLL cells were cultured alone (control), cocultured with NLCs in medium alone or in medium containing various concentrations of CAL-101. Displayed are representative contour plots that depict CLL cell viability after 48 hours and after staining with DiOC6 and PI (horizontal and vertical axes, respectively). The viable cell population is characterized by bright DiOC6 staining and PI exclusion, and is gated in the bottom right corner of each contour plot. The percentage of viable cells is displayed above each of these gates. (B) The bar diagram represents the mean relative viabilities of CLL cells cocultured with NLCs compared with CLL cells alone (control) and cocultured with NLCs plus various concentrations of CAL-101. Viabilities of CAL-101–treated samples were normalized to the viabilities of control samples at the respective timepoints (100%). Displayed are the means (± SEM) from 12 different patient samples, assessed after 24, 48, and 72 hours. CLL cell survival in the presence of NCLs was significantly inhibited by CAL-101, with P < .05, as indicated by the asterisks describing the comparison of results from each culture containing CAL-101 to the results from the control culture.
CAL-101 inhibits NLC- and BCR-induced secretion of the chemokines, CCL3, CCL4, and CXCL13
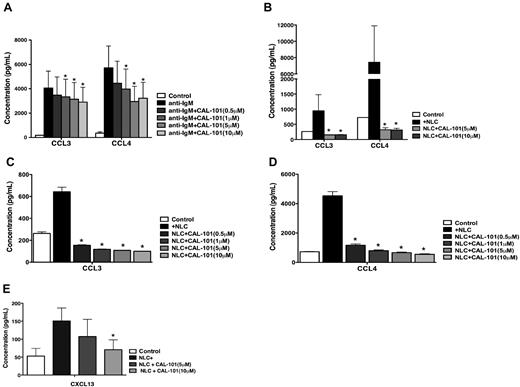
As illustrated in Figure 4A, activation of CLL cells via the BCR using anti-IgM resulted in marked increases in secretion of CCL3 and CCL4. In samples from 5 patients, this effect was significantly inhibited by CAL-101 in a dose-dependent manner.
NLC- and BCR-induced secretion of the chemokines, CCL3, CCL4, and CXCL13 by CLL cells is inhibited by CAL-101. (A) The bar diagram represents the mean supernatant concentrations of CLL3 and CCL4 from CLL cells cultured in complete medium (control), medium supplemented with 10 μg/mL of anti-IgM, or anti-IgM and various concentrations of CAL-101. Displayed are the mean (± SEM) supernatant concentrations from 5 different patient samples assessed after 24 hours. The secretion of CCL3 and CCL4 from CLL cells in the presence of anti-IgM mAbs was significantly inhibited by CAL-101, with P < .05, as indicated by the asterisks describing the comparison of results from each culture containing CAL-101 to the results from the culture containing anti-IgM alone. (B) This bar diagram represents the mean CLL cell supernatant concentrations for CCL3 and CCL4 from CLL cells cocultured with or without (controls) NLCs. Displayed are the means (± SEM) from 5 different patient samples, assessed after 24 hours. The secretion of CCL3 and CCL4 from CLL cells was significantly inhibited by CAL-101, with P < .05, as indicated by the asterisks, describing the comparison of results from each culture containing CAL-101 to the results from the control culture. Lower concentrations of CAL-101, which are indicated next to each bar diagram and depicted by different shades of gray, also significantly reduced CCL3 (C) and CCL4 (D) concentrations in CLL-NLC cocultures (n = 3). (E) This bar diagram represents mean (± SEM) supernatant CXCL13 concentrations from CLL cells cultured alone or cocultured with NLC from 5 different patients, assessed after 24 hours. The secretion of CXCL13 was reduced by CAL-101, with P < .05, as indicated by the asterisks describing the comparison of results from each culture containing CAL-101 to the results from control cultures.
NLC- and BCR-induced secretion of the chemokines, CCL3, CCL4, and CXCL13 by CLL cells is inhibited by CAL-101. (A) The bar diagram represents the mean supernatant concentrations of CLL3 and CCL4 from CLL cells cultured in complete medium (control), medium supplemented with 10 μg/mL of anti-IgM, or anti-IgM and various concentrations of CAL-101. Displayed are the mean (± SEM) supernatant concentrations from 5 different patient samples assessed after 24 hours. The secretion of CCL3 and CCL4 from CLL cells in the presence of anti-IgM mAbs was significantly inhibited by CAL-101, with P < .05, as indicated by the asterisks describing the comparison of results from each culture containing CAL-101 to the results from the culture containing anti-IgM alone. (B) This bar diagram represents the mean CLL cell supernatant concentrations for CCL3 and CCL4 from CLL cells cocultured with or without (controls) NLCs. Displayed are the means (± SEM) from 5 different patient samples, assessed after 24 hours. The secretion of CCL3 and CCL4 from CLL cells was significantly inhibited by CAL-101, with P < .05, as indicated by the asterisks, describing the comparison of results from each culture containing CAL-101 to the results from the control culture. Lower concentrations of CAL-101, which are indicated next to each bar diagram and depicted by different shades of gray, also significantly reduced CCL3 (C) and CCL4 (D) concentrations in CLL-NLC cocultures (n = 3). (E) This bar diagram represents mean (± SEM) supernatant CXCL13 concentrations from CLL cells cultured alone or cocultured with NLC from 5 different patients, assessed after 24 hours. The secretion of CXCL13 was reduced by CAL-101, with P < .05, as indicated by the asterisks describing the comparison of results from each culture containing CAL-101 to the results from control cultures.
As shown in Figure 4B through D, coculture with NLCs also induced the CLL cells to secrete high concentrations of CCL3 and CCL4 into the supernatants. Figure 4B shows the results from 5 patients, the respective mean (± SEM) CCL3 and CCL4 concentrations in supernatants of untreated CLL cells after NLC coculture for 24 hours were 943.3 (± 535.9) pg/mL and 7433.3 (± 4463.1) pg/mL, which are comparable with our previously experience.10 Treatment of CLL cells with CAL-101 almost completely abrogated the NLC-induced secretion of CCL3 and CCL4, reducing the respective mean (± SEM) values for CCL3 and CCL4 to 153 (± 3.3) and 320 (± 66) pg/mL. Treatment with lower concentrations of CAL-101 also reduced levels of CCL3 and CCL4 as shown in Figure 4C and D in 3 representative cases. Figure 4E shows that the level of CXCL13 in NLC cocultures decreased after treatment with CAL-101 from 151 (± 35) pg/mL to 70.7 (± 27) pg/mL.
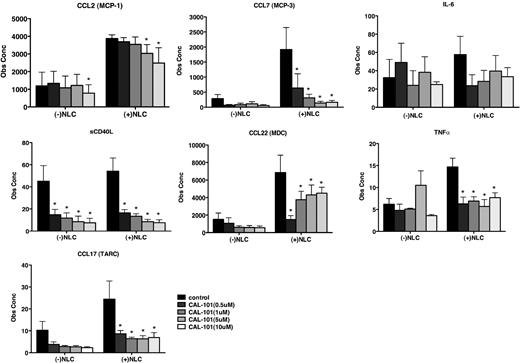
CAL-101 down-regulates secretion of other chemokines and cytokines in CLL-NLC cocultures
We next used a sensitive fluorescently labeled, microsphere bead array approach to assay CAL-101–induced changes in the secretion of chemokines and cytokines in CLL cells cocultured with NLCs. CLL cells from 7 patients were cultured with or without NLCs in various concentrations of CAL-101. Supernatants were harvested after 24 hours and assayed for protein concentrations via multiplex analysis (Figure 5). For all of the chemokine and cytokines examined (CCL2, CCL7, IL-6, sCD40L, CCL22, TNF-α, and CCL17), we noticed higher mean concentrations in supernatants from NLC cocultures. Increased levels were significantly down-modulated by CAL-101 treatment, most notably for CCL7, sCD40L, TNF-α, and CCL17 and CCL22. These data again suggest that CAL-101 disrupts central communication pathways between CLL cells and NLCs.
CAL-101 alters chemokine and cytokine secretion of CLL cells in coculture with NLCs. The bar diagrams represent the mean CLL cell supernatant concentrations for various chemokines and cytokines from CLL cells culture alone or cocultured with NLCs. Displayed are the means (± SEM) from 7 different patient samples, assessed after 24 hours. The secretion of some chemokines was significantly inhibited by CAL-101, with P < .05, as indicated by the asterisks describing the comparison of results from each culture containing CAL-101 to the results from the relevant control culture.
CAL-101 alters chemokine and cytokine secretion of CLL cells in coculture with NLCs. The bar diagrams represent the mean CLL cell supernatant concentrations for various chemokines and cytokines from CLL cells culture alone or cocultured with NLCs. Displayed are the means (± SEM) from 7 different patient samples, assessed after 24 hours. The secretion of some chemokines was significantly inhibited by CAL-101, with P < .05, as indicated by the asterisks describing the comparison of results from each culture containing CAL-101 to the results from the relevant control culture.
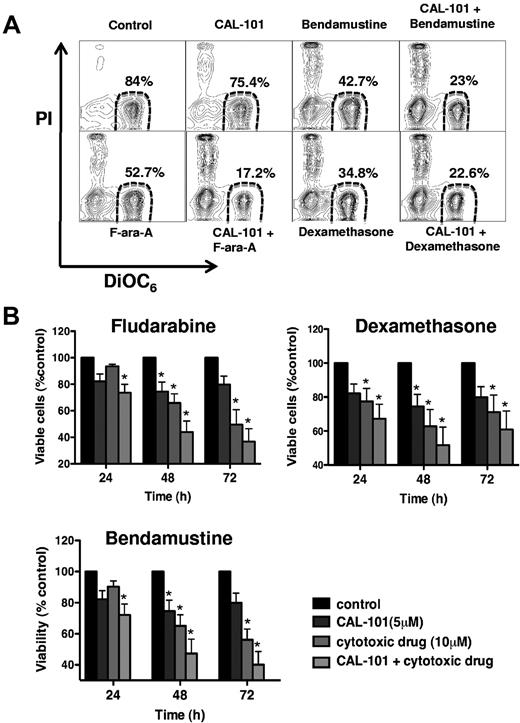
CAL-101 sensitizes CLL cells to cytotoxic drugs in MSC cocultures
Previous studies have demonstrated that MSCs can protect CLL cells from drug-induced apoptosis.23,25,26 Therefore, we evaluated whether CAL-101 could enhance CLL cell killing when these cells were cocultured with MSC in the presence of drugs that are cytotoxic to CLL cells (bendamustine, fludarabine, and dexamethasone). Figure 6A displays a representative case of CLL cells cocultured with MSC and treated with CAL-101 in the presence or absence of the cytotoxic agents. Figure 6B depicts the mean (± SEM) relative viabilities of CLL cells from 9 patients cocultured with MSC in the presence or absence of cytotoxic drugs, CAL-101, or the combination of both at 3 different timepoints. For example, after 72 hours, CLL cell viability relative to untreated controls was 93% (± 0.6%) with CAL-101 and 60% (± 8.7%) with bendamustine, but was reduced to 42.7% (± 11.4%) for the combination of the 2 drugs. Comparable results were seen for the combinations of CAL-101 and fludarabine or CAL-101 and dexamethasone. The displayed case (Figure 6A) and the results summarized in Figure 6B indicate that 5μM CAL-101 sensitized CLL cells to these cytotoxic agents, presumably by disrupting MSC-derived drug resistance signals. Testing of lower concentrations of CAL-101 (1μM, 0.5μM, and 0.1μM CAL-101) confirmed these data, showing induction of increased CLL cell death when cytotoxic drugs were combined with CAL-101 (see supplemental Figure 4).
CAL-101 enhances the activity of several different cytotoxic agents against CLL cells cocultured with MSCs. (A) CLL cells were cocultured with MSCs in medium alone (control) or in medium containing the indicated concentrations of CAL-101, dexamethasone, bendamustine, or fludarabine, or the drugs combined. Displayed are representative contour plots that depict CLL cell viability after 48 hours and after staining with DiOC6 and PI (horizontal and vertical axes, respectively). The viable cell population is characterized by bright DiOC6 staining and PI exclusion, and is gated in the lower right corner of each contour plot. The percentage of viable cells is displayed above each of these gates. (B) The bar diagram represents the mean relative viabilities of CLL cells cocultured with MSCs and the indicated concentrations of CAL-101, dexamethasone, bendamustine, fludarabine, or the drugs combined. Viabilities of drug-treated samples were normalized to the viabilities of control samples at the respective timepoints (100%). Displayed are the means (± SEM) from 9 different patient samples, assessed after 24, 48, and 72 hours. CLL cell survival in the presence of MSC was significantly reduced by combination therapy, with P < .05, as indicated by the asterisks describing the comparison of results from each drug-treated culture to the results from the control culture. Similar data were generated with lower CAL-101 concentration (1μM, 0.5μM, and 0.1μM; see supplemental Figure 4).
CAL-101 enhances the activity of several different cytotoxic agents against CLL cells cocultured with MSCs. (A) CLL cells were cocultured with MSCs in medium alone (control) or in medium containing the indicated concentrations of CAL-101, dexamethasone, bendamustine, or fludarabine, or the drugs combined. Displayed are representative contour plots that depict CLL cell viability after 48 hours and after staining with DiOC6 and PI (horizontal and vertical axes, respectively). The viable cell population is characterized by bright DiOC6 staining and PI exclusion, and is gated in the lower right corner of each contour plot. The percentage of viable cells is displayed above each of these gates. (B) The bar diagram represents the mean relative viabilities of CLL cells cocultured with MSCs and the indicated concentrations of CAL-101, dexamethasone, bendamustine, fludarabine, or the drugs combined. Viabilities of drug-treated samples were normalized to the viabilities of control samples at the respective timepoints (100%). Displayed are the means (± SEM) from 9 different patient samples, assessed after 24, 48, and 72 hours. CLL cell survival in the presence of MSC was significantly reduced by combination therapy, with P < .05, as indicated by the asterisks describing the comparison of results from each drug-treated culture to the results from the control culture. Similar data were generated with lower CAL-101 concentration (1μM, 0.5μM, and 0.1μM; see supplemental Figure 4).
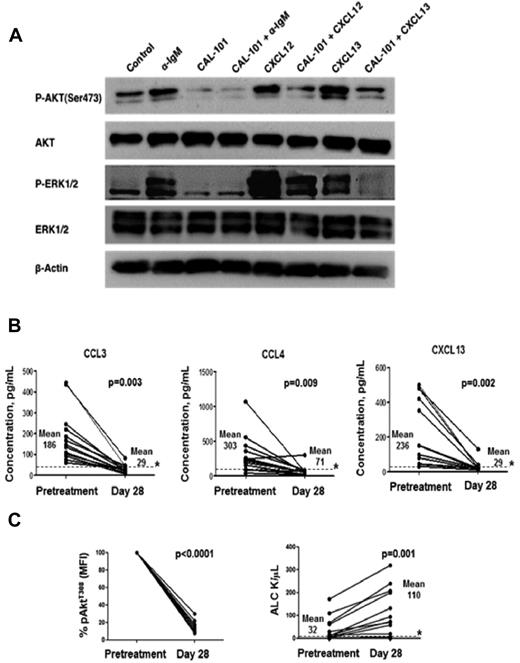
CAL-101 inhibits BCR-, CXCL12-, and CXCL13-induced AKT- and ERK-activation in CLL cells
To further evaluate the role of PI3Kδ in mediating signals from the tumor microenvironment, CLL cells were treated with anti-IgM to stimulate the cells through BCR activation, or with CXCL12 or CXCL13 to model CLL cell stimulation by stromal cells via CXCR4 or CXCR5 chemokine receptors, respectively. Stimulation was performed in the presence or absence of CAL-101. Figure 7A shows a representative immunoblot of 3 experiments with CLL cells from different patients, additional immunoblot data are displayed in the supplemental Figure 5. CAL-101 inhibited AKT and ERK phosphorylation in response to anti-IgM stimulation, and in response to CXCL12 or CXCL13, respectively. These data provide evidence that CAL-101 not only modulates signaling pathways downstream of the BCR, but also affects the signaling cascades downstream of CXCR4 and CXCR5, which are activated by CXCL12 or CXCL13, respectively.3,25 Akt kinase is a well-characterized effector of PI3K, and, on activation, becomes phosphorylated on 2 key residues: Thr308 (T308) of the activation loop and Ser473 (S473) in the hydrophobic motif of the C-terminal tail. The flow data in Figure 7C depict pAKT(T308) down-modulation after CAL-101 treatment in vivo, while the immunoblots display decreased pAKT(S473) in response to BCR-, CXCR4-, or CXCR5-activation when CLL cells were pre-treated with CAL-101 in vitro.
CAL-101 inhibits signaling downstream of the BCR, CXCR4 and CXCR5. In vivo, CAL-101 reduces plasma chemokine levels, impairs AKT activation, and induces lymphocytosis in CLL patients. (A) CLL cells were activated with anti-IgM, or CXCL12, or CXCL13 in the presence or absence of CAL-101. Lysates were probed with phospho-specific antibodies to AKT and ERK1/2, and antibodies for total AKT, ERK1/2, and actin. (B-C) Plasma chemokine levels, AKT phosphorylation, and lymphocyte counts were evaluated in 12 CLL patients pretreatment and after 28 days on treatment with CAL-101. (B) The line graphs represent concentrations of CCL3, CCL4, and CXCL13 in plasma samples from CLL patients before and after 28 days of CAL-101 treatment. Displayed are the individual values. * represents the upper limit of normal. Chemokine values in the plasma from patients were significantly reduced by CAL-101. (C) The bar diagram represents the mean relative phospho-AktT308 fluorescence intensity values (adjusted for isotype control) derived from circulating CLL cells in patients undergoing CAL-101 treatment. Displayed are the means (± SEM). Akt phosphorylation in CLL cells from patients was significantly inhibited by CAL-101. The line graphs indicate the absolute lymphocyte counts in patients with CLL undergoing CAL-101 treatment. Displayed are the individual values. * represents the upper limit of normal. Absolute lymphocyte counts were significantly increased by CAL-101.
CAL-101 inhibits signaling downstream of the BCR, CXCR4 and CXCR5. In vivo, CAL-101 reduces plasma chemokine levels, impairs AKT activation, and induces lymphocytosis in CLL patients. (A) CLL cells were activated with anti-IgM, or CXCL12, or CXCL13 in the presence or absence of CAL-101. Lysates were probed with phospho-specific antibodies to AKT and ERK1/2, and antibodies for total AKT, ERK1/2, and actin. (B-C) Plasma chemokine levels, AKT phosphorylation, and lymphocyte counts were evaluated in 12 CLL patients pretreatment and after 28 days on treatment with CAL-101. (B) The line graphs represent concentrations of CCL3, CCL4, and CXCL13 in plasma samples from CLL patients before and after 28 days of CAL-101 treatment. Displayed are the individual values. * represents the upper limit of normal. Chemokine values in the plasma from patients were significantly reduced by CAL-101. (C) The bar diagram represents the mean relative phospho-AktT308 fluorescence intensity values (adjusted for isotype control) derived from circulating CLL cells in patients undergoing CAL-101 treatment. Displayed are the means (± SEM). Akt phosphorylation in CLL cells from patients was significantly inhibited by CAL-101. The line graphs indicate the absolute lymphocyte counts in patients with CLL undergoing CAL-101 treatment. Displayed are the individual values. * represents the upper limit of normal. Absolute lymphocyte counts were significantly increased by CAL-101.
CAL-101 treatment modulates pAKT levels in CLL cells and reduces circulating chemokine levels in CLL patients in vivo
To corroborate our in vitro findings, we evaluated the effects of CAL-101 in CLL patients in vivo, assessing the effect of the drug on plasma concentrations of the chemokines CCL3, CCL4, and CXCL13, and on pAKT levels in circulating CLL cells before and during treatment with CAL-101. In 12 patients with CLL, evaluation of plasma samples obtained pre-treatment and after 28 days of daily treatment with CAL-101 showed nearly universal reductions in concentrations of circulating chemokines (Figure 7B). Mean (± SEM) values were reduced from 186 (± 34) to 29 (± 5.4) pg/mL for CCL3, from 303 (± 75) to 70 (± 19) pg/mL) for CCL4, and from 255 (± 55) to 24 (± 9.4) pg/mL for CXCL13; all of these changes were statistically significant. Before CAL-101 administration, detectable levels of pAKTT308 were observed in all 12 patients. During daily CAL-101 treatment, there was a significant reduction in pAKTT308 levels by day 28 of therapy (Figure 7C). Representative histograms that depict staining of gated CLL cells and normal lymphocytes with anti-pAKTT308 antibodies are displayed in the supplemental Figure 6. Concurrent with the reductions in plasma chemokine levels, there was a significant increase in peripheral blood lymphocyte counts (Figure 7C).
Discussion
In our in vitro evaluation of the interaction of CLL cells with supporting stromal cells, we found that PI3Kδ inhibition by CAL-101 disrupts crosstalk between the CLL and its microenvironment in several ways. We observed that CLL chemokine receptor function and signaling are modulated by CAL-101, causing diminished leukemia cell chemotaxis and migration beneath MSC. We noted that CAL-101 impairs CLL cell viability, both by disrupting BCR signaling but also by antagonizing support from NLCs and by interrupting paracrine secretion of chemokines by CLL cells (CCL3, CCL4) and NLCs (CXCL13). CAL-101 also reduced the exaggerated production of other chemokines and cytokines (including CCL7, sCD40L, TNF-α, and CCL17 and CCL22) that occurred when CLL cells were cocultured with NLCs. These findings have functional consequences as evidenced by our observations that CAL-101 disrupts the protective effect of MSC and thereby sensitizes CLL cells to cytotoxic drugs. Finally, our in vitro data correlate with clinical data that CAL-101 treatment markedly reduces PI3Kδ signaling in vivo, significantly depresses levels of circulating chemokines, and concurrently provokes a transient lymphocytosis in many patients. Each of these findings is likely to be relevant for understanding the clinical activity and for future development of this PI3Kδ inhibitor.
The effects of CAL-101 on CLL cell migration provide an explanation for the most striking clinical activity of CAL-101, which is characterized by a compartment shift of CLL cells from the tissues into the blood, resulting in a rapid lymph node shrinkage, accompanied by a marked lymphocytosis within the first weeks of treatment.22 Based on our data and the function of PI3Kδ in cells of B lymphocyte origin, this effect is likely caused by interference of CAL-101 with the migration and homing capabilities of these cells. Prior in vivo studies have demonstrated that PI3Kδ plays an important role in normal B-cell migration and homing to the lymphatic tissues.27 In these studies, B cells with a PI3Kδ gene deletion responded poorly to chemokines, especially to CXCL13, and exhibited reduced homing to lymphatic tissues in adoptive transfer experiments.27 These findings are consistent with our results that in CLL cells, CAL-101 inhibits chemotaxis and homing to MSCs (Figure 1). The notable clinical activity of CAL-101, the pronounced lymph node shrinkage and the early transient lymphocytosis because of mobilization of CLL cells from tissues into the blood is shared with other kinase inhibitors that target the BCR and Akt-mTOR signaling pathways. Clinical data have demonstrated this effect with inhibitors of spleen tyrosine kinase (Syk),28 Bruton's tyrosine kinase (Btk),29 and mTOR.30 This suggests that modulation of leukemia cell trafficking and homing via chemokine receptor function is a more general and potentially critical mechanism of action of this group of kinase inhibitors. What remains to be better defined are the relative contributions of CLL cell mobilization and direct CLL cell killing to the treatment effects of these drugs. Our data suggest that a major effect of such agents may be redistribution of CLL cells into the periphery that results in subsequent CLL cell death “by neglect,” meaning by deprivation of CLL cells from their supportive tissue microenvironment. These results, considered together with earlier work on CAL-101,19,20 would suggest that CLL cell mobilization accompanied by parallel direct inhibition of survival signals leads to transient lymphocytosis, which over time resolves because of reduced proliferation of new CLL cells and enhanced apoptosis of existing cells.
The experiments related to effects of CAL-101 on BCR- and NLC-induced activation and viability of CLL cells capture another central activity of this PI3Kδ inhibitor. Activation of CLL cells via the BCR and NLCs sets in motion a cascade of signaling events, resulting in enhanced CLL cell survival, increased migratory capabilities to chemokines, and paracrine secretion of chemokines to attract accessory cells, such as T cells. CAL-101 reverses the pro-survival effect of anti-IgM and reduces CLL cell viability to levels that are even lower than in controls, indicating that both induced and constitutive PI3Kδ activity contribute to CLL cell viability. The importance of BCR signaling for maintenance and expansion of CLL cells in the lymphatic tissue microenvironment recently has been substantiated by gene expression studies that demonstrate BCR signaling, NF-kappaB activation, and CLL cell proliferation in CLL cells isolated from lymph nodes, but not in cells obtained from other disease sites.8 These data, along with the early notion of proliferation centers (also called pseudofollicles),31 and the subsequent characterization of proliferation centers as sites of CLL cell proliferation and engagement with accessory cells, such as T cells,11,12 point toward the secondary lymphatic tissues as the principal site for hosting BCR- and T cell–driven CLL cell proliferation, accounting for a turnover of 0.1% to > 1.0% of the CLL clone per day.32
A novel and interesting aspect of this work is the evaluation of CCL3, CCL4, and CXCL13 as chemokines that tie together BCR signaling and the lymph node microenvironment. CCL3 and CCL4 are secreted by CLL cells after activation via the BCR and NLCs,10 and are among the most overexpressed chemokines in CLL cells isolated from the lymph node microenvironment.8 We demonstrated a substantial inhibition of CCL3 and CCL4 production by CAL-101 in assays with BCR- and NLC-stimulation, as well as in patients treated with CAL-101. These data corroborate that CCL3 and CCL4 function as surrogate markers of CLL cell activation, both in vitro and in vivo, and hence may also function as markers for response to therapy. Normalization of CCL3 and CCL4 levels after only 28 days of treatment with CAL-101 (Figure 7B), a timepoint when CLL patients typically still have high numbers of circulating CLL cells, suggests that, in the context of kinase inhibitor treatment, plasma levels of these chemokines reflect the activation status rather than the disease burden.
In vitro, CAL-101 substantially reduced supernatant concentrations of CXCL13, a chemokine that attracts CLL cells to sanctuary sites in lymph nodes.6 The relevance of this in vitro finding is supported by correlative in vivo results indicating significant drug-mediated reductions in circulating CXCL13 plasma concentrations among patients with CLL receiving CAL-101 treatment. These data bolster the concept that PI3Kδ inhibition in cells of the microenvironment may play an important role in the activity of CAL-101. Loss of CXCL13 production, coupled with reduced signaling via the CXCL13 receptor, CXCR5, may be particularly important in explaining the lymphocytosis observed during early treatment with drug.
In summary, our study demonstrates that CAL-101 inhibits signaling pathways critical for CLL cell migration and survival that may explain the pattern of treatment effects currently being observed in the clinic. In addition, CAL-101 abrogates stroma-mediated drug resistance and sensitizes CLL cells to cytotoxic drugs. Thereby, this study provides novel insight into the mechanism of action of CAL-101, which is critical for understanding the clinical activity of this kinase inhibitor. In addition, our results offer direction for the rationale design of new combination clinical trials of CAL-101 that take advantage of its unique properties in mobilizing CLL cells from sanctuary sites.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jerry Evarts for supplying CAL-101. They also wish to acknowledge the clinical investigators as part of our CAL-101 phase 1 study who provided CLL samples for analysis in this study.
The study was supported by CLL Global Research Foundation grants (to W.W. and J.B.), by an ASCO Career Development Award (to J.B.), and by Calistoga Pharmaceuticals Inc.
Authorship
Contribution: J.H. performed the experiments, analyzed the data, designed the figures, and wrote the paper with J.A.B.; B.J.L., S.A.M., and N.G. performed multiplex assays, ELISAs, helped with the experimental design, and reviewed the manuscript; M.S. assisted with the experiments and reviewed the manuscript; A.Y., H.K., L.M., S.O'B., W.W. and M.K. provided samples, helped with data interpretation and reviewed the manuscript; and J.A.B. designed the research, supervised the study, analyzed the data, and revised the paper.
Conflict-of-interest disclosure: S.A.M., A.S.Y., L.L.M., and B.J.L. are employees of Calistoga Pharmaceuticals. N.G. is a former employee of Calistoga Pharmaceuticals and hold equity interests in the company. The remaining authors declare no competing financial interests.
Correspondence: Jan A. Burger, MD, PhD, Department of Leukemia, Unit 428, The University of Texas M. D. Anderson Cancer Center, PO Box 301402, Houston, TX 77230-1402; e-mail: jaburger@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal