Abstract
The purpose of this article is to set forth our approach to diagnosing and managing the thalassemias, including β-thalassemia intermedia and β-thalassemia major. The article begins by briefly describing recent advances in our understanding of the pathophysiology of thalassemia. In the discussion on diagnosing the condition, we cover the development of improved diagnostic tools, including the use of very small fetal DNA samples to detect single point mutations with great reliability for prenatal diagnosis of homozygous thalassemia. In our description of treatment strategies, we focus on how we deal with clinical manifestations and long-term complications using the most effective current treatment methods for β-thalassemia. The discussion of disease management focuses on our use of transfusion therapy and the newly developed oral iron chelators, deferiprone and deferasirox. We also deal with splenectomy and how we manage endocrinopathies and cardiac complications. In addition, we describe our use of hematopoietic stem cell transplantation, which has produced cure rates as high as 97%, and the use of cord blood transplantation. Finally, we briefly touch on therapies that might be effective in the near future, including new fetal hemoglobin inducers and gene therapy.
Introduction
The term “thalassemia” is derived from the Greek words “Thalassa” (sea) and “Haema” (blood) and refers to disorders associated with defective synthesis of α- or β-globin subunits of hemoglobin (Hb) A (α2; β2), inherited as pathologic alleles of one or more of the globin genes located on chromosomes 11 (β) and 16 (α). More than 200 deletions or point mutations that impair transcription, processing, or translation of α- or β-globin mRNA have been identified. The clinical manifestations are diverse, ranging from absence of symptoms to profound fatal anemias in utero, or, if untreated, in early childhood.1
The thalassemia syndrome is classified according to which of the globin chains, α or β, is affected. These 2 major groups, α- and β-thalassemia, are subclassified according to absent (α° and β°) or reduced (α+ or β+) globin chain synthesis. In addition, where γ-chains together with α-chains compose fetal hemoglobin (HbF) in the fetus and δ chains in combination with α-chains compose hemoglobin A2 in adults, impaired synthesis of γ-globin or δ-globin chains can occur.
Although the switch from γ- to β-globin synthesis begins before birth, replacement of HbF by HbA occurs postnatally. Consequently, newborn infants with severe β-globin chain abnormalities are asymptomatic until 4-6 months of age. Complete absence of α-globin chains results in intrauterine failure and hydropic births, whereas fetuses with the lack or dysfunction of 3 α-genes, which is known as hemoglobin H (HbH) disease, will survive gestation.
Some mutations may also alter fetal to adult Hb switching, which occurs, for example, in hereditary persistence of HbF. Coinheritance of α- and γ-mutations as well as coinheritance of other hemoglobinopathies (eg, HbE, Hb Lepore, Constant Spring [CS], sickle cell hemoglobin, or HbS) may modify the clinical manifestations.1
Incidence
The thalassemias represent the most common monogenetic disorder worldwide. Because thalassemia heterozygosity confers some immunity against malaria, there is a particularly high incidence of thalassemia (2.5%-25%) in the Mediterranean basin, the Middle East, the tropical and subtropical regions of Africa, the Asian subcontinent, and Southeast Asia, where milder forms of the disease are most commonly seen. Cases of thalassemia also occur sporadically in virtually every ethnic group and geographic location.2,3
Pathophysiology
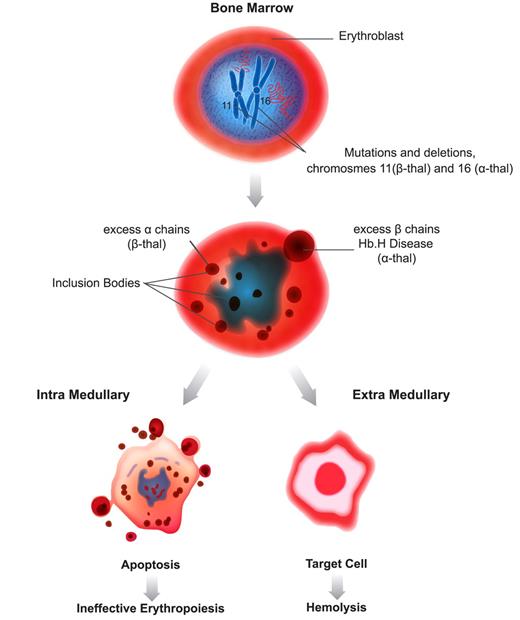
Although clinical spectra vary depending on coinheritance of other genetic modifiers, the underlying pathology among the types of thalassemia is similar.4 This pathology is characterized by decreased Hb production and red blood cell (RBC) survival, resulting from the excess of unaffected globin chain, which form unstable homotetramers that precipitate as inclusion bodies. α-Homotetramers in β-thalassemia are more unstable than β-homotetramers in α-thalassemia and therefore precipitate earlier in the RBC life span, causing marked RBC damage and severe hemolysis associated with ineffective erythropoiesis (IE) and extramedullary hemolysis.5 (Figure 1) In severe β-thalassemia, IE results in expanded marrow cavities that impinge on normal bone and cause distortion of the cranium, and of facial and long bones. In addition, erythroid activity proliferates in extramedullary hematopoietic sites, causing extensive lymphadenopathy, hepatosplenomegaly, and, in some cases, extramedullary tumors.1
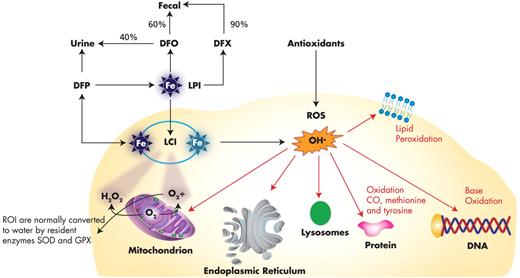
Severe IE, chronic anemia, and hypoxia also cause increased gastrointestinal (GI) tract iron absorption. Without transfusion support, ∼ 85% of patients with severe homozygous or compound heterozygous β-thalassemia will die by 5 years of age because of severe anemia.6 However, transfusions lead to progressive iron accumulation because of inadequate excretory pathways. When serum transferrin saturation exceeds 70%, free iron species, such as labile plasma iron, have been found in the plasma as well as labile iron pool in the RBCs. These iron species are mainly responsible for generating reactive oxygen species7 (Figure 2) with eventual tissue damage, organ dysfunction, and death. There have been attempts to ameliorate oxidative stress in thalassemic blood cells using antioxidants, but so far they have not met with clinically significant success.8,9 Iron chelation therapy has proven to be the only option to reduce morbidities and prolong survival into the fourth and fifth decades of life.
Amelioration of free iron species (LPI and LCI) by iron chelators and antioxidants. Labile plasma iron (LPI) is penetrating through the cell membrane with a consequent accumulation of labile cell iron (LCI). Both LPI and LCI react with reactive oxygen intermediate (ROI) producing noxious reactive oxygen species (ROS), for example, OH' radicals, which are highly reactive and oxidize DNA, proteins and lipid components of the cell. Deferiprone (DFP) chelates LCI alone or in combination with LPI by Deferiozamine (DFO). Deferasirox (DFX) mainly removes LPI.
Amelioration of free iron species (LPI and LCI) by iron chelators and antioxidants. Labile plasma iron (LPI) is penetrating through the cell membrane with a consequent accumulation of labile cell iron (LCI). Both LPI and LCI react with reactive oxygen intermediate (ROI) producing noxious reactive oxygen species (ROS), for example, OH' radicals, which are highly reactive and oxidize DNA, proteins and lipid components of the cell. Deferiprone (DFP) chelates LCI alone or in combination with LPI by Deferiozamine (DFO). Deferasirox (DFX) mainly removes LPI.
The α-thalassemias
Molecular studies using nucleic acid hybridization techniques and endonuclease analysis have identified loss of α-gene function related to gene deletion or nondeletional mutations causing hypofunctional genes and terminator codon mutations as responsible for the various α-thalassemia syndromes.1 Nearly 70 different nondeletional mutations exist that may be coinherited with deletional mutations or other genetic modifiers that result in variable genotypic and/or phenotypic expression.10
A diagnosis of α-thalassemia can be suspected based on factors, such as a family history of anemia and geographic and ethnic background, particularly if the patient comes from the Middle East, North Africa, and Southeast Asia, areas where α-thalassemia is common. The diagnosis is suspected in the presence of microcytic hypochromic anemia not because of iron deficiency, with normal HbA2 levels in Hb electrophoresis identified. Silent carriers of α-thalassemia and/or α-thalassemia trait are in general clinically asymptomatic and may present with either normal blood count and morphology or with mild microcytic hypochromic anemia. A differential diagnosis must be made to distinguish patients with iron deficiency anemia from those with α-thalassemia trait. No specific treatment is recommended unless the patient is anemic. Folic acid (1-5 mg/day) can be given when the diet is deficient in folate and/or in the presence of infection, malabsorption, and where the patient is pregnant.
HbH disease
Diagnosis of HbH disease is made using hemoglobin electrophoresis. Patients with HbH disease present with mild to moderate microcytic hypochromic anemia with Hb levels 8-10 g/dL. On physical examination, hepatosplenomegaly is commonly discovered. Exacerbation of the anemia can be induced by folic acid deficiency, acute infections, exposure to oxidative stress, and pregnancy. Treatment consists of folic acid supplementation (5 mg/day) and periodic blood transfusions when indicated. In more severe cases, some patients, especially those with compound heterozygotes for HbH and Hb CS, common in Southeast Asia, have more severe hemolytic anemia with moderate to severe IE. For these patients, transfusions may be required from infancy, with eventual splenectomy.10 Genotyping of 836 thalassemia patients in the United States by the National Institutes of Health Thalassemia Clinical Research Network identified 106 (12.7%) with HbH disease, 46 (5.5%) with a nondeletional α mutation, and 44 with HbH and Hb CS, most of them from the west coast.11
The β-thalassemias
β-thalassemia minor
In making a diagnosis of β-thalassemia minor, one must rule out the existence of iron deficiency, which may alter the usually elevated HbA2 levels. High levels of HbF are also seen, depending on the underlying genetic mutation. A carrier's RBC is microcytic (mean corpuscular volume < 79 fL) and hypochromic.
The clinical manifestations of β-thalassemia minor are usually mild, and patients with this condition generally have good quality of life. In the majority of carriers, the anemia is not clinically significant and does not require specific treatment, although carriers have occasionally been reported with splenomegaly, mild bone changes, leg ulcers, or cholelithiasis. In pregnant women, significant anemia (Hb < 7 g/dL) may develop (usually by the third trimester), requiring 1-5 mg/day of folic acid and supportive transfusion therapy.12 Couples and their close relatives should be evaluated for silent or atypical α- and β-mutations, and if they are detected, prenatal genetic counseling for diagnostic purposes should be provided.
β-thalassemia intermedia
Clinical manifestations
Nearly 10% of β-thalassemia patients have β-thalassemia intermedia (TI). Genetically, this group may have homozygous δβ-thalassemia, homozygous or compound heterozygous β° thalassemia, and/or β+ thalassemia mutations. These may present with or without the concurrent inheritance of an α-thalassemia gene deletion, mutation, or triplication, or of a γ-mutation. They have a moderate hemolytic anemia, maintaining Hb levels > 7 g/dL without transfusion support. In TI patients, the clinical phenotypes vary from those with β-thalassemia minor and from transfusion-dependent β- thalassemia major (TM) patients.13 The use of transfusions is what clinically divides the categories of β-TI from β-TM. When their transfusion requirements reach > 8 units per year, they are reclassified as β-TM. TI patients' clinical presentation typically occurs at 2-4 years of age, later than β-TM patients, and symptoms can include anemia, hyperbilirubinemia, and hepatosplenomegaly. These patients generally present with better growth, development, and sexual maturation than TM patients, and they typically live longer before dying of complications of chronic anemia with pulmonary hypertension, iron-induced cardiac disease, or liver failure.14 The majority of the patients will require transfusions at some point in their lives or when hemolytic or aplastic crises associated with acute infections, folate deficiency, hypersplenism, or pregnancy occur.
In some TI children, despite their having Hb levels > 7 g/dL, growth failure or cosmetic facial and bony abnormalities occur, which may not be reversible unless regular transfusions are started before the age of 6 or 7 years.1 In older patients, massive splenomegaly is often associated with hypersplenism, which contributes to progressive anemia neutropenia and thrombocytopenia, and it warrants a trial of regular transfusions to improve splenic size and function, although splenectomy may be required. TI patients who develop progressive anemia, fatigue, and cardiopulmonary complications also require regular transfusions to maintain Hb levels > 9-10 g/dL.15-17
TI treatment strategy
The need to identify complications that can be managed with transfusion support in TI patients is now being recognized because of the frequency of age-related complications associated with chronic anemia because of increased GI tract iron absorption that occurs even in untransfused patients.18 We think that, in TI patients whose ferritin levels are well above 500 μg/dL, monitoring of iron excess using only serum ferritin is insufficient,14 and we recommend annual assessments of liver iron concentration (LIC) by liver biopsy or by the more recently applied noninvasive T2* magnetic resonance imaging (MRI) beginning in late childhood or early adolescence.19 Iron chelation therapy is warranted when LIC exceeds 5-7 mg/g dry weight and to prevent serious endocrine and cardiac complications similar to those seen in TM patients.20 Monitoring for splenomegaly and hypersplenism is mandatory as a possible indication of the need for splenectomy. Other common complications include postsplenectomy thrombocytosis, cholelithiasis, leg ulcers, hyperuricemia, and aplastic crisis secondary to folic acid deficiency, which is an uncommon complication.
β-thalassemia major
β-thalassemia major (also called Cooley anemia, Mediterranean anemia, and von Jaksch anemia) denotes the homozygous or compound heterozygous forms of the disease, which are characterized by severe anemia (range, 1-7 g/dL of Hb), hemolysis, and massive IE.6 Clinical manifestations appear in infancy and include severe anemia characterized by extreme pallor, jaundice, or failure to thrive, accompanied by poor feeding, irritability, decreased activity, and/or increased somnolence. Hepatosplenomegaly and frontal bossing with the early signs of abnormal thalassemic facies are usually present.21
TM treatment strategies
Transfusion therapy
The decision to initiate a regular transfusion program in a child newly diagnosed with thalassemia must take into account both laboratory and clinical findings. An overlap of genotype and phenotype expression make the clinical assessment the most important step in distinguishing TM from TI. If the child is growing poorly and has developed facial or other bone abnormalities, and/or when Hb levels are < 7 g/dL, regular transfusions will be beneficial.1
Confounding factors that might aggravate the degree of anemia, including folic acid deficiency and acute febrile illness, blood loss, or coinheritance of glucose-6-phosphate dehydrogenase deficiency, need to be addressed simultaneously with transfusion therapy. If the child is folic acid replete and failing to thrive with no other factors to explain the Hb level of < 7 g/dL, a first transfusion is administered. The child is subsequently followed; and when the Hb level falls again to a level of < 7 g/dL, a regular monthly transfusion regimen is begun.
Before the first transfusion, patients' RBCs are typed for Rh and ABO antigens. At the same time, cytomegalovirus status should be obtained. Cytomegalovirus-negative blood products are recommended for potential candidates for curative stem cell transplantation (SCT). Parents and first-degree relatives should not be blood donors for these candidates. Hepatitis B vaccination is given before transfusion therapy, as is hepatitis A vaccine when age appropriate.1,22
The risk of transfusion-transmitted infections in thalassemia patients has been greatly reduced since screening for human immunodeficiency virus infections began in 1985 and for hepatitis C in 1991.22 However, new agents, such as West Nile Virus and babesiosis, which are not screened for, may contaminate the blood supply from asymptomatic donors.23
Transfusions of washed, leukocyte-depleted RBCs are recommended for all the patients to reduce the incidence of febrile and urticarial reactions as well as infectious cytomegalovirus contamination. If they are not available, frozen thawed RBCs should be administered. Once a pretransfusion Hb level > 9-10 g/dL is achieved, transfusions are administered monthly in infancy and subsequently at 2- to 4-week intervals.24,25 In clinically stable patients, ∼ 8-15 mL RBCs per kilogram of body weight can be infused over a span of 1-2 hours at each transfusion event.
If Hb levels are < 5 g/dL and/or in the presence of heart failure, smaller aliquots of RBCs (5 mL/kg) should be administered to prevent volume overload until the Hb level is gradually increased to 9 g/dL. A clinical record of all transfusion events should be monitored annually to identify hypersplenism. A record of weight, the amount of blood transfused at each visit, and the pretransfusion Hb level is needed to calculate the annual transfusion requirement.26
Managing TM complications
Cardiac complications
Cardiac failure and serious arrhythmias are the major causes of life-threatening morbidity and mortality in iron-overload patients.27 Before the availability of chelation therapy, cardiac disease was inevitable during the second decade and still occurs in older patients or those who are poorly compliant with chelation therapy.28 Therefore, cardiac function is monitored annually beginning at 7 or 8 years of age by electrocardiogram, echocardiogram, 24-hour Holter monitor, and recently by cardiac T2* MRI, which can detect preclinical cardiac iron accumulation.29
Pericarditis
Thalassemia patients are susceptible to benign pericarditis, possibly caused by viral and mycoplasmal organisms, bacterial or fungal infections, or associated with the engraftment syndrome in post-transplantation thalassemic patients.30 “Iron-induced” pericardial siderosis has also been postulated as a causative factor.31 Diagnosis is made by history and physical signs and is confirmed with serial electrocardiograms and chest x-ray and requires hospitalization if they are symptomatic. Pericarditis is best managed with bed rest and aspirin. Steroids may be helpful with engraftment syndrome and iron chelation with hemosiderosis. When a significantly large pericardial effusion is present, the patient should be hospitalized and observed. Pericardiocentesis and diuretics are recommended to prevent cardiac tamponade.32 Surgical intervention may be necessary if significant pericardial effusions recur.
Managing endocrinopathies
Growth and development
Normal growth and development can be achieved in the first decade by maintaining near-normal pretransfusion Hb levels of 9-10 g/dL.33 However, iron-induced damage to the hypothalamic pituitary axis can cause delayed pubertal growth and sexual development despite timely initiation of iron chelation in early childhood. Therefore, annual endocrine evaluations are recommended, including measures of pancreatic, thyroid, parathyroid, gonadal function, and bone health with nutritional counseling.34
Tanner staging should be performed every 6 months in the prepubescent child. Annual bone age films are performed to assess skeletal maturation. We begin annual monitoring between 8 and10 years of age for luteinizing hormone, follicular stimulating hormone, insulin-like growth factor, and insulin-like growth factor binding protein-3. Tests measuring these factors are required to make early diagnoses of growth hormone deficiency, which can be managed successfully with hormone replacement before the completion of puberty. If pubertal changes have not developed by 13 years of age in females, or 16 years of age in males, the use of gonadotropin releasing hormone and gonadal steroids may be necessary.35 Starting at 8-10 years of age, annual glucose tolerance testing for the early detection of insulin resistance is recommended to identify prediabetic or diabetic states caused by pancreatic destruction, which might benefit from metformin administration or indicate the need for insulin therapy.35
Bone disease
Although RBC transfusions suppress IE, making skeletal abnormalities less common today than in the past, bone health in thalassemia patients must be monitored to identify age-related low bone mass. Nearly 90% of TM patients, including 30% of those younger than 12 years, have low bone mass Z-score (≤ −2.0).36 For this reason, beginning in childhood, yearly studies that include bone mineral density as well as studies of calcium, vitamin D3 metabolism, and thyroid and parathyroid function should be performed.
Low bone mass is associated with a high prevalence of fractures in TM (17%) and TI (12%) patients, and the frequency increases with age, hypogonadism, and increased bone turnover.36 Some short-term success has been seen with the administration of pamidronate in patients with Z-/T-score < 2.5. Important preventive measures include age-appropriate calcium and vitamin D supplementation and timely use of hormonal supplementation.1
It seems that early administration of iron chelation is effective in preventing endocrine complications. According to the Thalassemia Clinical Research Network, 96% of chelated thalassemia patients with a median age of 20 years were free of hypoparathyroidism, 91% had no thyroid disease, and 90% were free of diabetes. Overall, 62% were free of any endocrinopathy.37 However, this is not always the case because some patients may develop endocrine complications despite chelation.
Hypercoagulable state
Because improvements in the medical management of patients with TM and TI have resulted in significant prolongation of life, previously undescribed complications are now being seen. These include the existence of a hypercoagulable state, particularly in splenectomized patients with TI who do not receive regular transfusions.38,39 Prothrombotic hemostatic anomalies, including low levels of coagulation inhibitors, such as protein C and protein S as well as thrombocytosis and platelet activation, have also been observed in these patients.40,41 Both venous and arterial events, including infrequent thrombotic events in the brain, have been described with a higher occurrence in TI than TM.42,43 However, the latter are largely subclinical.43 The addition of prophylactic antithrombotic therapy for high-risk patients with TI who have associated risk factors, such as surgery, immobilization, and pregnancy, should be considered, as should the use of antiplatelet aggregating agents for patients with significant thrombocytosis.42 However, until now, there are no recommendations based on clinical trials regarding if, when, or for whom prophylactic antithrombotic treatment is indicated.
Splenectomy
The severe hemolysis in TM and TI results in progressive overactivity of the spleen, which eventually aggravates the severity of the anemia and consequently increases transfusion requirements. After the initiation of a regular transfusion program from an early age, splenomegaly may be averted, but hypersplenism may nonetheless develop, usually in children between 5 and 10 years of age. The therapeutic rationale for splenectomy, particularly in patients with growth retardation and poor health, is to protect against the development of extramedullary hematopoiesis by improving the Hb level, decreasing the transfusion requirement, and consequently reducing iron overload (IO).44,45 It should be noted there are patients who are on regular transfusion programs who develop hypersplenism without splenomegaly. Therefore, we recommend splenectomy when the calculated annual transfusion requirement is > 200 to 220 mL RBCs/kg per year with a hematocrit of 70% (equal to 250-275 mL/kg per year of packed RBCs with a hematocrit of 60%.)46,47
The susceptibility to overwhelming infections after splenectomy can be reduced by immunization with pneumococcal and meningococcal vaccines before splenectomy and antimicrobial prophylaxis with penicillin after splenectomy. Fever over 38°C (101°F) developing in splenectomized patients with no focus of infection requires immediate intravenous broad-spectrum antibiotics. TI patients or those who have had previous thrombotic events should be carefully monitored for postsplenectomy thrombocytosis requiring thrombophilia prophylaxis or platelet deaggregating agents.48 However, before recommending splenectomy, one should bear in mind that, in a recent evaluation of 584 patients with TI, significantly higher rates of complications were documented in splenectomized patients.13
Iron chelation therapy
In cases of ongoing transfusion therapy, with each RBC unit containing ∼ 200 mg of iron, cumulative iron burden is an inevitable consequence. In TI and TM patients, the rate of transfusional and GI tract iron accumulation is generally 0.3-0.6 mg/kg per day.49 Increased GI tract iron absorption can result from severe anemia and IE, which down-regulate the synthesis of hepcidin, a protein that controls iron absorption from the GI tract and increases release of recycled iron from macrophages.50-52
To date, there are 3 major classes of iron chelators: hexadentate (deferoxamine [DFO], Desferal), in which 1 atom of iron is bound to 1 DFO molecule; bidentate (deferiprone, L1 [DFP]), in which 1 atom of iron is bound to 3 DFP molecules; and tridentate (deferasirox [DFX], Exjade), in which 1 atom of iron is bound to 2 DFX molecules.53,54
Parents are provided information by physicians about the currently available iron chelators, and together they make an informed decision about the chelator of choice for the child.
DFO, a naturally occurring sideraphore derived from Streptomyces pilosus with a high molecular weight of 657 and a very short half-life of 8-10 minutes, requires intravenous or subcutaneous parenteral administration. DFO enters hepatic parenchymal cells, chelates iron, and appears in the serum and bile as the iron chelator feroxamine. It also chelates iron released after catabolism of senescent RBCs and is excreted in the urine. The proportions and the long-term patient survival of DFO-chelated iron vary from patient to patient and are related to the degree of iron loading, chelator dose, frequency or duration, and IE activity.55 Maintaining normal ascorbic acid levels optimizes DFO iron excretion.56 Continuous slow subcutaneous infusions of DFO with a lightweight portable battery-operated pump enables longer exposure to circulating labile plasma iron.57
The initial recommended dose is 30-40 mg/kg per day for daily use 5-7 days each week in regularly transfused thalassemia patients. Chelation generally begins between 2 and 4 years of age, after 20-25 RBC units are transfused, with a serum ferritin level > 1000 μg/dL and an LIC > 3 mg Fe/g dry weight as measured by liver biopsy or by noninvasive hepatic T2*MRI. The efficiency of chelation can be relatively low during the first few years and may warrant gradual escalation of the daily DFO dose to 50 mg/kg and subsequently to 60 mg/kg in adolescents and adults. Those heavily IO adults previously naive to chelation should also be started on the higher DFO dose range. Dosage modifications may also be guided by annual monitoring of LIC with dose adjustments to maintain LIC of 3-7 mg Fe/g dry weight.56 Patients who are poorly complaint to administration of subcutaneous DFO can receive daily intravenous DFO using indwelling central venous lines.
DFP (L1) is a synthetic compound originally identified in the 1980s in London, hence the designation L1.58,59 It is absorbed by the GI tract and has a plasma half-life of 1.5-4 hours. The recommended daily dose is 75 mg/kg per day, which can be increased to 100 mg/kg per day, given orally in 3 divided doses with meals.60
DFP penetrates cell membranes more rapidly than DFO, expediting the chelation of toxic intracellular iron species. Initial clinical efficacy studies were encouraging, indicating that DFP is capable of rapidly removing intracellular iron, and more recent reports suggest its efficacy in removing iron from the heart, improving cardiac function, and preventing iron-induced cardiac disease.61-63
The sequential combination of DFP and DFO has an additive, if not synergistic, chelating effect. The “shuttle hypothesis” suggests that intracellular iron chelated by DFP may be transferred to DFO, a stronger chelator, in the plasma. (Figure 2.) Subsequently, DFP may reenter cells to bind with more iron, inducing greater iron excretion.54 Regular monitoring of blood counts on a weekly basis is mandatory because of the potential risk of agranulocytosis in 1% of the patients treated with DFP.64,65
DFX (Exjade), approved in 2005 for use in transfusional overload patients, is an orally ingested, highly bioavailable chelator that is absorbed in the GI tract.66 Because of its dose-dependent half-life of 12-18 hours, it can be taken once a day. Daily use of a single oral dose of 20-30 mg/kg per day results in dose-dependent decreases in LIC with similar trends in serum ferritin comparable with those achieved by subcutaneous 8-hour administration of 40-60 mg/kg per day DFO.67 The efficacy of DFX dosing is related to transfusional iron intake.26 Some patients may benefit by escalating the dose up to 40 mg/kg per day. Moreover, in a group of 114 patients who had cardiac IO, levels of cardiac iron measured by T2* MRI were decreased after 1 year of DFX.68
Close monthly monitoring of serum ferritin and creatinine levels and liver function is indicated. Interruption or discontinuation of DFX is required in cases of unexplained progressive increase in transaminase, progressive increase in serum creatinine, or progressive GI symptomatology (Table 1). Recent reports suggest that DFX is also effective in the removal of cardiac iron in hypertransfused rats and TM patients with abnormal MRI T2* cardiac iron.69,70 Experimental studies show that a combination of DFX with DFO chelation results in additive iron excretion.71
Comparison of the 3 leading iron-chelating drugs in the management of thalassemia
| Compound . | DFO . | DFP . | DFX . |
|---|---|---|---|
| Molecular weight, Da | 657 | 139 | 373 |
| Chelating properties | Hexadentate | Bidentate | Tridentate |
| Recommended dose | 30-60 mg/kg | 75-100 mg/kg | 20-40 mg/kg per day |
| Delivery | Subcutaneous or intravenous 8-12 h, 5-7 d/wk | Oral 3 times daily | Oral once daily |
| Half-life | 8-10 min | 1.5-4 h | 12-18 h |
| Excretion | 40%-60% fecal | 90% urinary | 90% fecal |
| Adverse effects | Ocular, auditory toxicity, growth retardation, local reactions, allergy | Gastrointestinal upset, arthralgia, neutropenia, agranulocytosis | Gastrointestinal upset, rash, ocular, auditory toxicity, reversible increases in creatinine, hepatitis |
| Compound . | DFO . | DFP . | DFX . |
|---|---|---|---|
| Molecular weight, Da | 657 | 139 | 373 |
| Chelating properties | Hexadentate | Bidentate | Tridentate |
| Recommended dose | 30-60 mg/kg | 75-100 mg/kg | 20-40 mg/kg per day |
| Delivery | Subcutaneous or intravenous 8-12 h, 5-7 d/wk | Oral 3 times daily | Oral once daily |
| Half-life | 8-10 min | 1.5-4 h | 12-18 h |
| Excretion | 40%-60% fecal | 90% urinary | 90% fecal |
| Adverse effects | Ocular, auditory toxicity, growth retardation, local reactions, allergy | Gastrointestinal upset, arthralgia, neutropenia, agranulocytosis | Gastrointestinal upset, rash, ocular, auditory toxicity, reversible increases in creatinine, hepatitis |
In some cases, patients who were not treated or insufficiently treated with iron chelators present, for the first time, with heart failure induced by IO. These patients should be started with DFO in a dose of 80 mg/kg by daily 24-hour continuous intravenous infusion together with DFP, where it is approved for use. This treatment has been shown to result in improvement in cardiac function. Concomitantly, cardiac function tests have to be monitored in an intensive care setting in collaboration with a cardiologist until significant improvement is achieved.72-74
If cardiac studies are abnormal but the patient is clinically well, we recommend maximizing the current chelation regimen.
Another unique group of patients is composed of pregnant women who require iron chelation. For these patients, it is recommended to delay chelation until the second trimester and to use subcutaneous DFO according to the guidelines of IO parameters. DFX is not approved for use during pregnancy.
Prevention: prenatal diagnosis
Prevention of severe α- or β-thalassemia births by prenatal diagnosis with termination of pregnancies has been available for > 2 decades, although it is among the most difficult ways to deal with the disease.75 Acceptance of prenatatal diagnosis and termination of affected fetuses are dependent on the early identification of couples at risk, culturally sensitive genetic counseling, the cost, and religious beliefs even when PCR technologies are available. Preimplantation genetic diagnosis is also currently feasible, although it is only available in some centers where conventional use of in vitro fertilization is also available. In this case, DNA of a cell from the blastomere is used for genetic diagnosis. However, successful diagnosis may be compromised by failure to amplify one of the 2 alleles in a heterozygous cell and/or by other complications associated with in vitro fertilization.76
Current PCR technologies and precise hybridization assays to detect single point mutations with great reliability using very small DNA samples have been developed. Adequate amounts of fetal DNA can be obtained safely around the 10th week of gestation by chorionic villus sampling and up to the 18th week of gestation by amniocentesis.1 New technology using fetal DNA obtained from maternal plasma or maternal peripheral blood has also been developed but is not routinely available.77,78
Cure: hematopoietic SCT
The first curative allogeneic SCT to a thalassemia patient from an human leukocyte antigen (HLA) identical sibling donor was reported in 1982.79 Since then, > 3000 successful transplantations have been reported.80 The probability of overall event-free survival has been recently reported as high as 89%-97% for patients with no advanced disease and of 80%-87% for patients with advanced disease.81
Donor selection is of great importance because transplantations may fail or be lethal resulting from immunologic complications. The best results have been obtained with HLA-matched siblings. The preparatory regimen includes administration of busulfan fludarabine and cyclophosphamide, which in combination can eradicate the thalassemia clone, enhance immunosuppression, and facilitate sustained allogeneic engraftment.82 There are several risk factors, including hepatomegaly > 2 cm, portal fibrosis, and inadequate iron chelation therapy, that can influence the outcome of SCT. Patients are typically classified into 3 risk groups: class 1, those with no risk factors; class 2, those with 1 or 2 risk factors; and class 3, those with all risk factors.81
The administration of cyclosporine and methylprednisolone together with a short course of methotrexate has been recommended as GVHD prophylaxis with an outcome of 8% moderate and 2% severe GVHD manifestations.83 Advances in conditioning regimens have considerably improved the outcomes of class 3 patients younger than 17 years. However, these favorable results have not been reproduced in older, more heavily iron-overloaded patients, and they remain at high risk for transplantation-related mortality.84
Approximately 10% of SCT patients are transfusion-free for years, although they experience persistent mixed hematopoietic chimerism.85 This suggests that only a few engrafted donor cells are sufficient for correction of donor phenotype. Approximately 30% subsequently reject their grafts.84 Those who deteriorate and require further transfusion support may benefit from a second transplantation with nonmyeloblative conditioning to restore normal Hb levels.81
Despite a successful engraftment, previously iron-overloaded patients may require phlebotomy after transplantation to prevent the risks of residual iron excess causing hepatic fibrosis or other endocrine complications.86 Moreover, growth failure and/or hypogonadism and infertility can develop after the chemotherapeutic preparative conditioning for transplantation or secondary to iron excess. Persistent iron excess can be normalized by phelobotomy after successful engraftment. Long-term post-transplantation survival in some patients may also be affected by previously acquired hepatitis C, which can be treated with ribavirin and peg-interferon.85 Rare cases of myelodysplastic syndrome and carcinoma have been reported in some centers.86,87
Another option is to use matched unrelated donor if a matched sibling is not available or when patients are not compliant with conventional therapy. In one series of 27 patients, 70% of 27 patients were alive and transfusion-independent for > 3 years using matched unrelated donor. However, 40% developed GVHD and a third had chronic GVHD.88 In another series of 49 thalassemic children from Thailand, there was no difference in the outcome of 28 patients transplanted from a related donor compared with 21 who received stem cells from unrelated donor.89 A few patients who failed the first transplantation underwent a second transplantation. Although the preliminary results are encouraging,90 this approach requires more clinical data before it can be recommended.
Cord blood transplantation
The potential benefits of umbilical cord blood (UCB) treatment are the low risk of viral contamination from a graft, the decreased incidence of acute and chronic GVHD, and easier accessibility. The small size or small number of stem cells in the UBC collection relative to the number required for engraftment are probably the main causes of failure of UCB transplantation; therefore, this procedure is being used mainly in pediatric patients.91 Some patients have received UCB transplantation in combination with bone marrow or peripheral progenitor cells.91
The use of UCB from unrelated donors has resulted in only 77% survival and 65% event-free survival, respectively, in 36 thalassemia patients.92 In these cases, it is suggested to store the patient's own bone marrow in case of a graft failure. The experience with UCB transplantation is encouraging, but additional data are required for definitive conclusions.
On the basis of all the available data to date, we think that every patient with a severe form of thalassemia should be offered the option for SCT. In addition, a check should be made for HLA-matched donors among family members when the use of cord blood and matched unrelated donor, the second best option, is suggested. In selected patients who fail a first transplantation, a second transplantation is also a possibility. Although SCT is the only curative available, its use is still limited because of the relatively high cost and the difficulty in identifying suitable donors.
Future therapies
Fetal Hb inducers
For many years, a major therapeutic goal has been to decrease the severity of anemia in β-thalassemia patients by the pharmacologic enhancement of the fetal globin gene expression to increase γ-globin chain production that would improve the excess α-chain imbalance. Several drugs, including erythropoietin, demethylating agents, such as 5-azacytidine, and short chain fatty acids, such as butyrate, have been studied individually and in various combinations.1,5 The short-chain fatty acid butyrate was reported to decrease transfusion requirements in transfusion-dependent β-thalassemia patients for 7 years.93 Erythropoietin administration is capable of increasing thalassemic erythropoiesis, mainly in patients with TI but also in those with E-β-thalassemia, without increasing HbF. Patients with low endogenous erythropoietin levels have been reported to respond to the combination of erythropoietin and butyrate.93 Hydroxyurea (HU), which is very effective in increasing HbF levels, has been used extensively for many years in patients with sickle cell anemia (SCA). However, the experience in thalassemia is limited. A substantial decrease in transfusion requirements and/or an increase in Hb levels, which may have been correlated with haplotypes, has been reported during a 6-year follow-up of 149 of 163 patients with β-thalassemia in Iran subsequent to their receiving a dose of 8-12 mg/kg per day.94,95
One of the major concerns is possible effects of HU on fertility, pregnancy or the risk of malignancy. However, the long-term experience with HU in SCA has ruled out these options.96 By and large, until now, the use of some of these agents has been limited by marginal therapeutic efficacy, high cost, insufficient clinical data, and/or difficulty of administration.5
Most recently, decitabine and HQK-1001, new fetal globin inducers that stimulate fetal globin induction through the proximal promoter and also exhibit erythropoietic-stimulatory effects, are being studied.93
Another potential strategy is to develop techniques to silence HbF suppression. Recently, the molecular basis of the HbF to HbA switch identified a variation in chromosome 11-encoding locus BCL11A, which was found to be associated with the level of HbF in patients with thalassemia and to be a regulator of γ-globin expression. Knockdown of BCL11A expression resulted in reactivation of HbF expression, which inversely correlated with the level of HbF.97
Gene therapy
Murine β-thalassemia models have been successfully cured with the use of a retroviral vector (TN39) transferring the human β-globin gene sequence and its promoter region into murine stem cells of TI and TM mice.98,99 β-Globin gene transfer into progenitor hematopoietic cells of humans is also being studied.100,101 However, concerns regarding gene transfer include the need for improved efficiency of gene delivery and mastery of vector stability, viral titers, nononcogenic insertion, the variable expression of globin genes, and the variable contributions of the β-thalassemia phenotype and other modifiers to the effectiveness of gene transfer.102
One regularly transfused patient with Hb-E/β° thalassemia has been reported who, after nonmyeloablative conditioning, received autologous bone marrow CD34+ cells transduced with a lentiviral vector expressing a βA-787Q globin gene, and has remained stable without transfusion support for 2 years.103 In addition, a phase 1 study of transfusion-dependent β-thalassemia patients using the TNS q.3.55 lentiviral vector encoding human β-globin gene after nonmyeloablative conditioning is planned. This approach may prevent graft rejection in patients who do not have identically matched HLA donors and therefore are at higher risk to develop GVHD and continuous immune suppression.104 Several other molecular approaches for gene therapy using different mutations of stop codons and aberrant splicing have also been described.102 Gene therapy is a promising approach to curing thalassemia but is still in the early investigational phase trials.
In conclusion, we have tried to describe the different clinical manifestations of thalassemia with the optimal care that is available today. However, very different treatment approaches exist worldwide depending on factors, such as socioeconomic conditions, cultural traditions, and the quality of available health care. Currently, in parts of the world where sufficient resources exist to support optimal transfusion and chelation programs, thalassemia patients are living longer and maintaining a good quality of life, with a select few being cured using bone marrow transplantation.26,27
Authorship
Contribution: E.A.R. and P.J.G. wrote the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eliezer A. Rachmilewitz, Department of Hematology, Wolfson Medical Center, Holon, Israel; e-mail: rachmilewitz@wolfson.health.gov.il; and Patricia J. Giardina, Division of Pediatric Hematology/Oncology, New York Presbyterian Hospital, New York, NY; e-mail: pjgiardi@med.cornell.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal