The article by Fuchs et al1 in this issue of Blood demonstrates that histones, possibly released from neutrophils in infection, or necrotic cells, can activate platelets directly, lead to thrombocytopenia in vivo and, importantly (see figure), that this platelet activation can be blocked by heparin. This study fits into our broader understanding of the links between the innate immune response and coagulation.
Innate immune responses trigger coagulation in a variety of ways, including through induction of procoagulant factors like tissue factor and down-regulation of anticoagulants like thrombomodulin.2 Coagulation is part of the innate immune response limiting the dissemination of bacteria in a variety of disease models, reviewed by Degen et al.3 One aspect of the innate immune response is the release of DNA from neutrophils. This DNA is bacteriacidal, probably in part because of the histones associated with the released DNA.4 This released DNA can lead to platelet activation and thrombosis.5 Activation of platelets by histones contributes to the thrombotic response that presumably aids in limiting the bacterial dissemination. In addition to serving as a platelet activator, histone interaction with polyphosphates released by the platelets leads to a > 20-fold increase in platelet procoagulant activity.6 Furthermore, the histones inhibit thrombomodulin activation of protein C, further augmenting the procoagulant response.7 Interestingly, the impact of the histones on the above responses is not simply related to charge, as there is considerable specificity with histone H4 being the most active in most of these responses despite having charge properties similar to the other histones.
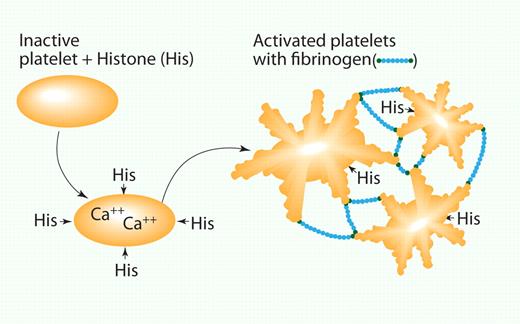
Histones activate platelets. Histones bind to the platelet, leading to a calcium flux and shape change. This in turn activates receptors on the platelet surface that bind fibrinogen and leads to platelet clumping. His indicates histones; and Fib, fibrinogen. Professional illustration by Paulette Dennis.
Histones activate platelets. Histones bind to the platelet, leading to a calcium flux and shape change. This in turn activates receptors on the platelet surface that bind fibrinogen and leads to platelet clumping. His indicates histones; and Fib, fibrinogen. Professional illustration by Paulette Dennis.
Histone release turns on a variety of innate immune responses including cytokine release and the activation of toll-like receptors 2,4, and 9.6,8,9 While this probably contributes to control of infection, excessive activation of these receptors by histones can contribute to organ damage in diverse situations such as Tylenol toxicity and reperfusion injury.8,9 Indeed, inhibiting histone-mediated triggering of these responses can protect against endotoxin-mediated death and reperfusion-mediated tissue injury. Thus, pharmacologic interventions to block the activities of circulating histones are potentially clinically important.
The observation by Fuchs et al that heparin can block histone-mediated platelet activation is important for a number of reasons. Presumably this blockade is because of charge-charge interactions. Currently, a variety of direct, nonheparin-based, anticoagulants are in clinical development for prevention of thrombosis. It is unlikely that the direct inhibitors will be able to prevent the direct platelet activation mediated by histones and hence, in certain situations involving substantial DNA release, there may be a disconnect between the anticipated and observed antithrombotic effects of these inhibitors. This could be particularly relevant in arterial thrombosis where the platelet is the major player and tissue necrosis could be contributing substantial histone levels regionally.
Conflict-of-interest disclosure: The author has a patent application on histones currently pending. ■
REFERENCES
Howard Hughes Funding

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal