In this issue of Blood, Ahmadi et al show that the expression of a transgenic TCR can be increased 10-fold or more by cotransfer of CD3 cDNA, thereby augmenting antitumor activity.1
Genetically modifying T cells to express transgenic TCR α and β chains of high affinity or a synthetic chimeric antigen receptor is an attractive strategy to generate tumor-directed antigen-specific T cells.2 Recent studies have shown encouraging clinical responses in melanoma patients treated with T cells genetically modified with melanoma-associated antigen recognized by T cells (MART-1)–specific TCRs,3 colon cancer patients treated with CEA-specific TCRs,4 and patients with NY-ESO-1–positive tumors treated with autologous T cells transduced with a NY-ESO-1 TCR.5
One major limitation of TCR gene transfer is the development of hybrid TCRs that contain a mixture of native and transgenic receptors that may result in inefficient expression of the transferred TCRs and consequent loss (and occasionally unwanted gain) of function. Several different strategies have been developed to increase the expression of introduced α and β chains by reducing the mispairing with endogenous TCR chains.2 Ahmadi et al take a new approach. They hypothesize that it is endogenous CD3 components of the TCRs that are rate-limiting for αβ TCR expression and, hence, for tumor-directed T-cell function after the introduction of transgenic TCRs. In preclinical models, they evaluated transgenic TCRs, with specificity for Wilms Tumor antigen 1 and influenza nucleoprotein, and found that cotransduction of CD3 and αβ TCR cDNA produced a 1-log increase in TCR surface expression and increased in vitro cytokine production on exposure to tumor antigen compared with T cells modified with the αβ TCR only (see figure). They then evaluated this strategy in murine models and found that when T cells were genetically modified with both the TCRs and CD3, they infiltrated tumors more rapidly and in larger numbers than cells transduced with the TCRs alone. After tumor clearance, the TCR+CD3–modified T cells persisted at higher levels than T cells containing only the TCRs, and mounted a more effective memory response when rechallenged with antigen. Taken together, these results suggest that transfer of additional exogenous CD3 molecules may effectively enhance the activity and persistence of TCR gene–modified T cells.
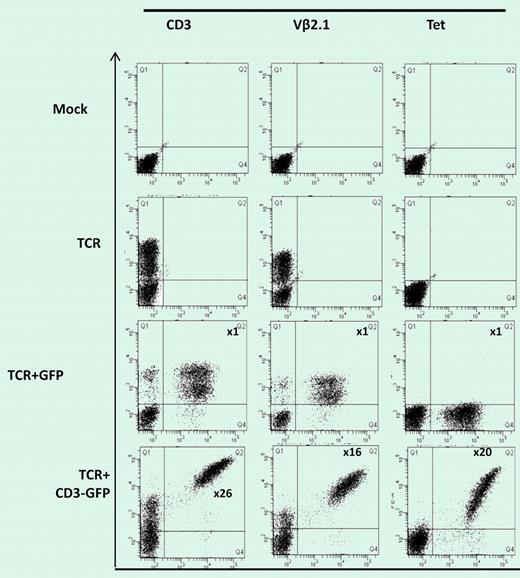
Figure (shown in more detail in Figure 1B in Ahmadi et al1 ) shows 58α-β- T lymphoma cells mock-transduced or transduced with WT1-TCR, WT1-TCR+control-GFP, or WT1-TCR+CD3-GFP, then stained with antibodies against CD3, Vβ2.1, or with HLA-A2/pWT126 tetramer. The numbers in the panels in TCR+CD3-GFP–transduced cells indicate the fold increase in the level of CD3 or TCR expression (measured by mean fluorescence intensity) in cells expressing TCR+CD3-GFP (Q2) compared to the cells expressing TCR only (Q1). No increase in CD3 or TCR expression was seen in cells transduced with TCR+control-GFP.
Figure (shown in more detail in Figure 1B in Ahmadi et al1 ) shows 58α-β- T lymphoma cells mock-transduced or transduced with WT1-TCR, WT1-TCR+control-GFP, or WT1-TCR+CD3-GFP, then stained with antibodies against CD3, Vβ2.1, or with HLA-A2/pWT126 tetramer. The numbers in the panels in TCR+CD3-GFP–transduced cells indicate the fold increase in the level of CD3 or TCR expression (measured by mean fluorescence intensity) in cells expressing TCR+CD3-GFP (Q2) compared to the cells expressing TCR only (Q1). No increase in CD3 or TCR expression was seen in cells transduced with TCR+control-GFP.
A number of concerns remain for translating this work to humans. First, we do not know whether the molar concentration of CD3 is similarly limiting for the expression of transgenic human αβ TCRs. If CD3 is indeed limiting, then the enhanced expression and functionality we achieve by cotransfection of CD3 and αβ TCRs may be accompanied by enhanced toxicity. Thus, enhanced expression may increase the risk of mispairing of endogenous and transgenic TCRs, and increase the risk of unwanted gain of function. It is, however, encouraging that the autoreactivity observed with such gain-of-function mispairing6 has yet to be observed in clinical trials,7,8 and indeed was absent in Ahmadi et al's murine studies. Even without gain-of-function mispairing, however, T cells with transgenic TCRs may produce on-target antigen but off-target organ toxicities, such as the damage to melanin-expressing cells in the inner ear and the retina observed using MART1-specific TCRs9 and the severe colitis observed in recipients of T cells expressing transgenic CEA-specific TCRs.4 These toxicities may become more frequent and more severe if we simply increase overall TCR expression.
The above concerns notwithstanding, there will undoubtedly be much interest in exploring whether CD3-mediated “easing” of αβ TCR expression really will benefit human T-cell therapies of cancer.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health