Abstract
Essential thrombocythemia (ET) and polycythemia vera (PV) are characterized by persistent platelet activation. The mechanisms involved in their clearance are poorly characterized. In the present study, we report that leukocytes were actively involved in platelet disposal in 51 patients with ET and 30 with PV, but not in 70 age- and sex-matched controls. The fraction of circulating neutrophils and monocytes that had phagocytosed platelets, as assessed by flow cytometry, was significantly higher in patients with PV or ET, independently of hydroxyurea treatment, than in controls. Platelet phagocytosis by circulating leukocytes was confirmed by confocal and electron microscopy. The lack of effect of hydroxyurea, which disrupts the P-selectin/P-selectin glycoprotein ligand 1 (PSGL-1) interaction, suggests a P-selectin–independent mechanism. This hypothesis was confirmed in an ad hoc animal model based on the in vivo injection of activated platelets from P-selectin+/+ and P-selectin−/− mice. P-selectin expression was associated with an earlier and effective clearance of platelets by neutrophils. A second delayed, P-selectin–independent phase actively involved monocytes. Our results suggest that phagocytic clearance of platelets by leukocytes occurs in PV and ET, possibly involving P-selectin–dependent and -independent pathways, thus representing a novel mechanism to remove activated platelets from the circulation.
Introduction
Platelets and leukocytes interact productively: the formation of heterotypic aggregates is a feature of acute coronary syndromes and of other systemic inflammatory, neoplastic, and autoimmune diseases.1-8 We have previously demonstrated that circulating neutrophils phagocytose activated platelets.7,9 This clearance program depends on the activation state of platelets, because it requires the initial interaction of platelet P-selectin with P-selectin glycoprotein ligand 1 (PSGL-1), its counter receptor on neutrophils. As a consequence, leukocytes rearrange the actin-based cytoskeleton and degranulate and transactivate β2 integrins, with firm adhesion to tethered platelets. Finally, active engulfment takes place, which is dependent on the recognition of phosphatidylserine.7,9 Activated platelets expose anionic phospholipids10,11 and tissue factor,12-15 providing a template for the organization of the coagulation cascade.16-18 Their active removal by phagocytes might represent a protective mechanism against thrombosis. Approximately 15% of circulating neutrophils contain phagocytosed platelets in the very early phase of acute myocardial infarction, and the extent of platelet phagocytosis is correlated with markers of platelet activation, including expression of P-selectin.7 P-selectin–expressing platelets in patients with acute myocardial infarction are not detectable after a relatively short time.3,6 A clinical condition characterized by persistent activation of circulating platelets may represent an informative clinical setting to assess their phagocytic clearance by circulating leukocytes.
Persistent platelet activation, reflected by increased numbers of platelets expressing P-selectin and of platelet-leukocyte heterotypic aggregates, is a well-documented characteristic of patients with essential thrombocythemia (ET) and polycythemia vera (PV).4,5,19-22 In the present study, we analyzed the phagocytosis of platelets by neutrophils and monocytes in patients with PV and ET, in sex- and age- matched control subjects, and in an ad hoc animal model. The results of our study indicate that phagocytic clearance of activated platelets indeed occurs in PV and ET, thus representing a novel mechanism to remove activated platelets from the circulation.
Methods
Patients and controls
Eighty-one consecutive patients (51 ET and 30 PV, 47 men and 34 women, median age 64 years, age range 36-89 years) were studied. All patients were being treated with acetylsalicylic acid (100 mg daily) and 56 patients were being treated with hydroxyurea according to risk-oriented therapy (age, generic cardiovascular risk factors, and prior cardiovascular event). Twenty-one patients had a positive history for arterial (n = 14) or venous (n = 7) thrombotic episodes, which recurred in 5 while they were on hydroxyurea treatment. Patients with a diagnosis of PV and ET according to World Health Organization diagnostic criteria were included in the study. The Janus kinase 2 (JAK2) V617F detection was assessed in the peripheral blood using a DNA tetraprimer amplification refractory mutation system assay, as described previously.23,24 Measurement of the allele burden was assessed with semiquantitative PCR. Patients were considered homozygous for the mutation when the mutant allele burden was > 75%. The JAK2 mutation was identified in 27 PV patients and in 38 ET patients (Table 1). Seven patients were also studied before and after hydroxyurea treatment (treatment duration, 1-5 months). A sex- and age-matched control group of 70 control subjects with normal blood cell counts (43 men and 27 women, median age 65 years, age range 36-89 years) was also studied: 38 of them (median age 66.5, range 52-88) were on treatment with acetylsalicylic acid (100 mg daily). The institutional ethics committee of the San Raffaele Scientific Institute (Milan, Italy) approved the study. All patients and controls gave their informed consent for the study in accordance with the Declaration of Helsinki.
Characteristics of patients with PV and ET
| . | PV . | ET . |
|---|---|---|
| N | 30 | 51 |
| Sex (M/F) | 20/10 | 27/24 |
| Median age, y (range) | 64 (43-88) | 64 (36-89) |
| JAK2 mutation (positive/wild-type) | 27/3 | 38/13 |
| Aspirin treatment, n (%) | 30 (100) | 51 (100) |
| Hydroxyurea treatment, n (%) | 20 (71) | 36 (77) |
| History of thrombosis, n (%) | 9 (30) | 12 (23.5) |
| . | PV . | ET . |
|---|---|---|
| N | 30 | 51 |
| Sex (M/F) | 20/10 | 27/24 |
| Median age, y (range) | 64 (43-88) | 64 (36-89) |
| JAK2 mutation (positive/wild-type) | 27/3 | 38/13 |
| Aspirin treatment, n (%) | 30 (100) | 51 (100) |
| Hydroxyurea treatment, n (%) | 20 (71) | 36 (77) |
| History of thrombosis, n (%) | 9 (30) | 12 (23.5) |
Reagents and mAbs
Thrombofix, mAbs against human CD14 (clone RMO52), human CD42a (platelet glycoprotein Ib, clone SZ2), human CD45 (clone J33), CD61 (platelet glycoprotein IIIa, clone SZ21), human CD66b (clone 80H3), human MPO (clone CLB-MPO-1), and human VWF and its isotype-matched control mAb (clone 679.1Mc7) were from Instrumentation Laboratories. mAbs against human tissue factor (CD142, clone HTF), human P-selectin–specific AK-4 mAb, mouse CD61 (clone 2C9.G2), mouse CD14 (clone mC5-3), mouse CD45 (clone 30-F11), and isotype-matched control Abs were from Beckman Coulter. mAbs against human fibrinogen (clone HYB 051-04) and its isotype-matched control mAb (clone MOPC-21) were from the Antibody Shop. mAbs against mouse neutrophils (clone 7/4) and its isotypic control were from Abcam. The Zenon IgG Labeling kits and 2′,7′-bis-(2-carboxyethyl)-5 (6)-carboxy-fluorescein triacetoxy methyl ester (BCECF-AM) were from Invitrogen and the Fix & Perm kit was from Caltag. The anti–human P-selectin (clone AK-4 mAb), anti–mouse CD61 (clone 2C9.G2), anti–mouse CD14 (clone mC5-3), and anti–mouse CD45 (clone 30-F11), and the appropriate isotypic control mAbs were from BD.
Human blood sampling and processing
Venous blood was drawn through a 19-gauge butterfly needle. After discarding the first 3-5 mL of blood, 1.8 mL was carefully collected in tubes containing 0.2 mL of sodium citrate (109 mM), and a cocktail containing Na2EDTA (50mM), N-ethylmaleimide (60mM), and aprotinin (2000 KIU/mL) to limit in vitro cellular activation as much as possible.1,2,7,25
Flow cytometry
Blood samples were immediately fixed with equal volumes of Thrombofix. Samples were analyzed on a Gallios flow cytometer (Beckman Coulter). The activation of platelets and leukocytes, the proportion of platelet-neutrophil heterotypic aggregates and the presence of phagocytosed platelets in neutrophils were assessed by 4 parametric flow cytometry.1,2,7 Briefly, to assess leukocyte-platelet aggregates aliquots of whole blood were labeled with mAbs against CD45, CD14, CD66b, and CD61. Neutrophils were identified within the CD45+ population based on: (1) the expression of CD66b, (2) the absence of CD14, and (3) their orthogonal light scatter. Neutrophils with adherent platelets were identified by the expression of the CD61 platelet antigen. The presence of intracellular platelets in neutrophils was determined as described previously.7 Briefly, samples were labeled with anti-CD45, anti-CD66b, and anti-CD14 mAbs, permeabilized with Fix & Perm, and labeled with anti-CD61 and anti-VWF mAbs. The fraction of neutrophils (CD66b+ cells) or monocytes (CD14+ cells) with intracellular platelet antigens was calculated by subtracting the percentage of platelet antigen–positive, nonpermeabilized leukocytes (ie, neutrophils or monocytes with adherent platelets on their membrane) from the percentage of platelet antigen–positive, permeabilized neutrophils or monocytes (ie, neutrophils or monocytes with adherent platelets plus neutrophils or monocytes with adherent platelets and phagocytosed platelets; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To determine neutrophil and monocyte tissue factor expression and cell-associated fibrinogen, samples were labeled with mAbs against CD45, CD14, CD66b, and tissue factor or fibrinogen. To determine the myeloperoxidase content of neutrophils, samples were labeled with anti-CD45 and anti-CD66b permeabilized with the Fix & Perm kit, and labeled with mAbs against myeloperoxidase.7 The platelet expression of P-selectin, tissue factor, and platelet-associated fibrinogen were assessed using mAbs against the CD61 platelet antigen and against P-selectin, tissue factor, or fibrinogen, respectively.
Confocal microscopy and electron microscopy
For confocal microscopy, whole blood samples were treated with FACS lysing solution, fixed and permeabilized with Fix & Perm, and labeled with mAbs against the platelet glycoprotein Ib (labeled with Zenon IgG Labeling Kits with Alexa Fluor 546, which stains red). Hoechst stain (blue) was used for counterstaining nuclei. Samples were then washed and plated on glass coverslips before analysis with a Leica TCS SP2 laser scanning confocal microscope, with a 63× objective and a numeric aperture of 1.4. For electron microscopy, neutrophils were isolated as described previously,1,7,26,27 and fixed in 2 steps: first with 4% paraformaldehyde and 2.5% glutaraldehyde in 125mM cacodylate buffer at 4°C for 30 minutes, and then with 2% OsO4 in 125mM cacodylate buffer for 1 hour. Samples were then washed, dehydrated, and embedded in Epon. Conventional thin sections were collected on uncoated grids, stained with uranil and lead citrate, and examined on a Leo912 electron microscope.
Animal model
Female C57BL/6N (wild-type) or C57BL/6N-knockout P-selectin mice (P-sel−/−) have been described and characterized previously28 and were kindly provided by Dr Virgilio Evangelista (Santa Maria Imbaro, Italy). Blood was retrieved by cardiopuncture and platelets were isolated as described previously7 and labeled with the intracellular BECEF dye before activation with thrombin. Resting or activated fluorescent platelets (200 μL of a 5 × 105 platelets/μL suspension) from wild-type (n = 3) or P-sel−/− (n = 3) mice were injected in the tail vein of synergic wild-type mice (n = 5 for wild-type resting platelets, n = 5 for wild-type activated platelets, and n = 5 for P-sel−/− activated platelets). After 1, 2, 3, and 24 hours, 30- to 50-μL aliquots of blood were retrieved from the tail vein and used for flow cytometry determinations. Animals were killed 24 hours after injection. Neutrophils (identified within CD45- and CD7/4-positive cells) and monocytes (identified within CD45- and mC5-3–positive cells) with intracellular fluorescent (BCECF+) platelets were identified and quantified by flow cytometry, as described previously.7 Fluorescent platelets in solid organs retrieved at microscopy, including the spleen, were revealed by confocal microscopy, as described previously.7 All procedures were performed according to protocols approved by the animal care and use committee of the Fondazione San Raffaele del Monte Tabor (permit IACUC424) and communicated to the Ministry of Health and local authorities according to Italian law. Procedures were performed under anesthesia and all efforts were made to minimize suffering.
Statistical analysis
Results are reported as means ± SEM unless otherwise indicated. Statistical analysis was performed by ANOVA, followed by the Bonferroni multiple comparison test and the Tukey test, as appropriate, using Prism Version 4.00 software (GraphPad). All tests were 2-sided and P < .05 was considered statistically significant.
Results
Markers of platelet and leukocyte activation in PV and ET patients
We observed that compared with control subjects, PV and ET patients had: (1) significantly lower myeloperoxidase neutrophil content (a marker of neutrophil degranulation); (2) more blood monocytes and neutrophils with bound fibrinogen (which is dependent on leukocyte β2 integrin transactivation) and expressing tissue factor (a marker of a prothrombotic profile); (3) more platelets expressing P-selectin, fibrinogen, and tissue factor; and (4) more platelet-neutrophil and platelet-monocyte heterotypic aggregates (Table 2). The percentage of neutrophils with bound fibrinogen was significantly higher in ET compared with PV patients. The myeloperoxidase content of neutrophils was lower in carriers of the JAK2 mutation than in carriers of wild-type JAK2 (Table 2). The latter result reflects the activation of neutrophils in PV and ET patients associated with JAK2 mutation, which has been demonstrated previously.19,20
Markers of platelet and leukocyte activation and presence of intracellular platelets in circulating leukocytes
| . | Healthy controls . | PV + ET patients . | PV . | ET . | JAK2 mutation . | History of thrombosis . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| No . | Yes . | |||||||||
| Negative . | Positive . | . | All . | One episode . | Two episodes . | |||||
| N | 70 | 81 | 30 | 51 | 17 | 64 | 60 | 21 | 16 | 5 |
| Median age, y (range) | 65 (36-89) | 64 (36-88) | 64 (43-88) | 64 (36-89) | 59 (41-88) | 64 (36-89) | 64 (36-89) | 67 (41-88) | 66 (41-81) | 78 (53-88) |
| White blood cells, ×109/L | 6.2 ± 0.7 | 7.7 ± 0.4* | 8.0 ± 0.4* | 7.6 ± 0.5* | 8.0 ± 1.1* | 7.7 ± 0.4* | 7.9 ± 0.5* | 7.3 ± 0.5* | 7.1 ± 0.6* | 8.0 ± 0.9* |
| Granulocytes, ×109/L | 3.5 ± 0.3 | 5.6 ± 0.3* | 5.9 ± 0.4* | 5.5 ± 4.9* | 5.6 ± 0.9* | 5.7 ± 0.3* | 5.8 ± 0.4* | 5.4 ± 0.4* | 5.3 ± 0.4* | 5.4 ± 0.9* |
| Platelets, ×109/L | 223.1 ± 20.5 | 464.5 ± 35.9* | 347.1 ± 45.6* | 515 ± 45.1*† | 545.1 ± 78.0* | 450 ± 39.9*‡ | 467.9 ± 43.2* | 452.1 ± 58.7* | 482.6 ± 80.7* | 391.0 ± 49.5 |
| Platelets expressing P-selectin | 6.8 ± 0.8 | 23.9 ± 1.5* | 23.4 ± 1.8* | 24.2 ± 2.1* | 25.3 ± 3.1* | 23.5 ± 1.7* | 22.2 ± 1.6* | 29.0 ± 3.2*§ | 26.5 ± 3.5*§ | 36.1 ± 6.7*§ |
| Platelets expressing tissue factor | 3.9 ± 0.8 | 25.2 ± 3.2* | 31.4 ± 5.7* | 20.4 ± 3.3*† | 31.5 ± 7.9* | 24.0 ± 3.5*‡ | 21.6 ± 3.7* | 36.9 ± 6.3*§ | 35.0 ± 6.1*§ | 38.6 ± 5.0*§ |
| Platelets with bound fibrinogen | 3.1 ± 0.6 | 32.1 ± 2.2* | 33.5 ± 3.0* | 31.0 ± 3.1* | 20.4 ± 5.4* | 33.1 ± 2.4*‡ | 27.8 ± 2.2* | 44.2 ± 4.1*§ | 43.2 ± 5.1*§ | 48.0 ± 1.7*§ |
| Neutrophil MPO content | 159.9 ± 8.4 | 86.2 ± 8.0* | 98.2 ± 9.9* | 77.5 ± 11.6* | 98.6 ± 11.9* | 74.2 ± 6.7*‡ | 107.2 ± 6.7* | 55.1 ± 3.4*§ | 57.2 ± 5.8*§ | 52.1 ± 5.9*§ |
| Neutrophils with bound fibrinogen | 3.1 ± 0.8 | 28.1 ± 3.0* | 23.3 ± 3.7* | 29.9 ± 3.3*† | 29.1 ± 4.5* | 27.8 ± 3.6* | 19.7 ± 2.8* | 43.8 ± 4.8*§ | 43.4 ± 5.9*§ | 45.0 ± 8.4*§ |
| Neutrophils expressing tissue factor | 2.9 ± 0.6 | 21.1 ± 1.9* | 21.4 ± 3.3* | 20.8 ± 2.2* | 29.3 ± 4.7* | 19.5 ± 2.0*‡ | 18.1 ± 1.7* | 29.1 ± 4.8*§ | 29.3 ± 5.4*§ | 28.1 ± 12.2*§ |
| Monocytes with bound fibrinogen | 2.1 ± 0.4 | 22.8 ± 2.5* | 28.4 ± 4.2* | 21.1 ± 3.1*† | 21.2 ± 4.6* | 23.2 ± 2.9* | 19.7 ± 3.2* | 28.6 ± 3.8*§ | 30.2 ± 4.8*§ | 23.7 ± 5.7*§¶ |
| Monocytes expressing tissue factor | 2.4 ± 0.4 | 20.0 ± 2.0* | 21.3 ± 3.3* | 18.8 ± 2.5* | 23.0 ± 4.8* | 19.4 ± 2.3* | 18.5 ± 2.3* | 23.9 ± 4.3*§ | 25.6 ± 4.9*§ | 17.1 ± 9.7*§¶ |
| Platelet-neutrophil aggregates | 6.8 ± 0.5 | 19.8 ± 1.3* | 19.4 ± 2.1* | 20.0 ± 1.7* | 21.8 ± 2.6* | 19.2 ± 1.5* | 16.3 ± 1.0* | 29.5 ± 3.5*§ | 28.1 ± 3.2*§ | 30.6 ± 9.5*§ |
| Platelet-monocyte aggregates | 5.1 ± 0.9 | 15.4 ± 1.2* | 14.4 ± 1.8* | 16.0 ± 1.6* | 20.1 ± 2.7* | 14.2 ± 1.3*‡ | 14.9 ± 1.3* | 17.1 ± 2.7*§ | 17.3 ± 3.1*§ | 16.2 ± 5.1*§ |
| Neutrophils with intracellular platelets | 2.2 ± 0.4 | 39.4 ± .5.0* | 36.1 ± 4.0* | 41.0 ± 3.2* | 42.3 ± 5.8* | 38.7 ± 2.9* | 48.6 ± 2.4* | 13.6 ± 2.3*§ | 16.5 ± 2.7*§ | 4.6 ± 0.9*§¶ |
| Monocytes with intracellular platelets | 0.9 ± 0.2 | 30.7 ± 2.7* | 28.2 ± 4.7* | 32.3 ± 3.3* | 37.4 ± 5.5* | 29.1 ± 3.1* | 36.3 ± 3.2* | 15.1 ± 3.6*§ | 15.8 ± 4.5*§ | 11.4 ± 4.3*§¶ |
| . | Healthy controls . | PV + ET patients . | PV . | ET . | JAK2 mutation . | History of thrombosis . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| No . | Yes . | |||||||||
| Negative . | Positive . | . | All . | One episode . | Two episodes . | |||||
| N | 70 | 81 | 30 | 51 | 17 | 64 | 60 | 21 | 16 | 5 |
| Median age, y (range) | 65 (36-89) | 64 (36-88) | 64 (43-88) | 64 (36-89) | 59 (41-88) | 64 (36-89) | 64 (36-89) | 67 (41-88) | 66 (41-81) | 78 (53-88) |
| White blood cells, ×109/L | 6.2 ± 0.7 | 7.7 ± 0.4* | 8.0 ± 0.4* | 7.6 ± 0.5* | 8.0 ± 1.1* | 7.7 ± 0.4* | 7.9 ± 0.5* | 7.3 ± 0.5* | 7.1 ± 0.6* | 8.0 ± 0.9* |
| Granulocytes, ×109/L | 3.5 ± 0.3 | 5.6 ± 0.3* | 5.9 ± 0.4* | 5.5 ± 4.9* | 5.6 ± 0.9* | 5.7 ± 0.3* | 5.8 ± 0.4* | 5.4 ± 0.4* | 5.3 ± 0.4* | 5.4 ± 0.9* |
| Platelets, ×109/L | 223.1 ± 20.5 | 464.5 ± 35.9* | 347.1 ± 45.6* | 515 ± 45.1*† | 545.1 ± 78.0* | 450 ± 39.9*‡ | 467.9 ± 43.2* | 452.1 ± 58.7* | 482.6 ± 80.7* | 391.0 ± 49.5 |
| Platelets expressing P-selectin | 6.8 ± 0.8 | 23.9 ± 1.5* | 23.4 ± 1.8* | 24.2 ± 2.1* | 25.3 ± 3.1* | 23.5 ± 1.7* | 22.2 ± 1.6* | 29.0 ± 3.2*§ | 26.5 ± 3.5*§ | 36.1 ± 6.7*§ |
| Platelets expressing tissue factor | 3.9 ± 0.8 | 25.2 ± 3.2* | 31.4 ± 5.7* | 20.4 ± 3.3*† | 31.5 ± 7.9* | 24.0 ± 3.5*‡ | 21.6 ± 3.7* | 36.9 ± 6.3*§ | 35.0 ± 6.1*§ | 38.6 ± 5.0*§ |
| Platelets with bound fibrinogen | 3.1 ± 0.6 | 32.1 ± 2.2* | 33.5 ± 3.0* | 31.0 ± 3.1* | 20.4 ± 5.4* | 33.1 ± 2.4*‡ | 27.8 ± 2.2* | 44.2 ± 4.1*§ | 43.2 ± 5.1*§ | 48.0 ± 1.7*§ |
| Neutrophil MPO content | 159.9 ± 8.4 | 86.2 ± 8.0* | 98.2 ± 9.9* | 77.5 ± 11.6* | 98.6 ± 11.9* | 74.2 ± 6.7*‡ | 107.2 ± 6.7* | 55.1 ± 3.4*§ | 57.2 ± 5.8*§ | 52.1 ± 5.9*§ |
| Neutrophils with bound fibrinogen | 3.1 ± 0.8 | 28.1 ± 3.0* | 23.3 ± 3.7* | 29.9 ± 3.3*† | 29.1 ± 4.5* | 27.8 ± 3.6* | 19.7 ± 2.8* | 43.8 ± 4.8*§ | 43.4 ± 5.9*§ | 45.0 ± 8.4*§ |
| Neutrophils expressing tissue factor | 2.9 ± 0.6 | 21.1 ± 1.9* | 21.4 ± 3.3* | 20.8 ± 2.2* | 29.3 ± 4.7* | 19.5 ± 2.0*‡ | 18.1 ± 1.7* | 29.1 ± 4.8*§ | 29.3 ± 5.4*§ | 28.1 ± 12.2*§ |
| Monocytes with bound fibrinogen | 2.1 ± 0.4 | 22.8 ± 2.5* | 28.4 ± 4.2* | 21.1 ± 3.1*† | 21.2 ± 4.6* | 23.2 ± 2.9* | 19.7 ± 3.2* | 28.6 ± 3.8*§ | 30.2 ± 4.8*§ | 23.7 ± 5.7*§¶ |
| Monocytes expressing tissue factor | 2.4 ± 0.4 | 20.0 ± 2.0* | 21.3 ± 3.3* | 18.8 ± 2.5* | 23.0 ± 4.8* | 19.4 ± 2.3* | 18.5 ± 2.3* | 23.9 ± 4.3*§ | 25.6 ± 4.9*§ | 17.1 ± 9.7*§¶ |
| Platelet-neutrophil aggregates | 6.8 ± 0.5 | 19.8 ± 1.3* | 19.4 ± 2.1* | 20.0 ± 1.7* | 21.8 ± 2.6* | 19.2 ± 1.5* | 16.3 ± 1.0* | 29.5 ± 3.5*§ | 28.1 ± 3.2*§ | 30.6 ± 9.5*§ |
| Platelet-monocyte aggregates | 5.1 ± 0.9 | 15.4 ± 1.2* | 14.4 ± 1.8* | 16.0 ± 1.6* | 20.1 ± 2.7* | 14.2 ± 1.3*‡ | 14.9 ± 1.3* | 17.1 ± 2.7*§ | 17.3 ± 3.1*§ | 16.2 ± 5.1*§ |
| Neutrophils with intracellular platelets | 2.2 ± 0.4 | 39.4 ± .5.0* | 36.1 ± 4.0* | 41.0 ± 3.2* | 42.3 ± 5.8* | 38.7 ± 2.9* | 48.6 ± 2.4* | 13.6 ± 2.3*§ | 16.5 ± 2.7*§ | 4.6 ± 0.9*§¶ |
| Monocytes with intracellular platelets | 0.9 ± 0.2 | 30.7 ± 2.7* | 28.2 ± 4.7* | 32.3 ± 3.3* | 37.4 ± 5.5* | 29.1 ± 3.1* | 36.3 ± 3.2* | 15.1 ± 3.6*§ | 15.8 ± 4.5*§ | 11.4 ± 4.3*§¶ |
Results are expressed as mean ± SEM of the percentage of CD61+ platelets that express P-selectin, tissue factor; and fibrinogen; mean ± SEM arbitrary units of myeloperoxidase (MPO) fluorescence within CD66b+ neutrophils; mean ± SEM percentage of CD66b+ neutrophils that express tissue factor or fibrinogen, that form heterotypic aggregates with platelets, or that contain intracellular platelets; or mean ± SEM of the percentage of CD14+ monocytes that express tissue factor or fibrinogen, that form heterotypic aggregates with platelets, or that contain intracellular platelets.
Statistically different from control, P < 05.
Statistically different from patients with PV, P < .05.
Statistically different from patients without the JAK2 mutation, P < .05.
Statistically different from patients without previous thrombosis, P < .05.
Statistically different from patients with a single episode of thrombosis, P < .05.
Platelet phagocytosis by neutrophils and monocytes in PV and ET patients
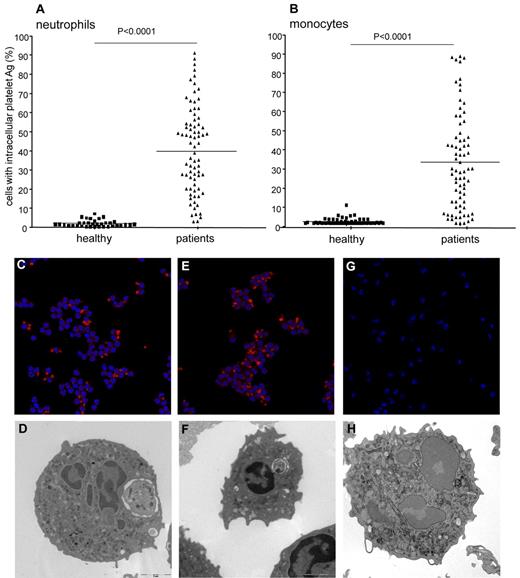
More neutrophils and monocytes from PV or ET patients contained the intracellular platelet antigens CD61 and VWF compared with leukocytes derived from sex- and age-matched control subjects (Figure 1). This feature was independent of the clinical diagnosis (PV or ET), of the JAK2 genotype (Figure 1 and Table 2), and of other markers of cellular activation such as neutrophil myeloperoxidase content, extent of fibrinogen binding, and tissue factor expression. In contrast, the fraction of neutrophils with intracellular platelets was inversely correlated with the fraction of neutrophils with adherent extracellular platelets (heterotypic aggregates; r = −0.62, P = .0002). Confocal and electron microscopy of samples from the first 20 studied patients (8 PV and 12 ET) and from 10 healthy controls confirmed that leukocytes containing whole platelets within phagocytic vacuoles were frequent in patients and extremely rare in healthy subjects (Figure 1). There was a linear correlation between the number of neutrophils with intracellular platelets and that of monocytes with intracellular platelets (r = 0.63, P = .0003). The fraction of circulating leukocytes that had phagocytosed platelets was significantly higher in patients with PV (CD61+ neutrophils, 36.3% ± 4.3%; VWF+ neutrophils, 33.2% ± 5.3%; CD61+ monocytes, 27.8% ± 4.9%; VWF+ monocytes, 23.6% ± 7.3%) or ET (CD61+ neutrophils, 41.8% ± 3.3%; VWF+ neutrophils, 39.1% ± 5.6%; CD61+ monocytes, 27.8% ± 4.9%; VWF+ monocytes, 23.6% ± 3.8%) than in controls (CD61+ neutrophils, 2.2% ± 0.4%; VWF+ neutrophils, 1.8% ± 0.4%; CD61+ monocytes, 0.9% ± 0.2%; VWF+ monocytes, 0.5% ± 0.1%; Figure 1 and Tables 2–3).
Phagocytosed platelets in leukocytes from patients with PV or ET. The presence of neutrophils and monocytes with intracellular platelets was assessed by flow cytometry and confocal and electron microscopy, as described in “Flow cytometry” and “Confocal microscopy and electron microscopy.” For confocal microscopy, platelets were identified based on the expression of the glycoprotein Ib (red). Hoechst stain (blue) was used for counterstaining nuclei. (A) Symbols represent the fraction of neutrophils with intracellular platelet antigens. Horizontal lines indicate mean values. (B) Symbols represent the percentage of monocytes with intracellular platelet antigens. Horizontal lines indicate mean values. Representative confocal (panels C,E,G) and electron microscopy (panels D,F,H) images of neutrophils from a patient with PV (panels C-D), from a patient with ET (panels E-F), and from a healthy control (panels G-H) are shown.
Phagocytosed platelets in leukocytes from patients with PV or ET. The presence of neutrophils and monocytes with intracellular platelets was assessed by flow cytometry and confocal and electron microscopy, as described in “Flow cytometry” and “Confocal microscopy and electron microscopy.” For confocal microscopy, platelets were identified based on the expression of the glycoprotein Ib (red). Hoechst stain (blue) was used for counterstaining nuclei. (A) Symbols represent the fraction of neutrophils with intracellular platelet antigens. Horizontal lines indicate mean values. (B) Symbols represent the percentage of monocytes with intracellular platelet antigens. Horizontal lines indicate mean values. Representative confocal (panels C,E,G) and electron microscopy (panels D,F,H) images of neutrophils from a patient with PV (panels C-D), from a patient with ET (panels E-F), and from a healthy control (panels G-H) are shown.
Markers of activation of platelets and neutrophils in PV or ET patients: relationship with treatment
| . | Controls . | PV and ET patients . | ||||
|---|---|---|---|---|---|---|
| No previous thrombosis . | Previous thrombosis . | |||||
| ASA yes HU no . | ASA yes HU yes . | All ASA yes HU yes . | Arterial ASA yes HU yes . | Venous ASA yes HU yes . | ||
| N | 70 | 25 | 35 | 21 | 14 | 7 |
| Median age, yr (range) | 65 (36-89) | 57 (46-89) | 64 (36-87) | 67 (41-88) | 66 (41-88) | 67 (62-83 |
| White blood cells, ×109/L | 6.2 ± 0.7 | 9.2 ± 0.9* | 7.1 ± 0.4*† | 7.3 ± 0.5 | 7.0 ± 0.6 | 7.8 ± 0.9*† |
| Granulocytes, ×109/L | 3.5 ± 0.3 | 6.8 ± 0.7* | 5.1 ± 0.4*† | 5.4 ± 0.4 | 5.1 ± 0.4 | 5.9 ± 0.9*† |
| Platelets, ×109/L | 223.1 ± 20.5 | 581.0 ± 79.1* | 387.1 ± 41.3*† | 452.1 ± 58.7* | 535.5 ± 75.4* | 327.0 ± 54.2*†‡ |
| Platelets expressing P-selectin | 6.8 ± 0.8 | 21.6 ± 2.3* | 22.6 ± 2.3* | 29.0 ± 3.2*†‡ | 28.3 ± 3.8*†‡ | 30.3 ± 6.1*†‡ |
| Platelets expressing tissue factor | 3.9 ± 0.8 | 20.3 ± 2.9* | 22.8 ± 4.2* | 36.9 ± 6.3*†‡ | 38.6 ± 6.9*†‡ | 31.1 ± 8.8*†‡ |
| Platelets with bound fibrinogen | 3.1 ± 0.6 | 24.2 ± 3.8* | 30.0 ± 2.7* | 44.2 ± 4.1*†‡ | 46.0 ± 4.0*†‡ | 39.3 ± 11.7*† |
| Neutrophil MPO content | 159.9 ± 8.4 | 88.6 ± 9.8* | 123.1 ± 8.2*† | 55.1 ± 3.4*†‡ | 55.3 ± 2.9*†‡ | 54.9 ± 3.0*†‡ |
| Neutrophils with bound fibrinogen | 3.1 ± 0.8 | 18.4 ± 3.7* | 20.5 ± 4.1* | 43.8 ± 4.8*†‡ | 40.4 ± 7.1*†‡ | 48.1 ± 6.1*†‡ |
| Neutrophils expressing tissue factor | 2.9 ± 0.6 | 22.8 ± 3.0* | 14.1 ± 2.1*† | 29.1 ± 4.8*‡ | 29.6 ± 6.1*†‡ | 28.1 ± 8.3*‡ |
| Monocytes with bound fibrinogen | 2.1 ± 0.4 | 18.9 ± 3.7* | 20.2 ± 4.7* | 28.6 ± 3.8*† | 30.9 ± 6.1*†‡ | 25.5 ± 4.2* |
| Monocytes expressing tissue factor | 2.4 ± 0.4 | 20.1 ± 3.6* | 17.6 ± 3.0* | 23.9 ± 4.3* | 25.5 ± 5.5*‡ | 20.6 ± 7.7* |
| Platelet-neutrophil aggregates | 6.8 ± 0.5 | 18.6 ± 1.7* | 11.8 ± 1.1*† | 29.5 ± 3.5*†‡ | 25.7 ± 3.3*†‡ | 37.0 ± 7.6*†‡ |
| Platelet-monocyte aggregates | 5.1 ± 0.9 | 20.4 ± 2.5* | 11.0 ± 1.1*† | 17.1 ± 2.7*† | 14.7 ± 3.0* | 21.9 ± 5.3*‡ |
| Neutrophils with intracellular platelets | 2.2 ± 0.4 | 48.4 ± 3.1* | 47.8 ± 3.4* | 13.6 ± 2.3*†‡ | 14.3 ± 3.0*† | 12.0 ± 3.8*†‡ |
| Monocytes with intracellular platelets | 0.9 ± 0.2 | 42.4 ± 4.9* | 36.1 ± 4.1* | 15.1 ± 3.6*†‡ | 16.4 ± 4.7*†‡ | 12.4 ± 5.4*†‡ |
| . | Controls . | PV and ET patients . | ||||
|---|---|---|---|---|---|---|
| No previous thrombosis . | Previous thrombosis . | |||||
| ASA yes HU no . | ASA yes HU yes . | All ASA yes HU yes . | Arterial ASA yes HU yes . | Venous ASA yes HU yes . | ||
| N | 70 | 25 | 35 | 21 | 14 | 7 |
| Median age, yr (range) | 65 (36-89) | 57 (46-89) | 64 (36-87) | 67 (41-88) | 66 (41-88) | 67 (62-83 |
| White blood cells, ×109/L | 6.2 ± 0.7 | 9.2 ± 0.9* | 7.1 ± 0.4*† | 7.3 ± 0.5 | 7.0 ± 0.6 | 7.8 ± 0.9*† |
| Granulocytes, ×109/L | 3.5 ± 0.3 | 6.8 ± 0.7* | 5.1 ± 0.4*† | 5.4 ± 0.4 | 5.1 ± 0.4 | 5.9 ± 0.9*† |
| Platelets, ×109/L | 223.1 ± 20.5 | 581.0 ± 79.1* | 387.1 ± 41.3*† | 452.1 ± 58.7* | 535.5 ± 75.4* | 327.0 ± 54.2*†‡ |
| Platelets expressing P-selectin | 6.8 ± 0.8 | 21.6 ± 2.3* | 22.6 ± 2.3* | 29.0 ± 3.2*†‡ | 28.3 ± 3.8*†‡ | 30.3 ± 6.1*†‡ |
| Platelets expressing tissue factor | 3.9 ± 0.8 | 20.3 ± 2.9* | 22.8 ± 4.2* | 36.9 ± 6.3*†‡ | 38.6 ± 6.9*†‡ | 31.1 ± 8.8*†‡ |
| Platelets with bound fibrinogen | 3.1 ± 0.6 | 24.2 ± 3.8* | 30.0 ± 2.7* | 44.2 ± 4.1*†‡ | 46.0 ± 4.0*†‡ | 39.3 ± 11.7*† |
| Neutrophil MPO content | 159.9 ± 8.4 | 88.6 ± 9.8* | 123.1 ± 8.2*† | 55.1 ± 3.4*†‡ | 55.3 ± 2.9*†‡ | 54.9 ± 3.0*†‡ |
| Neutrophils with bound fibrinogen | 3.1 ± 0.8 | 18.4 ± 3.7* | 20.5 ± 4.1* | 43.8 ± 4.8*†‡ | 40.4 ± 7.1*†‡ | 48.1 ± 6.1*†‡ |
| Neutrophils expressing tissue factor | 2.9 ± 0.6 | 22.8 ± 3.0* | 14.1 ± 2.1*† | 29.1 ± 4.8*‡ | 29.6 ± 6.1*†‡ | 28.1 ± 8.3*‡ |
| Monocytes with bound fibrinogen | 2.1 ± 0.4 | 18.9 ± 3.7* | 20.2 ± 4.7* | 28.6 ± 3.8*† | 30.9 ± 6.1*†‡ | 25.5 ± 4.2* |
| Monocytes expressing tissue factor | 2.4 ± 0.4 | 20.1 ± 3.6* | 17.6 ± 3.0* | 23.9 ± 4.3* | 25.5 ± 5.5*‡ | 20.6 ± 7.7* |
| Platelet-neutrophil aggregates | 6.8 ± 0.5 | 18.6 ± 1.7* | 11.8 ± 1.1*† | 29.5 ± 3.5*†‡ | 25.7 ± 3.3*†‡ | 37.0 ± 7.6*†‡ |
| Platelet-monocyte aggregates | 5.1 ± 0.9 | 20.4 ± 2.5* | 11.0 ± 1.1*† | 17.1 ± 2.7*† | 14.7 ± 3.0* | 21.9 ± 5.3*‡ |
| Neutrophils with intracellular platelets | 2.2 ± 0.4 | 48.4 ± 3.1* | 47.8 ± 3.4* | 13.6 ± 2.3*†‡ | 14.3 ± 3.0*† | 12.0 ± 3.8*†‡ |
| Monocytes with intracellular platelets | 0.9 ± 0.2 | 42.4 ± 4.9* | 36.1 ± 4.1* | 15.1 ± 3.6*†‡ | 16.4 ± 4.7*†‡ | 12.4 ± 5.4*†‡ |
Results are expressed as mean ± SEM of the percentage of CD61+ platelets expressing P-selectin, tissue factor, and fibrinogen; mean ± SEM arbitrary units of myeloperoxidase (MPO) fluorescence within CD66b+ neutrophils; mean ± SEM of the percentage of CD66b+ neutrophils that express tissue factor or fibrinogen, that form heterotypic aggregates with platelets, or that contain intracellular platelets; or mean ± SEM of the percentage of CD14+ monocytes that express tissue factor or fibrinogen, that form heterotypic aggregates with platelets, or that contain intracellular platelets.
ASA indicates acetylsalicylic acid; and HU, hydroxyurea.
Statistically different from controls, P < .05.
Statistically different from patients not treated with HU, P < .05.
Statistically different from patients without previous thrombosis treated with HU, P < .05.
Defective platelet phagocytosis by leukocytes in patients with history of thrombosis
A lower fraction of neutrophils (13.6% ± 2.3%) and monocytes (15.1% ± 3.6%) containing phagocytosed platelets (Figure 2 and Tables 2–3) was detectable in patients with at least one previous thromboembolic episode (P < .01). Personal history for thrombosis was significantly associated with: (1) lower neutrophil myeloperoxidase content; (2) a higher fraction of neutrophils expressing tissue factor on their surface; (3) a higher percentage of neutrophils with bound fibrinogen; (4) more platelet-neutrophil heterotypic aggregates; and (5) a higher percentage of platelets expressing tissue factor and fibrinogen (Figure 2 and Table 3). These features were significantly more frequent in patients who experienced 2 episodes of thrombosis and were independent of the type of thrombotic episodes (arterial or venous; Figure 2 and Tables 2–3).
Phagocytosed platelets in leukocytes from patients with PV or ET with or without a history of thrombosis. The presence of leukocytes with intracellular platelets was assessed as described in “Flow cytometry.” Symbols represent the percentage of neutrophils (A) or monocytes (B) with intracellular platelet antigens. Grey triangles indicate patients with a single thrombotic episode. Black circles indicate patients with 2 thrombotic episodes (n = 5 of 21). Horizontal lines indicate mean values.
Phagocytosed platelets in leukocytes from patients with PV or ET with or without a history of thrombosis. The presence of leukocytes with intracellular platelets was assessed as described in “Flow cytometry.” Symbols represent the percentage of neutrophils (A) or monocytes (B) with intracellular platelet antigens. Grey triangles indicate patients with a single thrombotic episode. Black circles indicate patients with 2 thrombotic episodes (n = 5 of 21). Horizontal lines indicate mean values.
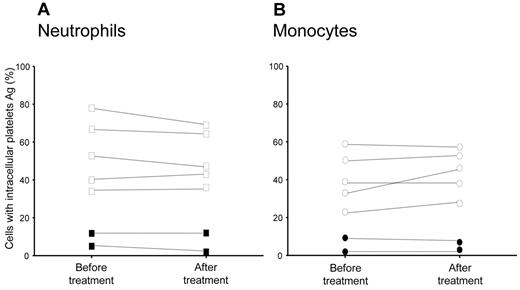
The extent of platelet phagocytosis by neutrophils was significantly associated with neutrophil count (r = 0.62, P < .0001), with the exception of patients with previous thromboembolic episodes (a single thromboembolic episode, r = 0.37; 2 episodes, r = 0.24; supplemental Figure 2). Differences between patients with or without previous thrombosis were not accounted for by treatment with hydroxyurea (Table 3). Platelet clearance did not significantly change in samples analyzed before treatment with cytoreductive agents and samples from the same patients taken after receiving hydroxyurea (n = 7), suggesting that the cytoreductive regimen did not directly influence the extent of the phenomenon (Figure 3).
Hydroxyurea treatment and platelet clearance in PV and ET patients. The presence of leukocytes with intracellular platelets was assessed by flow cytometry in the blood of 7 patients studied before and after hydroxyurea treatment, as described in the text. Symbols represent the percentage of neutrophils (A) and monocytes (B) with intracellular platelet antigens. Filled symbols indicate patients with previous thrombotic episodes.
Hydroxyurea treatment and platelet clearance in PV and ET patients. The presence of leukocytes with intracellular platelets was assessed by flow cytometry in the blood of 7 patients studied before and after hydroxyurea treatment, as described in the text. Symbols represent the percentage of neutrophils (A) and monocytes (B) with intracellular platelet antigens. Filled symbols indicate patients with previous thrombotic episodes.
P-selectin–dependent and -independent mechanisms of platelet clearance by leukocytes
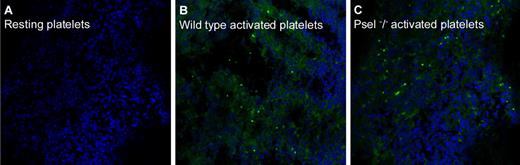
The apparent lack of effect of hydroxyurea, which disrupts P-selectin/PSGL-1 cross-talk,1,29,30 on platelet phagocytosis is consistent with a P-selectin–independent mechanism. To verify this hypothesis, we injected syngeneic mice with activated fluorescent platelets that express (wild-type) or did not express (P-sel−/−) P-selectin, thus mimicking the relative overload with activated platelets that is a hallmark of PV and ET. Intracellular platelets in blood neutrophils and monocytes, as well as the accumulation of fluorescent platelets in the spleen, were then assessed. Table 4 shows that both neutrophils and monocytes, albeit with different kinetics, were involved in the phagocytosis of activated platelets, and that, especially 24 hours after injection, activated P-sel−/− platelets had also been phagocytosed. Fluorescent wild-type and P-sel−/− platelets accumulated in the spleen of injected mice, as expected. In contrast, resting fluorescent platelets were not detectable (Figure 4). These results suggest that both P-selectin–dependent and -independent mechanisms are involved in the process of phagocytosis of activated platelets by leukocytes.
In vivo phagocytosis of activated platelets by leukocytes: role of P-selectin
| . | . | Platelet-neutrophil heterotypic aggregates, % 7/4+ cells . | Neutrophils with intracellular platelets, % 7/4+ cells . | Platetelet-monocyte heterotypic aggregates, % CD14+ cells . | Monocytes with intracellular platelets, % CD14+ cells . |
|---|---|---|---|---|---|
| Untreated | 1.1 ± 0.1 | 1.0 ± 0.8 | 1.7 ± 0.9 | 0.9 ± 0.3 | |
| 1 h | Resting WT | 3.7 ± 1.2 | 2.0 ± 0.8 | 2.9 ± 1.1 | 1.1 ± 0.7 |
| Activated WT | 21.0 ± 4.0† | 73.0 ± 5.9† | 62.0 ± 9.2† | 2.0 ± 2.6 | |
| Activated P-sel−/− | 7.5 ± 2.4* | 5.3 ± 2.9* | 7.7 ± 2.1† | 9.0 ± 1.0† | |
| 3 h | Resting WT | 3.1 ± 0.8 | 4.1 ± 0.9 | 2.2 ± 0.8 | 1.6 ± 0.3 |
| Activated WT | 16.0 ± 1.7† | 23.0 ± 2.0† | 47.3 ± 11.0† | 17.7 ± 8.3† | |
| Activated P-sel−/− | 8.5 ± 2.4*† | 7.3 ± 4.5*† | 9.0 ± 2.6* | 12.7 ± 5.9 † | |
| 24 h | Resting WT | 2.5 ± 1.8 | 6.3 ± 1.5 | 3.1 ± 1.3 | 1.0 ± 0.9 |
| Activated WT | 5.1 ± 0.3 | 6.3 ± 1.5 | 38.3 ± 12.5† | 66.0 ± 7.9† | |
| Activated P-sel−/− | 7.3 ± 1.0* | 43.5 ± 3.7*† | 7.0 ± 1.0* | 46.5 ± 4.5† |
| . | . | Platelet-neutrophil heterotypic aggregates, % 7/4+ cells . | Neutrophils with intracellular platelets, % 7/4+ cells . | Platetelet-monocyte heterotypic aggregates, % CD14+ cells . | Monocytes with intracellular platelets, % CD14+ cells . |
|---|---|---|---|---|---|
| Untreated | 1.1 ± 0.1 | 1.0 ± 0.8 | 1.7 ± 0.9 | 0.9 ± 0.3 | |
| 1 h | Resting WT | 3.7 ± 1.2 | 2.0 ± 0.8 | 2.9 ± 1.1 | 1.1 ± 0.7 |
| Activated WT | 21.0 ± 4.0† | 73.0 ± 5.9† | 62.0 ± 9.2† | 2.0 ± 2.6 | |
| Activated P-sel−/− | 7.5 ± 2.4* | 5.3 ± 2.9* | 7.7 ± 2.1† | 9.0 ± 1.0† | |
| 3 h | Resting WT | 3.1 ± 0.8 | 4.1 ± 0.9 | 2.2 ± 0.8 | 1.6 ± 0.3 |
| Activated WT | 16.0 ± 1.7† | 23.0 ± 2.0† | 47.3 ± 11.0† | 17.7 ± 8.3† | |
| Activated P-sel−/− | 8.5 ± 2.4*† | 7.3 ± 4.5*† | 9.0 ± 2.6* | 12.7 ± 5.9 † | |
| 24 h | Resting WT | 2.5 ± 1.8 | 6.3 ± 1.5 | 3.1 ± 1.3 | 1.0 ± 0.9 |
| Activated WT | 5.1 ± 0.3 | 6.3 ± 1.5 | 38.3 ± 12.5† | 66.0 ± 7.9† | |
| Activated P-sel−/− | 7.3 ± 1.0* | 43.5 ± 3.7*† | 7.0 ± 1.0* | 46.5 ± 4.5† |
Fluorescent platelets from wild type (WT) or P-sel−/− mice were activated (or not) and injected in the tail vein of WT mice. Leukocytes with intracellular fluorescent platelets were identified by flow cytometry at different times after injection; see text for experimental details. Results are expressed as mean ± SEM.
Statistically different from mice injected with activated WT platelets, P < .05.
Statistically different from mice injected with resting platelets, P < .05.
Spleen platelet accumulation. Fluorescent platelets from wild-type or from P-sel−/− mice were injected in the tail vein of wild-type synergic mice. Animals were killed 24 hours after platelet injection and their spleens retrieved, fixed, and included in optimal cutting temperature medium. Fluorescent platelets in the tissue were then revealed by confocal microscopy. See text for experimental details. (A) Mice injected with wild-type resting platelets. (B) Mice injected with wild-type activated platelets. (C) Mice injected with P-sel−/− activated platelets.
Spleen platelet accumulation. Fluorescent platelets from wild-type or from P-sel−/− mice were injected in the tail vein of wild-type synergic mice. Animals were killed 24 hours after platelet injection and their spleens retrieved, fixed, and included in optimal cutting temperature medium. Fluorescent platelets in the tissue were then revealed by confocal microscopy. See text for experimental details. (A) Mice injected with wild-type resting platelets. (B) Mice injected with wild-type activated platelets. (C) Mice injected with P-sel−/− activated platelets.
Discussion
Neutrophils that have phagocytosed platelets can be traced with relative ease in the circulating blood in the very early (4-6 hours) phase of acute myocardial infarction.7 Because platelets expressing P-selectin in these patients disappear after a relatively short time,3,6 we proposed that the active removal of activated platelets by neutrophils is involved in their accelerated turnover.7,9 This prompted us to investigate whether leukocytes could contribute to platelet disposal in clinical conditions characterized by persistent activation of circulating platelets and in which deregulated platelet function plays a key role in the natural history of the disease.
Consistent with this scenario, the results of the present study demonstrate that a high percentage of leukocytes from patients with PV or ET contain platelets in phagocytic vacuoles. Evidence from electron micrographs and results obtained using mAbs against various platelet antigens (CD61 and VWF) suggest that whole platelets, rather than their microparticles, are present in the phagocytic vacuoles of neutrophils. However, we cannot rule out the possibility that platelet-derived microparticles, which represent a key particulate thrombogenic substrate,29-34 are cleared through a similar mechanism.
Overall, our results suggest that the extensive interaction between activated platelets and leukocytes, which represents a hallmark of myeloproliferative diseases, is associated with an effective phagocytic clearance. This finding also hints at a hypothetical homeostatic function.31 Indeed, previous observations indicated that clearance of primed or activated platelets might prevent thrombosis.31,32 Conversely, platelet-leukocyte heterotypic aggregates associate with a history of thrombosis in patients with myeloproliferative disorders,4 and accelerates and enhances the formation of atherosclerotic lesions and the expression of inflammatory cytokines in atherosclerosis-prone, apoE-deficient mice.33 Indeed, the percentages of circulating platelets expressing tissue factor and fibrinogen, as well as the fraction of platelet-neutrophil and platelet-monocyte heterotypic aggregates, were significantly higher in our patients with a history of thrombosis.
The actual cause of the less-effective clearance of platelets in PV or ET patients with previous thrombosis is not yet clear. The massive leukocyte activation observed in patients with previous thromboembolic events could contribute to a lower phagocytic capacity.7 In vitro experiments have indicated that preactivated neutrophils have a lower capacity to remove activated platelets.7 We did not find an association with the JAK2 mutation, which increases the risk of thrombosis in ET patients34,35 or hydroxyurea treatment, which decreases the incidence of thrombotic events.36-38 As expected,19,20 the JAK2 mutation was associated with the activation state of circulating leukocytes in our patients. This suggests that the two events are not mechanistically correlated, and possibly represent independent factors to explain the incidence of thrombosis in PV and ET patients. However, the number of patients we have studied so far is not sufficient to draw any unambiguous conclusions.
In summary, our results suggest that the disposal of activated platelet by neutrophils and monocytes is a relevant and apparently stable feature of patients with PV and ET. However, the design of our retrospective, case-control study does not allow firm conclusions on the nature of the observed association. Large-scale, prospective studies will be necessary to verify the actual clinical relevance of the phenomenon we describe in the present study.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Virgilio Evangelista (Consorgio Mario Negri Sud, Santa Maria Imbaro) for kindly providing P-sel−/− mice; Drs Maria Carla Panzeri and Cesare Covino of the San Raffaele Microscopy facility (Alembic) for the electron and confocal microscopy studies; and Prof Attilio Maseri for much scientific discussion and unfailing support.
This work was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC), by the Ministero della Salute, and by the Ministry of Education, Universities and Research.
Authorship
Contribution: N.M. designed and performed the experiments, analyzed the data, and wrote the manuscript; E.A.F. performed the experiments; S.M. and M.P. selected the patients and organized and analyzed the clinical data; L.C. contributed to the mouse model of platelet clearance; F. Lunghi, F. Lussana, and G.P. selected the patients; P.R.-Q. participated in discussions on experimental strategy; M.C. and F.C. supervised the patient selection, participated in discussions on experimental and clinical strategy, supervised the data analysis, and wrote the manuscript; and A.A.M. defined the experimental strategy, supervised the data analysis, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Norma Maugeri, San Raffaele Institute, via Olgettina 58, 20132 Milano, Italy; e-mail: maugeri.norma@hsr.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal