Abstract
One-third of all splenic marginal zone lymphomas (SMZL) use the IgH VH1-02 gene. These cases are usually not associated with hepatitis C virus infection. Of interest, the rearranged VH1-02 genes display similar complementarity determining regions 3, a finding confirmed by our study. The latter suggests that these SMZL may produce antibodies with similar reactivity. We produced recombinant antibodies from 5 SMZL cases with VH1-02 gene rearrangement to study the binding reactivity of these antibodies. Surprisingly, the recombinant antibodies demonstrated poly- and self-reactivity as demonstrated by their reactivity with nuclear, cytoplasmic, as well as membranous antigens expressed by human cells and by reactivity with human serum. This polyreactivity was specific as demonstrated by ELISA. The antibodies did not react with proteins on the cell surface that are induced by apoptosis as shown for antibodies produced by chronic lymphatic leukemia with VH1-02 gene rearrangement. The results indicate that a common subset of SMZL arises from polyreactive B cells, a subset of marginal zone B cells that are important in the immunologic defense against infection.
Introduction
Marginal zone lymphomas are believed to arise from B lymphocytes residing in the marginal zone of the B-cell zone in secondary lymphoid tissue. The origin and function of human marginal zone B lymphocytes have not completely been elucidated, but marginal zone B cells likely have a function in the T-independent immune response.1 Depending mainly on the organ in which marginal zone lymphoma arises, 3 distinct subtypes of marginal zone lymphoma are recognized: nodal, extra-nodal, and splenic marginal zone lymphomas (SMZL), arising from lymph nodes, mucosal sites, and the spleen, respectively. Together, those marginal zone lymphomas represent ∼ 8% of all lymphomas and are the third most-frequent lymphoma type.2
SMZL as well as the extranodal marginal zone lymphomas are clinically low grade or indolent, whereas nodal marginal zone lymphomas have a more aggressive clinical course.3 In contrast to nodal and extranodal marginal zone lymphomas, SMZLs are mostly systemic and are diagnosed with BM infiltration. A subset of extranodal marginal zone lymphomas occurring in the stomach and lung are characterized by the translocation t(11;18)(q21;q21) or variant translocation t(14;18)(q32;q21), resulting in overexpression of the MALT1 gene, and a minority of those lymphomas can show either a translocation t(3;14)(p14;q32), t(1;14)(p22;q32), or BCL10 mutation. Primary genetic alterations have not been demonstrated for nodal or SMZL. However, frequent deletion of the long arm of chromosome 7(del 7q31-32) as well as trisomy 3 have been documented in SMZL.4 The importance of these genetic findings for the pathogenesis of SMZL is not clear.5
Extranodal marginal zone lymphomas may occur secondary to chronic infection or chronic inflammation.6 Extranodal marginal zone lymphoma arising in the stomach may occur secondarily to Helicobacter pylori–associated gastritis.7 This led to the successful use of antibiotics for its treatment.8 Similarly, marginal zone lymphoma arising in the thyroid gland and salivary gland can be secondary to chronic inflammation associated with autoimmune disease, such as Hashimoto thyroiditis or Sjögren syndrome, respectively.9,10 Other examples are the association of Borrelia burgdorferi infection, Chlamydia psittaci infection, and Campylobacter jejuni infection with secondary development of marginal zone lymphoma in the skin, orbita, and jejunum, respectively.11-13
SMZL may occur secondarily to hepatitis C virus infection and can be successfully treated with antiviral drugs.14 The IgH variable gene (VH) 1-69 is preferentially used in hepatitis C virus–associated SMZL.15-17 This is likely a consequence of the infection and subsequent antigen-selective pressure.18 However, many cases of SMZL, including those arising in nonendemic countries such as Norway, are not associated with hepatitis C virus infection. As part of a search for other potential causal agents, we have produced antibodies from several cases of SMZL that are not associated with hepatitis C virus infection and that use the VH1-02 gene, a gene found in one-fifth to one-third of all SMZL.19-23 We have studied the reactivity of these antibodies.
Methods
We have used archival frozen tissue of SMZL cases and cloned the respective IgH and Ig light chain variable genes that are expressed by the lymphoma cells into appropriate vectors for expression. The specificity of the in vitro–produced Igs was investigated. The study was approved by the University Hospital of Oslo institutional review board (reference 09/5693) and the regional ethics committee (reference 2009/1814).
Recombinant antibody synthesis
Isolation of RNA and cDNA synthesis.
Frozen tissue of 10 cases of hepatitis C virus–negative SMZL was retrieved from the archive of the Department of Pathology at the Norwegian Radium Hospital. The pathology and cytogenetic characteristics of 6 of these cases that were used for antibody production are given in Table 1. Total RNA was extracted from the samples by use of the Trizol-method (Invitrogen). A total of 1.5 μg of RNA and oligo(dT) primers were used for cDNA synthesis with the ProtoscriptTM First Strand cDNA Synthesis Kit (New England BioLabs), according to the instructions of the manufacturer.
Immunophenotypic and cytogenetic characteristics of the SMZL cases
| Case . | Sex . | Age, y . | Immunophenotype . | Cytogenetic findings . |
|---|---|---|---|---|
| Case 10 | Male | 55 | CD19+, CD20+, CD22+, FMC7+, IgM+, IgK+, CD5−, CD10−, CD23− | 46,XY,t(4;14)(p13;q32),dup(12)(q14q23)[15] |
| Case 25 | Female | 56 | CD19+, CD20+, IgM+, IgK+, CD5−, CD10−, CD23− | 47,XX,+3[4]/46,XX[10] |
| Case 103 | Female | 55 | CD19+, CD20+, CD22+, FMC7+, IgM+, IgK+, CD5−, CD10−, CD23− | 46,XX[25] |
| Case 129 | Female | 52 | CD19+, CD20+, CD22+, FMC7+, IgM+, IgK+, CD5-, CD10- | 46,XX,del(7)(q33),add(13)(p13),add(14)(q32)[3]/46,XX[2] |
| Case 152 | Female | 51 | CD19+, CD20+, IgM+, IgK+, CD5−, CD10− | 47,XX,+add(3)(p21),del(7)(q11q22orq22q32)[8]/46,XX[7] |
| Case 255 | Female | 62 | CD19+, CD20+, FMC7+, IgM+, IgK+, CD5−, CD10− | 47,XX,+X,der(15)t(11;15)[4]/47,XX,+add(12)(p11)[2]/46,XX[2] |
| Case . | Sex . | Age, y . | Immunophenotype . | Cytogenetic findings . |
|---|---|---|---|---|
| Case 10 | Male | 55 | CD19+, CD20+, CD22+, FMC7+, IgM+, IgK+, CD5−, CD10−, CD23− | 46,XY,t(4;14)(p13;q32),dup(12)(q14q23)[15] |
| Case 25 | Female | 56 | CD19+, CD20+, IgM+, IgK+, CD5−, CD10−, CD23− | 47,XX,+3[4]/46,XX[10] |
| Case 103 | Female | 55 | CD19+, CD20+, CD22+, FMC7+, IgM+, IgK+, CD5−, CD10−, CD23− | 46,XX[25] |
| Case 129 | Female | 52 | CD19+, CD20+, CD22+, FMC7+, IgM+, IgK+, CD5-, CD10- | 46,XX,del(7)(q33),add(13)(p13),add(14)(q32)[3]/46,XX[2] |
| Case 152 | Female | 51 | CD19+, CD20+, IgM+, IgK+, CD5−, CD10− | 47,XX,+add(3)(p21),del(7)(q11q22orq22q32)[8]/46,XX[7] |
| Case 255 | Female | 62 | CD19+, CD20+, FMC7+, IgM+, IgK+, CD5−, CD10− | 47,XX,+X,der(15)t(11;15)[4]/47,XX,+add(12)(p11)[2]/46,XX[2] |
Sequence analysis of IgV genes.
The IgVH and IgVL genes were first amplified with IgVH and IgVL consensus primers. These primers are complementary to the leader sequence/framework 1 (FR-1) and FR-4/constant (C) regions of VH and VL.24 The IgVH and IgVL PCR products of the first-round reaction were subsequently reamplified in a second PCR by use of the primers used in the first round of PCR that were extended with restriction enzyme-recognition sites.24 IgV PCR products were directly sequenced with the same primers as were used for the first PCR. After subcloning of the PCR products (see next paragraph), between 9 and 19 clones per case were additionally sequenced to determine the homology with the sequence obtained by direct sequencing. This was performed to obtain relevant clones for antibody production and to study ongoing somatic hypermutation. Sequencing was performed with the ABI Prism Dye Terminator Cycle Sequencing Kit (Applied Biosystems), and products were analyzed with the ABI 3100 Genetic Analyzer (Applied Biosystems). Analysis of IgV sequences was performed by use of the web-based IMGT (EBI) and IgBLAST (National Center for Biotechnology Information) services. Ongoing mutation was defined as the presence of 1 or more identical nucleotide differences in at least 2 clones compared with sister clones. Antigen-selective pressure was analyzed according to Lossos et al.25
IgVH and IgVL gene constructs and antibody synthesis.
Recombinant IgH gene constructs consisted of a CMV promoter, a mouse Ig leader signal sequence, the rearranged IgVH gene from the respective SMZL, a human CHγ1 region, and mouse CH2-CH3-γ2b (Fcγ2b) region (supplemental Figure 1, available on the Blood Web site; see the Supplemental Material link at the top of the online article). An Igγ constant region was chosen because of the readily available anti-γ secondary antibodies used in the various tests we performed. Recombinant Ig light chain constructs consisted of a CMV promoter, a mouse Ig leader signal sequence, the rearranged IgVL gene from the respective SMZL, and a human Cκ region (supplemental Figure 1). The negative control construct contained only a mouse Ig CH2-CH3 γ2b fragment (Fcγ2b).
The constructs for the heavy chains were made as follows: first, the IgVH PCR products were digested with BsmI and BsiWI restriction enzymes and inserted into a pLNOH2 vector that is a derivative of the pcDNA3 vector (Invitrogen), as described by Norderhaug et al.26 Subsequently, the inserted IgVH gene together with an upstream leader signal sequence and the downstream human CHγ1 sequence from the pLNOH2 vector were amplified using a forward primer (5′-CCG GGG GAT CCT CAC CAT GGG ATG GAG CTG TAT-3′) and a reverse primer (5′-TGT GGA TCC TTT GTC ACA AGA TTT GGG CTC-3′). This PCR fragment was then inserted in-frame in a pcDNA1-Fc expression vector27 between the upstream CMV promoter and the downstream mouse CH2-CH3-γ2b gene sequence. The constructs for the light chains were made by inserting the IgVL gene fragment into a variant of the pLNOH2 vector containing a CMV promoter, a mouse Ig leader sequence, and a human Ck sequence.26 IgH and IgL constructs were transformed into TOP10 competent cells (Invitrogen) and from each case at least 10 clones were selected. Only the plasmids containing IgV sequences that were identical with the patient IgV sequences were used. The 2 plasmids carrying the respective IgH and IgL gene were cotransfected into HEK293T cell line by use of the lipofectAMINE transfection reagent (Invitrogen). Transfection was performed according to manufacturer's instructions. The recombinant antibodies were purified with protein A beads as described previously27 ; proper Ig synthesis was confirmed by 8% SDS-PAGE and Western blotting with a mouse anti-IgG antibody. The yield of the respective antibodies was different according to the case. In particular, Ab255 was difficult to produce, and not all tests could be performed with this antibody. In 1 case (case 152), we found 2 different expressed VL genes; hence, we produced 2 different monoclonal antibodies using the same heavy chain and either of the 2 light chains genes, respectively.
Immunohistochemical analysis
Tissue sections from normal tissues as well as from cell-blocks with human pancreatic carcinoma cell lines MIA PaCa-2 (American Type Culture Collection [ATCC]) and PANC-1 (ATCC) were immunohistochemically stained with the recombinant antibodies. Normal tissues included lymph node, tonsil, spleen, BM, appendix, salivary gland, pancreas, kidney, skin, stomach, uterus, and lung. These tissue types were selected because most may be infiltrated by mucosa-associated lymphoid tissue that contains marginal zone B cells and thus hypothetically may be a source of autoantigen reacting with marginal zone B cells. Both frozen and formalin-fixed tissues were available. We fixed 5-μm frozen sections with acetone, and they were stored at −70°C until further used for staining. Then, 4- to 5-μm formalin-fixed sections were dried overnight and stored at 4-8°C until further used. Sections were stained with the peroxidase-labeled EnVision+ system according to the instructions of the manufacturer (DakoCytomation A/S). Negative controls were performed by omitting the primary antibody as well as using a recombinant mouse Ig Fcγ2b control.
Flow cytometry
The following cell lines were analyzed by flow cytometry: B-cell lines REH (German Collection of Microorganisms and Cell Cultures [DMSZ]), NALM-6 (DMSZ), KARPAS-422 (DMSZ), Ramos (ATCC), Daudi (ATCC), and SU-DHL-6 (DMSZ); T-cell line Jurkat (ATCC); cervical carcinoma cell line HELA (DMSZ); pancreatic carcinoma cell line PANC-1 (ATCC); breast carcinoma cell line T-47D (ATCC); ovarian carcinoma cell line NIH:OVCAR-828 ; hepatocellular carcinoma cell line HEP-G2 (DMSZ); and HEK293T cells. In addition, BM aspirate, peripheral blood, as well as lymph node cell suspensions, were analyzed. Flow cytometry analysis was performed on a FACSCalibur flow cytometer (Becton Dickinson). The data were analyzed with the CellQuest 3.3 (Becton Dickinson) and FlowJo 7.2.2 (TreeStar) software. A total of 10 000 or more gated events were analyzed per sample.
To test whether the recombinant antibodies bound to surface protein, HEK293T cells were pretreated with Trypsin EDTA solution (PAA Laboratories) containing 0.5 mg/mL trypsin and 0.22 mg/mL EDTA in Ca- and Mg-free PBS, for 30 minutes at 37°C. Control cells were incubated with only PBS before staining. Furthermore, to test whether the recombinant antibodies reacted with a glycosylated epitope, HEK293T cells were submitted to the following treatments before staining; first, 1 μg/mL of tunicamycin (Sigma-Aldrich) was added to the cell culture overnight, a procedure that blocks the formation of N-glycosylation; second, cells were treated with 5 units of N-glycosidase (Roche Diagnostics); third, cells were treated with 10 units of O-glycosidase (Roche Diagnostics).
Apoptosis assay
The recombinant antibodies derived from SMZL were used in an apoptosis assay to test whether cell surface expression of the antigen detected by the recombinant antibodies was increased by apoptosis. The latter has recently been demonstrated for antibodies expressed by chronic lymphatic leukemia (CLL).29 We used an apoptosis assay for Jurkat cells as well as for NIH:OVCAR-8 cells, as described previously in detail by Chu et al29 and Dong et al,30 respectively.
Western blotting
Mouse pancreatic β cell line MIN6,31 HEK293T, HEP-G2, and HELA cell lines were analyzed by Western blotting. The details of the method are described in the supplement materials.
ELISA
Coating of microtiter plates.
Crude cell lysates of HEK293T and PANC1 were prepared as described for Western blotting analysis. Human insulin, lipopolysaccharide, (ssDNA), and BSA were purchased from Sigma-Aldrich. Human serum albumin (HSA) was purchased from Baxter International and was passed through a PD-10 column (GE Healthcare) that was equilibrated with PBS containing 0.01% NaN3,. Thyroglobulin (TG), mouse anti–progastrin-releasing peptide (anti-proGRP) IgG2b E149, anti–neuron-specific enolase IgG2b, and anti-TG IgG1 E41 antibodies were a kind gift from Dr Elisabeth Paus (Medical Biochemistry Department, the Norwegian Radium Hospital).32 A total of 100 μL of a 100 μg/mL of ssDNA solution and a 10 μg/mL solution of human insulin, lipopolysaccharide, HAS, and TG, respectively, in a PBS-0.01% NaN3 solution were applied to 96-well Trans Maxi FluoroNunc plates (Nunc) and allowed to bind to the plates for 20 hours at 37°C. Blocking was performed with a 2% BSA solution but was repeated with a 1% (vol/vol) fish-skin gelatin (Sigma-Aldrich) solution as the recombinant antibodies also bound to some degree to coated BSA.
Assay for antibody binding.
A total of 100 μL of the respective recombinant antibodies in a PBS-0.1% BSA or a PBS-0.1% gelatin solution at pH 7.3 was incubated with the coated plates overnight at 4° C. The plates were subsequently washed 6 times in a 1296-02 Delfia Platewasher (PerkinElmer) before adding 100 μL of a HRP-linked rabbit anti–mouse Ig, diluted 1:2000 in PBS-0.1% gelatin. It was first excluded that our recombinant monoclonal antibodies bound to this rabbit IgG antibody. The antibody was incubated for 1 hour. After washing 6 times, 100 μL of BM Blue POD chromogenic substrate (Roche) diluted 1:2 in 0.1M Na-acetate buffer pH 6.0 was added, and this solution was incubated for 30 minutes. Subsequently, 50 μL of 2 M H2SO4 was added, and the color reaction was quantitated by reading the light absorption at 450 nm.
Inhibition of antibody binding.
Biotinylated TG bound on streptavidin-coated plates (PerkinElmer) were used. For this purpose, purified TG was reacted with 20 × molar excess of Sulfo-NHS-LC biotin (Thermo Fisher Scientific Inc). Free biotin was removed by gel filtration on a PD-10 column. A total of 100 μL of a 5 μg/mL biotinylated TG solution in PBS-0.05% Tween 20 buffer was added to the streptavidin-coated wells. TG was incubated for 30 minutes and washed 3 times before use. A total of 110 μL Ab10 or Ab25 of a 3 μg/mL solution in PBS-0.1%BSA-0.025%Tween 20 solution was preincubated with 110 μL of the respective inhibitors in PBS-0.05%Tween 20 buffer overnight. A total of 100 μL of this antibody solution was subsequently added to the solid-phase TG. The plates were incubated at room temperature for 1 hour while shaking. Thereafter, the wells were washed 6 times and developed with HRP-linked rabbit anti–mouse antibody as described previously. The results are presented as percentage of binding in the absence of inhibitor. All results were corrected for control values in the absence of primary antibody. The positive control experiment consisted of using anti-TG E41 antibody, which showed specific binding to TG and showed inhibition with none of the inhibitors but TG (results not shown).
Results
Rearranged IgVH and IgVL genes reveal similar antigen-binding regions of SMZL with VH1-02 gene usage
The rearranged genes of all SMZL cases showed somatic mutations in the VH region, with homologies to the respective germline genes ranging from 96.18% to 89.82%. All rearranged VH and VL genes were in frame and hence potentially functional. Six cases used the same VH1-02*04 gene, whereas the remaining 4 cases used either VH1-18, VH4-59, or VH3-30 genes. Five of the 6 cases with VH1-02 gene rearrangement as well as 2 of the other cases used the same HD3-3*01 gene, whereas 1 case with VH1-02 gene rearrangement used the highly homologous HD3-9*01 gene (Table 2). HJ gene use was variable (Table 2). The presence of somatic mutations and intraclonal sequence variation is given in Table 2. The characteristics of the somatic hypermutations are given in Table 3. None of the cases showed antigen-selective pressure. GenBank accession numbers are JF894276-JF894288.
Ig gene sequence characteristics
| Case . | VH gene . | Homology/clones/ intraclonal variation (y/n) . | DH gene . | JH gene . | VH-CDR3 length . | VL gene . | Homology . | JL gene . |
|---|---|---|---|---|---|---|---|---|
| Case 10 | IGHV1-02*04 | 93.40/10/y | HD3-3*01 | HJ3*02 | 22 | IGKV3-20*01 | 92.91 | KJ2*01 |
| Case 25 | IGHV1-02*04 | 95.14/10/n | HD3-3*01 | HJ2*01 | 22 | IGKV1-17*01 | 97.49 | KJ1*01 |
| Case 103 | IGHV1-02*04 | 95.88/10/n | HD3-3*01 | HJ6*02 | 21 | IGKV1-17*01 | 97.13 | KJ1*01 |
| Case 129 | IGHV1-02*04 | 94.79/9/y | HD3-3*01 | HJ6*03 | 22 | IGKV1-27*01 | 94.44 | KJ4*01 |
| Case 152 | IGHV1-02*04 | 95.83/19/n | HD3-3*01 | HJ4*03 | 22 | IGKV1-9*01 | 96.70 | KJ4*01 |
| IGV4-1*01 | 94.95 | KJ2*02 | ||||||
| Case 255 | IGHV1-02*04 | 96.18/10/n | HD3-9*01 | HJ6*03 | 23 | GKV3-17*01 | 97.85 | KJ4*01 |
| Case . | VH gene . | Homology/clones/ intraclonal variation (y/n) . | DH gene . | JH gene . | VH-CDR3 length . | VL gene . | Homology . | JL gene . |
|---|---|---|---|---|---|---|---|---|
| Case 10 | IGHV1-02*04 | 93.40/10/y | HD3-3*01 | HJ3*02 | 22 | IGKV3-20*01 | 92.91 | KJ2*01 |
| Case 25 | IGHV1-02*04 | 95.14/10/n | HD3-3*01 | HJ2*01 | 22 | IGKV1-17*01 | 97.49 | KJ1*01 |
| Case 103 | IGHV1-02*04 | 95.88/10/n | HD3-3*01 | HJ6*02 | 21 | IGKV1-17*01 | 97.13 | KJ1*01 |
| Case 129 | IGHV1-02*04 | 94.79/9/y | HD3-3*01 | HJ6*03 | 22 | IGKV1-27*01 | 94.44 | KJ4*01 |
| Case 152 | IGHV1-02*04 | 95.83/19/n | HD3-3*01 | HJ4*03 | 22 | IGKV1-9*01 | 96.70 | KJ4*01 |
| IGV4-1*01 | 94.95 | KJ2*02 | ||||||
| Case 255 | IGHV1-02*04 | 96.18/10/n | HD3-9*01 | HJ6*03 | 23 | GKV3-17*01 | 97.85 | KJ4*01 |
Homology is given as the percentage of identical nucleic acids compared with the closest germline gene sequence. The length of the CDR3 regions was determined according to the IGMT numbering (http://imgt.cines.fr/). Case 152 displays 2 functionally rearranged immunoglobulin light chains.
DJ indicates IgH diversity gene; JH, IgH junction gene; JL, Ig light chain junction gene; n, no; VH, IgH variable gene; VL, Ig light chain variable gene; and y, yes.
Somatic hypermutation characteristics
| Case . | FR/CDR . | R . | S . | P . |
|---|---|---|---|---|
| Case 10 | FR | 10 | 4 | .221 |
| CDR | 2 | 3 | .701 | |
| Case 25 | FR | 7 | 5 | .198 |
| CDR | 1 | 1 | .785 | |
| Case 103 | FR | 4 | 2 | .095 |
| CDR | 2 | 2 | .334 | |
| Case 129 | FR | 8 | 4 | .182 |
| CDR | 3 | 1 | .346 | |
| Case 152 | FR | 7 | 3 | .549 |
| CDR | 1 | 0 | .687 | |
| Case 255 | FR | 5 | 2 | .15 |
| CDR | 2 | 2 | .383 |
| Case . | FR/CDR . | R . | S . | P . |
|---|---|---|---|---|
| Case 10 | FR | 10 | 4 | .221 |
| CDR | 2 | 3 | .701 | |
| Case 25 | FR | 7 | 5 | .198 |
| CDR | 1 | 1 | .785 | |
| Case 103 | FR | 4 | 2 | .095 |
| CDR | 2 | 2 | .334 | |
| Case 129 | FR | 8 | 4 | .182 |
| CDR | 3 | 1 | .346 | |
| Case 152 | FR | 7 | 3 | .549 |
| CDR | 1 | 0 | .687 | |
| Case 255 | FR | 5 | 2 | .15 |
| CDR | 2 | 2 | .383 |
CDR indicates IgH variable gene complementarity determining regions; FR, IgH variable gene framework regions; R, replacement mutations; and S, silent mutations.
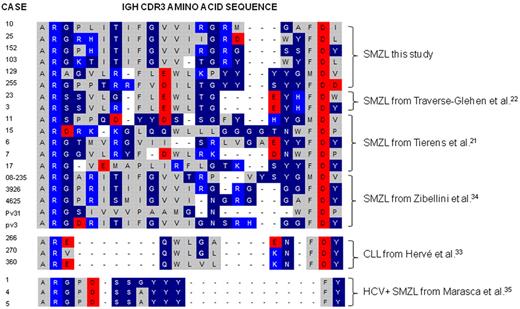
The deduced amino acid sequence of the heavy chain complementarity determining regions 3 (CDR3) showed a high degree of sequence homology among the 6 cases using the VH1-02 gene, with several conserved or semiconserved amino acids. These CDR3 regions differ in length and amino acid composition compared with previously published cases of CLL that use the VH1-02 gene.33 The average length of the CDR3 segment of the 6 SMZL was 22 amino acids, compared with 13 amino acids for CLL cases (Figure 1). With respect to the DH segments, 5 of the SMZL cases used the HD3-3*01 gene, whereas 1 case used the similar HD3-9*01. By contrast, previously published CLL cases used the D6-19 gene.33 In view of the CDR3 region similarities and the likelihood that those antibodies recognized similar antigens, only cases that used the VH1-02 gene (cases 10, 25, 103, 129, 152, and 255, respectively) were used for recombinant antibody production. The rearranged Ig VL genes of the 6 cases were either IGKV1 genes or IGKV3 genes (Table 1). IGKJ gene usage is variable (Table 1). We obtained useful in vitro produced recombinant antibodies from cases 10, 25, 129, 152, and 255 but not from case 103. Because 2 different rearranged light chains were obtained from case 152, 2 antibodies containing the respective light chains were produced.
Amino acid sequence alignment of IgH CDR3. The IgH CDR3 of SMZL cases using the VH1-02 gene from the current study are compared with those of published series of SMZL and CLL with VH1-02 usage.21,22,33-35 The bottom 3 sequences are published VH1-69 IgH CDR3 sequences from marginal zone lymphomas in patients with hepatitis C virus infection. The alignment shows that the majority of SMZL cases that use the VH1-02 have considerably longer IgH CDR3 segments than CLL cases that use this gene. Of interest, SMZLs that use VH1-69 in patients with hepatitis C virus infections show different, short, and stereotyped sequences. Amino acids are color-coded as follows: gray for nonpolar, dark blue for uncharged polar, light blue for basic, and red for acidic amino acids.
Amino acid sequence alignment of IgH CDR3. The IgH CDR3 of SMZL cases using the VH1-02 gene from the current study are compared with those of published series of SMZL and CLL with VH1-02 usage.21,22,33-35 The bottom 3 sequences are published VH1-69 IgH CDR3 sequences from marginal zone lymphomas in patients with hepatitis C virus infection. The alignment shows that the majority of SMZL cases that use the VH1-02 have considerably longer IgH CDR3 segments than CLL cases that use this gene. Of interest, SMZLs that use VH1-69 in patients with hepatitis C virus infections show different, short, and stereotyped sequences. Amino acids are color-coded as follows: gray for nonpolar, dark blue for uncharged polar, light blue for basic, and red for acidic amino acids.
The SMZL recombinant antibodies react with autoantigens present in the cytoplasm of diverse cell types and on the cell membrane of epithelial cells
Four of the 5 recombinant antibodies, from cases 10, 25, 129, and 255, respectively, reacted very similarly with human tissues as tested with immunohistochemistry (Figure 2). Negative controls were consistently negative. The 2 antibodies derived from case 152 did not show detectable reactivity with any of the tissues tested.
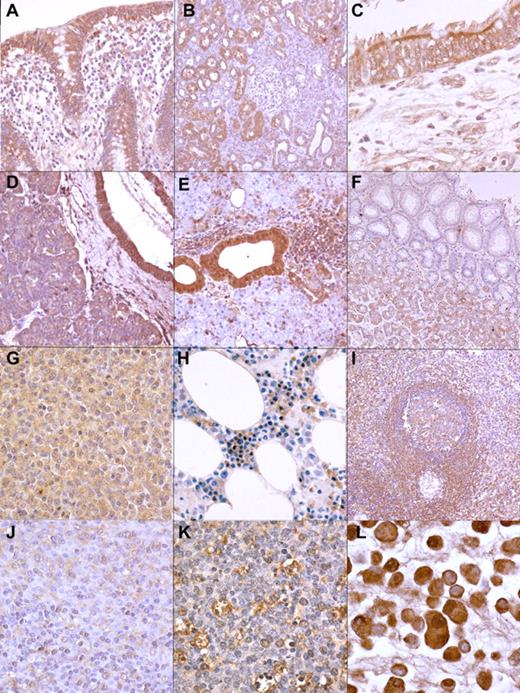
Reactivity of the recombinant antibodies in diverse tissues and cells. The antibodies stain cytoplasmic autoantigens in a diverse array of cell types (immunoperoxidase staining): (A) colonic epithelial cells in the appendix (mAb 25; magnification ×200); (B) tubular epithelial cells in the kidney (mAb 129; magnification ×100); (C) respiratory epithelial cells in the bronchus (mAb 10; ×400); (D) pancreatic ductal epithelial cells as well as exocrine secretory cells (mAb 129; ×200); (E) ductal epithelial cells in the salivary gland (mAb 25; ×200); (F) acinar cells in the stomach (mAb 10; ×100); (G) SMZL cells (mAb 10; ×400); (H) red cell precursor cells in the bone marrow (mAb 10; ×400); (I) marginal zone B lymphocytes in the spleen (mAb 129; ×100); (J) a subpopulation of monocytoid B cells in the lymph node (mAb 129; ×100);(K) tingible body macrophages in germinal centers from a lymph node (mAb 25; ×400); (L) cells of pancreatic carcinoma cell line PANC1 (mAb 25; ×400).
Reactivity of the recombinant antibodies in diverse tissues and cells. The antibodies stain cytoplasmic autoantigens in a diverse array of cell types (immunoperoxidase staining): (A) colonic epithelial cells in the appendix (mAb 25; magnification ×200); (B) tubular epithelial cells in the kidney (mAb 129; magnification ×100); (C) respiratory epithelial cells in the bronchus (mAb 10; ×400); (D) pancreatic ductal epithelial cells as well as exocrine secretory cells (mAb 129; ×200); (E) ductal epithelial cells in the salivary gland (mAb 25; ×200); (F) acinar cells in the stomach (mAb 10; ×100); (G) SMZL cells (mAb 10; ×400); (H) red cell precursor cells in the bone marrow (mAb 10; ×400); (I) marginal zone B lymphocytes in the spleen (mAb 129; ×100); (J) a subpopulation of monocytoid B cells in the lymph node (mAb 129; ×100);(K) tingible body macrophages in germinal centers from a lymph node (mAb 25; ×400); (L) cells of pancreatic carcinoma cell line PANC1 (mAb 25; ×400).
Glandular tissues showed the most intense staining: strong cytoplasmic staining was seen in salivary gland and pancreas ducts, whereas moderate-to-weak staining was observed in acinar cells. Staining was also observed in gastric glandular epithelium but not in surface epithelium. Intestinal epithelium was devoid of staining. Bronchial epithelium by contrast showed intense cytoplasmic staining, but no staining was observed in alveolar epithelium. Kidney tubular epithelium also showed cytoplasmic staining, but no staining was noted in collecting ducts or glomeruli. Further, smooth muscle cells in diverse organs showed slight cytoplasmic staining. In addition, staining was observed in lymphoid tissues from tonsil, spleen, appendix, and lymph nodes. There was moderate cytoplasmic positivity in germinal center B cells and marginal zone B cells but not in mantle B cells. Other lymphoid cells did not stain. However, the cytoplasm of macrophages, especially within the germinal center, also stained. Sinus-lining cells in the spleen showed moderate-to-weak cytoplasmic staining. BM hematopoietic cells did not show any staining with the antibodies, except for the red cell series. In particular, normoblasts showed cytoplasmic positivity. The antibodies reacted also with pancreatic cell lines PANC-1 and MIA PaCa-2. Predominantly cytoplasmic staining was seen in these cell lines (Figure 2).
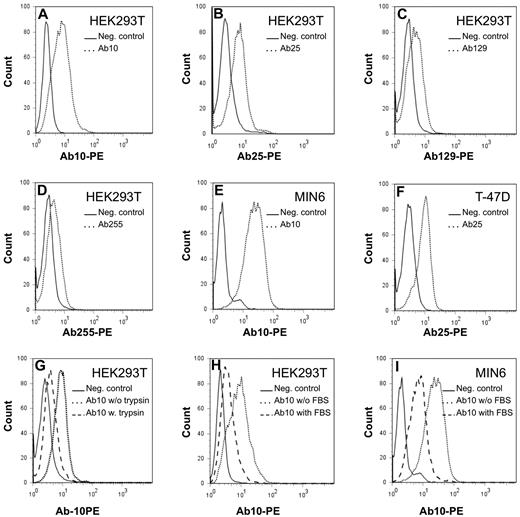
The antibody reactivity with different cancer cell lines was also tested by flow cytometry. The antibodies 10, 25, 129, and 255 bound to the surface of human embryonal kidney cell line HEK293T cells, the breast carcinoma cell line T-47D, the human ovarian carcinoma cell line NIH:OVCAR-8, and the mouse pancreatic β cell line MIN6 (Figure 3). Of interest, weaker binding was observed after trypsin treatment of the cells (Figure 3). In addition, treatment of the cells with tunicamycin, N-glycosidase, or O-glycosidase failed to reduce reactivity with the recombinant antibodies. Together, the results indicate that the cell-surface epitopes recognized by the recombinant antibodies are most likely peptides. The cell surface of lymphoma cell lines and other tested epithelial cell lines did not stain with the recombinant antibodies (data not shown). However, all cell lines did stain after permeabilization of the cell membrane. These data are in agreement with the ubiquitous intracellular localization of the antigen recognized by the recombinant antibodies as also shown by immunohistochemistry. Flow cytometric analysis of BM, peripheral blood, and lymph node cell suspensions could not reveal cell surface expression in hematopoietic cells, including red blood cells and lymphoid cells. Antibodies from case 152 did not react with any of the cell lines.
Reactivity of the recombinant antibodies with cell lines by the use of flow cytometry. The recombinant antibodies stain the cell surface of cell lines HEK293T, MIN6, and T-47D (panels A-F). Dotted lines represent specific staining with the recombinant antibodies, whereas continuous lines represent staining with a negative control mouse Igγ2b Fc fragment. Trypsin treatment reduces specific surface antibody binding (dashed line, panel G). Incubation of the cell lines with antibody diluted in fetal bovine serum reduces specific antibody binding as demonstrated in 2 of the cell lines (dashed line, panels H and J).
Reactivity of the recombinant antibodies with cell lines by the use of flow cytometry. The recombinant antibodies stain the cell surface of cell lines HEK293T, MIN6, and T-47D (panels A-F). Dotted lines represent specific staining with the recombinant antibodies, whereas continuous lines represent staining with a negative control mouse Igγ2b Fc fragment. Trypsin treatment reduces specific surface antibody binding (dashed line, panel G). Incubation of the cell lines with antibody diluted in fetal bovine serum reduces specific antibody binding as demonstrated in 2 of the cell lines (dashed line, panels H and J).
Induction of apoptosis does not induce reactivity with the recombinant antibodies on the cell surface of Jurkat cells and does not up-regulate reactivity on the cell surface of NIH:OVCAR-8 cells
Live as well as apoptotic Jurkat cells did not show cell surface reaction with the recombinant antibodies. Induction of apoptosis in cell line NIH:OVCAR-8 did not result in increased binding of the recombinant antibodies on the cell surface (supplemental Figure 2). This is unlike antibodies produced by CLL that bind to nonmuscle myosin IIA, that is transported to the cell surface during apoptosis.29
The antibodies are polyreactive
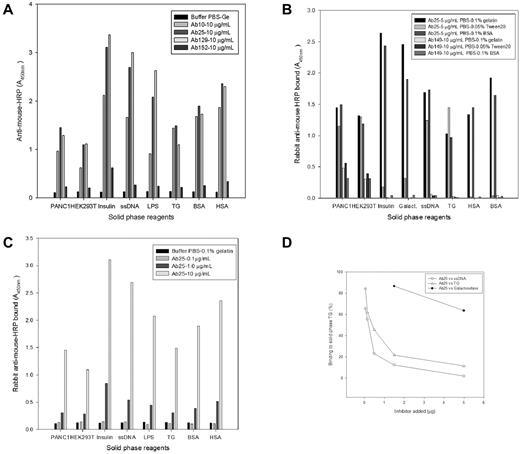
Flow cytometry (Figure 3) and Western blot analysis (supplemental Figure 3) using primary antibodies diluted with 5% milk or serum greatly reduced reactivity. These findings suggested reactivity of the antibodies with epitopes in milk and serum. Western blots that used antibodies diluted in a solution only containing BSA showed reactivity with a range of proteins, including a predominant protein with a molecular mass of approximately 55-60 kDa (supplemental Figure 3). These findings combined with the ubiquitous reactivity of the antibodies with epitopes in the cell nucleus, cell cytoplasm, and cell membrane suggested that the antibodies were polyreactive. ELISAs performed with antibodies 10, 25, and 129 showed that these antibodies strongly bound to galactosidase, insulin, lipopolysaccharide, TG, coated BSA, and coated HSA as well as to crude cell lysates of PANC-1 and HEK293T cells, albeit with different affinities (Figure 4A). Antibody 152 showed only limited binding to insulin and served as a negative control (Figure 4A). The weak or restricted binding of antibody 152 was in accordance with the results from immunohistochemistry and flow cytometry. A recombinant mouse Ig Fcγ2b control (results not shown) and irrelevant mouse monoclonal IgG2b anti-NSE or anti-proGRP (Figure 4B) did not bind the solid-phase reagents, as expected. Anti-proGRP did bind to the cell lysates, as expected, because these cells show limited proGRP expression. Binding of the recombinant antibodies to BSA and HSA was inhibited by the addition of Tween 20 to the assay buffer. The latter also reduced the binding to insulin and galactosidase but did not inhibit it.
ELISA. Solid-phase reagents PANC1 cell lysate, HEK293T lysate, insulin, ssDNA, lipopolysaccharide (LPS), TG, BSA, and HSA are given in the x-axis, respectively, whereas light absorption is indicated in the y-axis. (A) Reaction of control buffer and 10 μg/mL of the 4 different antibodies Ab10, Ab25, Ab129, and Ab152, respectively, with the solid-phase reagents. All antibodies, except Ab152 and control buffer, react with the solid phase reagents. (B) Reaction of Ab25 and a mouse monoclonal Ab149, anti-proGRP, with the solid-phase reagents using different buffers. Ab25, but not Ab149, reacts with the reagents. Ab149 reacts solely with the cell lysates, as expected. The addition of Tween20 inhibits the reaction of Ab25 with HSA, BSA and reduces the reaction with insulin and galactosidase. Ab25 reacts in a dose-dependent fashion with the solid phase reagents (panel C) and its binding to TG can be blocked by TG and heterologous binding reagents ssDNA and galactosidase (panel D).
ELISA. Solid-phase reagents PANC1 cell lysate, HEK293T lysate, insulin, ssDNA, lipopolysaccharide (LPS), TG, BSA, and HSA are given in the x-axis, respectively, whereas light absorption is indicated in the y-axis. (A) Reaction of control buffer and 10 μg/mL of the 4 different antibodies Ab10, Ab25, Ab129, and Ab152, respectively, with the solid-phase reagents. All antibodies, except Ab152 and control buffer, react with the solid phase reagents. (B) Reaction of Ab25 and a mouse monoclonal Ab149, anti-proGRP, with the solid-phase reagents using different buffers. Ab25, but not Ab149, reacts with the reagents. Ab149 reacts solely with the cell lysates, as expected. The addition of Tween20 inhibits the reaction of Ab25 with HSA, BSA and reduces the reaction with insulin and galactosidase. Ab25 reacts in a dose-dependent fashion with the solid phase reagents (panel C) and its binding to TG can be blocked by TG and heterologous binding reagents ssDNA and galactosidase (panel D).
Of interest, the addition of albumin to the buffer did not affect reactivity of the recombinant antibodies with coated albumin. However, addition of denatured albumin to the buffer completely removed reactivity with coated albumin (results not shown). Thus, the recombinant antibodies seemed only to react with denatured or similarly modified albumin. This likely explains why the addition of albumin to the recombinant antibodies for use in flow cytometry and Western blotting did not affect the results. The polyreactivity was dose-dependent, showing binding at a minimal concentration of 1 μg/mL (Figure 4C, supplemental Figure 4). In addition, binding to TG could be inhibited by preincubation of the antibodies with other antigens. Indeed, homologous incubation with TG as well as heterologous incubation with ssDNA and galactosidase gave dose-dependent inhibition of antibody binding to TG as shown in Figure 4D. Insulin, lipopolysaccharide, human IgG, and BSA gave < 5% inhibition tested at 5 μg (data not shown). Results with Ab25 and Ab10 were very similar in all dose-response and inhibition tests performed.
Discussion
SMZLs show a restricted use of the IgH variable genes and show a pattern of somatic mutations, which together could indicate selection by antigen.19-23,36,37 The Ig VH1-02 gene is one of the more common genes used by SMZL.19-23 It has recently been shown that these cases more often are associated with alterations of chromosome arms 7q or 14q or, in general, with an abnormal karyotype,38 as were the cases reported in the present study. Studies of patients from Spain, England, Greece, France, and Italy show a different frequency of VH1-02 restriction in SMZL, that is, 18/35 (51%), 5/23 (22%), 3/43 (7%), 8/35 (23%), and 12/59 (20%), respectively, of patients.20-23,37 The limited series of Norwegian cases reported in the present study shows a VH1-02 restriction in 6/10 (60%) of the patients. The cases with VH1-02 restriction reported here are not associated with hepatitis C virus infection, similar to previously reported series of SMZL cases with VH1-02 gene rearrangement.23
Analysis of the heavy chain CDR3 regions in our cases revealed a similar amino acid sequence, although this was not as pronounced as reported for the several subsets of CLL with stereotyped antigen receptors.39 Of note, the SMZL cases showed a restricted use of the IGHD3-3*01 or very homologous IGHD3-9*01 diversity genes, although different JH genes are used. The CDR3 regions were of similar length, between 21 and 23 amino acids according to the IMGT unique numbering system, and display an amino acid composition with comparable chemical properties, especially rich in hydrophobic amino acids. Sequences of previously published rearranged VH1-02 genes in SMZL patients also show CDR3 regions with similar properties (Figure 1). These properties are beyond what could be expected by random Ig V-D-J recombination. We therefore hypothesized that the Igs produced by a subset of SMZL showing VH1-02 restriction showed a similar reactivity. The relatively long and hydrophobic IgH CDR3 regions were also suggestive that the Igs were polyreactive.40 To investigate this, we produced recombinant antibodies from the rearranged Ig genes in 5 of those lymphoma patients.
Four of the recombinant monoclonal antibodies (10, 25, 129, and 255) synthesized from SMZL showed similar reactivity with tissues and cells as demonstrated by immunohistochemistry, by flow cytometry as well as by Western blotting. Two antibodies, of the fifth case (antibodies 152), consistently showed very weak reactivity or none at all. The antibodies reacted with a cytoplasmic antigen in a diverse array of cell types; epithelial cells from diverse organs, as well as mesenchymal cells such as smooth muscle cells, erythroid precursors, lymphoid cells and macrophages. Flow cytometry revealed, in addition to cytoplasmic staining, membranous staining in a subset of epithelial cell lines. Flow cytometry did not show membranous staining in hematopoietic cells, including red blood cells and lymphoid cells. By Western blotting analysis the antibodies recognized multiple proteins, with a predominant reactivity with a 55- to 60-kDa protein. Of interest, reactivity of the antibodies with the latter protein could be blocked by preincubation of the antibody with either serum or milk, suggesting that the same antigen or another reactive protein was present in these fluids. Together, these results suggest that the recombinant antibodies were polyreactive. The latter was confirmed by ELISA. Polyreactivity with insulin, TG, lipopolysaccharide, galactosidase, and ssDNA were documented. These results are very similar to those obtained in a recent elegant study by Craig et al.41 This study has shown that MALT lymphoma B cells also produce polyreactive Igs. However, the VH gene restriction, being predominantly VH1-69, CDR3 sequences as well as the binding characteristics of the antibodies, are different compared with the characteristics of the antibodies of SMZL reported in our study here.
The preferential usage of the same VH1-02 gene, the presence of somatic mutations in these genes, the highly homologous CDR3 segments in 6/10 of our SMZL cases as well as in published series of SMZL and the similar pattern of polyreactivity of the recombinant antibodies strongly indicate that these malignant clones have been selected for. However, the amino acid sequence differs between some cases of previously published series compared with the ones reported here. Whether this indicates that not all cases of SMZL using the VH1-02 gene react with similar antigens remains to be demonstrated. It is of interest that the frequency of VH1-02 use by SMZL is different among series reported from different geographic regions.20-23,37 The frequency of cases with VH1-02 gene rearrangement is particularly low in the Greek series.37 The latter might indicate that the selection of malignant clones is geographically determined. Stereotyped antigen receptors have been well recognized in chronic lymphocytic leukemia.39,42-45 CLL expresses stereotyped antigen receptors in ∼ 20% of the cases and up to 48 subsets of stereotyped receptors have been demonstrated.39 One minor CLL subset, subset number 28, also uses the VH1-02 gene as observed in SMZL and reacts with antigen exposed on apoptotic cells.29 However, the stereotyped CDR3 sequence of this CLL subset is different from the one in SMZL. In CLL, the IGHD1-26 D-gene is used, whereas the IGHD3-3*01 gene is used in SMZL, resulting in shorter CDR3 sequences in the former. In addition, our results indicate that the recombinant SMZL antibodies do not react with antigens on apoptotic cells, in contrast to what has been demonstrated in CLL.29
The reactivity of the SMZL antibodies with ubiquitous autoantigens might suggest that at least a subset of SMZL arises from a natural antibody producing B cell in the splenic marginal zone. Natural antibodies are largely polyreactive and are produced by B cells that localize, among other organs, in the splenic marginal zone, at least in rodents.46 Furthermore, recruitment and selection of B cells in the splenic marginal zone in rodents are dependent on polyreactivity with autoantigens.47 In rodents, polyreactive antibodies have antibacterial activity in the presence of complement, suggesting a role in the natural immunity against bacterial infection.46 Whether human SMZL derives from natural antibody producing cells is uncertain. SMZLs, including the cases in this study, express somatically mutated IgM antibodies, a feature shared with most human splenic marginal zone B cells and circulating IgM+ memory B cells.
The presence of mutations in SMZL is a strong argument against an origin from natural antibody producing B cells but may in itself be compatible with an origin from IgM+ memory cells. However, IgM+ memory B cells are selected against self-reactivity and rarely produce autoreactive antibodies.48 In addition, IgM+ memory B cells, in contrast to naive B cells and switched-memory B cells, rarely show a rearranged VH1-2 gene.49 Together these data do not seem to favor an origin from IgM+ memory B cells. However, this hypothesis cannot be totally ruled out. The VH1-2 subset of SMZL might arise from IgM+ memory B cells that have escaped the negative selection process. The latter remains to be demonstrated. Of interest, physiologic selection for polyreactivity has recently been demonstrated for IgG+ memory B cells in HIV infection.50 Polyreactivity may increase the affinity of otherwise high-affinity antibodies to HIV-gp140 by low-affinity heteroligation to more abundant self-antigens in circumstances in which the specific antigen, in this case HIV-gp140, is not present at such density to allow homoligation.50 Whether the SMZL B-cell receptor is stimulated by the autoantigens and might stimulate lymphoma growth needs to be investigated as well. The reactivity with autoantigen in serum is intriguing in this respect. The, at least partially, open circulation, unique to the spleen, exposes cells to serum proteins, perhaps leading to BCR stimulation and lymphoma growth. The observation that surgical resection of the spleen in patients with SMZL may result in disease remission might support this hypothesis.51
In conclusion, the findings by Craig et al41 and our results suggest that MALT lymphoma and SMZL with VH1-02 gene rearrangement produce polyreactive antibodies that react against self-antigens. These lymphomas likely derive from polyreactive B cells. In addition, the restricted usage of VH genes and CDR3 regions in these respective lymphoma types suggests antigen-driven selection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Peter Krajci for his critical advice concerning the antibody constructs used in this study. They also thank Agniezska Malecka for excellent technical assistance.
This study was supported by funds from the Norwegian Cancer Association.
Authorship
Contribution: A.A.W. designed part of the research, made the antibody constructs, performed immunohistochemical analyses and Western blots, cultured cells, and helped write the manuscript; H.-C.A. transfected cells, produced and purified the recombinant antibodies, performed Western blots and flowcytometric analyses, interpreted the data, and helped write the manuscript; K.N. designed and performed the enzyme-linked immunosorbent assays and interpreted the data; G.T. cultured cells, analyzed Ig gene sequences, and interpreted the data; A.T. designed part of the research, performed some of the flowcytometric analyses, and interpreted the data; V.W. performed some of the flowcytometric analyses, including the apoptosis tests, and interpreted the data; U.R. performed some of the flowcytometric analyses and interpreted the data; H.P.D. cultured and treated cells for one of the apoptosis tests and interpreted the data; S.H. performed cytogenetic analysis on the lymphomas and interpreted the results; A.B. performed immuno-electron microscopic analyses and interpreted the data; and J.D. conceived and supervised the study and helped write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Abdirashid A. Warsame, MSc, Department of Pathology, Oslo University Hospitals, The Norwegian Radium Hospital, Montebello N-0310, Oslo, Norway; e-mail: abdirashid.ali.warsame@radiumhospitalet.no.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal