Abstract
This is the first study to investigate the efficacy of intravenous iron in treating fatigue in nonanemic patients with low serum ferritin concentration. In a randomized, double-blinded, placebo-controlled study, 90 premenopausal women presenting with fatigue, serum ferritin ≤ 50 ng/mL, and hemoglobin ≥ 120 g/L were randomized to receive either 800 mg of intravenous iron (III)–hydroxide sucrose or intravenous placebo. Fatigue and serum iron status were assessed at baseline and after 6 and 12 weeks. Median fatigue at baseline was 4.5 (on a 0-10 scale). Fatigue decreased during the initial 6 weeks by 1.1 in the iron group compared with 0.7 in the placebo group (P = .07). Efficacy of iron was bound to depleted iron stores: In patients with baseline serum ferritin ≤ 15 ng/mL, fatigue decreased by 1.8 in the iron group compared with 0.4 in the placebo group (P = .005), and 82% of iron-treated compared with 47% of placebo-treated patients reported improved fatigue (P = .03). Drug-associated adverse events were observed in 21% of iron-treated patients and in 7% of placebo-treated patients (P = .05); none of these events was serious. Intravenous administration of iron improved fatigue in iron-deficient, nonanemic women with a good safety and tolerability profile. The efficacy of intravenous iron was bound to a serum ferritin concentration ≤ 15 ng/mL. This study was registered at the International Standard Randomized Controlled Trial Number Register (www.isrctn.org) as ISRCTN78430425.
Medscape EDUCATION Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 3450.
Disclosures
This study was funded by Vifor Pharma (Villars-sur-Glâne, Switzerland). The sponsor of the study was involved in the trial design and was responsible for data collection and storage. The authors had full access to all data and were responsible for the analysis and interpretation of the data presented in this publication. Christian Breymann is a consulting expert for Vifor Pharma in the field of obstetrics and gynecology. The remaining authors, the Associate Editor Martin S. Tallman, and the CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe the effects of intravenous iron on fatigue and on ferritin concentrations, on the basis of findings of a randomized controlled trial in nonanemic, premenopausal women.
Describe the adverse effects of intravenous iron, on the basis of findings of this randomized controlled trial in nonanemic, premenopausal women.
Describe clinical implications of these findings.
Release date: September 22, 2011; Expiration date: September 22, 2012
Introduction
Fatigue is a common symptom in general practice, affecting up to one-third of the population.1-5 Similarly, iron deficiency is a disorder affecting approximately one-fourth of menstruating women, as indicated by a French survey defining iron deficiency as serum ferritin concentrations below 15 ng/mL.6-8 Women are particularly at risk of developing either or both of these conditions.9,10
Several studies have suggested that iron deficiency can cause fatigue symptoms.11-13 Clinical studies indicate an association between the status of body iron stores and aerobic adaptation during exercise14-16 as well as with cognitive function.17,18 Recently, Anker et al demonstrated that intravenous iron improved clinical disease, functional capacity, and quality of life in iron-deficient, nonanemic patients with chronic heart failure.14
Verdon et al demonstrated that oral iron supplementation improved fatigue in a subgroup of premenopausal nonanemic women with iron deficiency.13 Beutler et al showed that women with normal hemoglobin concentration but depleted or reduced bone marrow iron stores experienced improvements in fatigue after oral iron therapy.11 However, oral iron administration has limitations: it is often accompanied by gastrointestinal side effects and only ∼ 10% of orally administered iron is absorbed by the body.6
Investigation of any therapeutic approach to reducing fatigue can be influenced by a strong placebo effect,19 making a placebo-controlled and blinded study design essential for drawing conclusions relevant for medical practice. Prior studies analyzing the effect of iron supplementation on fatigue improvement were performed with oral iron, which can result in an unintended unblinding of patients because of intestinal side effects and stool coloring and thus influence study results. This is the first study to investigate the efficacy of intravenous iron in the treatment of fatigue in nonanemic female patients with low serum ferritin concentration.
Methods
The FERRIM trial was conducted at 4 study centers in Switzerland (Policlinic of Internal Medicine, University Hospital Basel; Division of Internal Medicine, University Hospital Zurich; Gynaekologie & Geburtshilfe, Seefeld, Zurich; and Ambulatorium Wiesendamm, Basel) in accordance with the Declaration of Helsinki and the Guidelines on Good Clinical Practice. The protocol and all amendments were approved by the local ethics committees at the centers. All patients included in the study gave written informed consent. This study was registered at International Standard Randomized Controlled Trial Number Register (www.isrctn.org) as ISRCTN78430425.
Inclusion and exclusion criteria
Premenopausal, menstruating women ≥ 18 years of age who presented with fatigue were evaluated for inclusion in the study. Inclusion criteria were serum ferritin concentration ≤ 50 ng/mL, hemoglobin concentration ≥ 120 g/L, and adequate contraception for the study period. Exclusion criteria were pregnancy, intake of gestagens repressing menstruation, physical or mental disorders, medication affecting physical or mental performance, iron treatment in the 4 weeks before enrollment, and history of hypersensitivity to any iron medication. Patients were withdrawn from the study if serum ferritin concentration exceeded 800 ng/mL or if transferrin saturation exceeded 50% during the study period.20
Study design
The FERRIM trial was a randomized, double-blinded, placebo-controlled study. Patients were randomized to receive either a cumulative intravenous dose of 800 mg of iron as iron (III)–hydroxide sucrose or intravenous placebo for 2 weeks. The primary study objective was to determine the efficacy of iron compared with placebo in decreasing fatigue 6 weeks after treatment initiation. Secondary objectives were to determine the impact of iron supplementation on serum ferritin and hemoglobin concentration and to confirm the safety of intravenous iron sucrose. Patients were followed up for 12 weeks. Subgroups of the study population were analyzed to investigate the influence of the serum iron status at baseline on the efficacy of iron treatment.
Randomization and blinding
The randomization schedule was generated by Cardinal Health Germany GmbH (Schorndorf, Germany). In total, 172 randomization numbers were generated.
The control group received placebo (0.9% saline). It was ensured through organizational measures that neither the patient nor the investigator could become aware of whether the active group (dark-brown solution) or placebo (colorless solution) was administered. The study medication was prepared and administered by a staff member other than the investigator. Both the infusion bag and the injection site were covered and nontransparent tubing was used, ensuring that the patient could not see the infusion solution at any time. The investigator was not present during the infusion.
Study medication
Treatment was delivered for 4 days during the first 2 weeks of the study by a physician's assistant who injected either 4 infusions containing 200 mg of iron as iron (III)–hydroxide sucrose (Venofer; Vifor Pharma) in 200 mL of 0.9% saline or 4 infusions of 200 mL of 0.9% saline over a minimum period of 10 minutes each. The physician's assistant took all necessary precautions to ensure that the patient could not see nor draw any conclusion as to the nature of the solution administered. The work of the physician's assistant was done completely independently of the study physicians.
Fatigue scores
Fatigue was assessed at baseline, and after 6 and 12 weeks using the Brief Fatigue Inventory questionnaire (BFI).21,22 Patients were asked to rate the severity of fatigue on a scale from 0 (no fatigue) to 10 (maximum imaginable fatigue) by answering standardized questions. Three questions addressed the level of fatigue at its worst, its usual, and its current level during the last 24 hours; 6 questions were aimed at gauging the interference of fatigue with aspects of daily life, including general activity, mood, walking ability, work/housework, relations with other people, and enjoyment of life. According to the original publication validating the BFI, total fatigue score was obtained by calculating the mean of the resulting scores for these questions.21,22
In addition, change in fatigue was assessed 6 and 12 weeks after treatment initiation by the Short Performance Inventory (SPI) questionnaire. Patients were asked to categorize their current level of fatigue compared with the situation at baseline as improved (slightly better, much better, or completely resolved) or not improved.
Laboratory parameters
The concentrations of hemoglobin, iron, serum ferritin, transferrin, and C-reactive protein were measured at baseline and after 6 and 12 weeks. Levels of creatinine, alanine aminotransferase, aspartate aminotransferase, and thyroid-stimulating hormone were determined at baseline. Analyses were done in a central laboratory (Rothen Medizinische Laboratorien, Basel, Switzerland).
Adverse events
Adverse events were reported by the patients or assessed by the study physicians at each visit. Adverse events were classified as “serious” or “not serious” and as “drug-associated” or “not drug-associated.” All patients who received at least 1 dose of study medication were included in the analysis.
Statistical analysis
Results with parametric distribution are presented as mean values (± 1 SD) and results with nonparametric distribution as median values (quartiles Q1, Q3). Comparisons between the study groups were performed with the t test, the Mann-Whitney U test, or the χ2 test. Correlations were determined according to the method of Pearson. Intention-to-treat analysis included all patients randomized and patients treated at least once and with at least one post-baseline evaluation according to the study protocol. Missing values were imputed according to the baseline observation and the last observation carried forward principle.23 All tests were performed double-sided with a significance level of 5% (P = .05).
Results
Study population and baseline characteristics
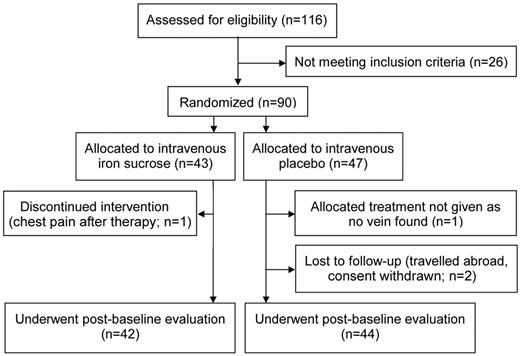
Of 116 premenopausal women presenting with fatigue, 90 were included in the study. Median fatigue score of the study population was 4.5 at baseline as assessed on a scale from 0-10 using the BFI questionnaire. The majority of study patients presented with fatigue levels previously defined as “low” or “moderate.”21,22 Of the 90 patients, 43 (48%) were randomized to receive intravenous iron (4 × 200 mg iron as iron [III]–hydroxide sucrose) and 47 (52%) to receive intravenous placebo (4 × 200 mL of 0.9% saline). Forty-two iron-treated patients and 44 placebo-treated patients underwent postbaseline evaluation (Figure 1).
At baseline, there was no significant difference between the iron and the placebo group with regard to fatigue (4.0 vs 4.7, P > .10), serum ferritin concentration (24 vs 20 ng/mL, P > .10), or transferrin saturation (20 vs 25%, P > .10). The study groups were also well randomized with regard to age, height, body weight, blood pressure, heart rate, and laboratory parameters (Figure 2 and Table 1).
Baseline characteristics
| . | Iron group . | Placebo group . | P . |
|---|---|---|---|
| Age, y | 31 ± 8 | 32 ± 7 | ns |
| Height, cm | 166 ± 6 | 166 ± 6 | ns |
| Body weight, kg | 60 ± 8 | 59 ± 7 | ns |
| Blood pressure systolic, mmHg | 117 ± 13 | 117 ± 13 | ns |
| Blood pressure diastolic, mmHg | 73 ± 8 | 72 ± 10 | ns |
| Heart rate, 1/min | 72 ± 10 | 71 ± 9 | ns |
| Hemoglobin, g/L | 133 ± 6 | 133 ± 7 | ns |
| Mean red blood cell volume, fl | 90 ± 5 | 90 ± 5 | ns |
| Serum ferritin concentration, ng/mL | 24 (10, 32) | 20 (14, 28) | ns |
| Transferrin saturation, % | 20 (14, 30) | 25 (16, 32) | ns |
| Creatinine, μmol/L | 77 (72, 82) | 78 (72, 84) | ns |
| Alanine aminotransferase, U/L | 17 (13, 21) | 17 (15, 23) | ns |
| Aspartate aminotransferase, U/L | 20 (17, 26) | 21 (18, 24) | ns |
| Thyroid-stimulating hormone, mU/L | 1.5 (1.0, 2.0) | 1.4 (0.8, 2.0) | ns |
| C-reactive protein, mg/L | 1.1 (1.1, 1.1) | 1.1 (1.1, 1.1) | ns |
| . | Iron group . | Placebo group . | P . |
|---|---|---|---|
| Age, y | 31 ± 8 | 32 ± 7 | ns |
| Height, cm | 166 ± 6 | 166 ± 6 | ns |
| Body weight, kg | 60 ± 8 | 59 ± 7 | ns |
| Blood pressure systolic, mmHg | 117 ± 13 | 117 ± 13 | ns |
| Blood pressure diastolic, mmHg | 73 ± 8 | 72 ± 10 | ns |
| Heart rate, 1/min | 72 ± 10 | 71 ± 9 | ns |
| Hemoglobin, g/L | 133 ± 6 | 133 ± 7 | ns |
| Mean red blood cell volume, fl | 90 ± 5 | 90 ± 5 | ns |
| Serum ferritin concentration, ng/mL | 24 (10, 32) | 20 (14, 28) | ns |
| Transferrin saturation, % | 20 (14, 30) | 25 (16, 32) | ns |
| Creatinine, μmol/L | 77 (72, 82) | 78 (72, 84) | ns |
| Alanine aminotransferase, U/L | 17 (13, 21) | 17 (15, 23) | ns |
| Aspartate aminotransferase, U/L | 20 (17, 26) | 21 (18, 24) | ns |
| Thyroid-stimulating hormone, mU/L | 1.5 (1.0, 2.0) | 1.4 (0.8, 2.0) | ns |
| C-reactive protein, mg/L | 1.1 (1.1, 1.1) | 1.1 (1.1, 1.1) | ns |
Results are presented as means ± 1 SD or as median values (quartiles Q1, Q3).
P values were calculated by t test or the Mann-Whitney U test.
ns indicates not significant and no trend (P > .10).
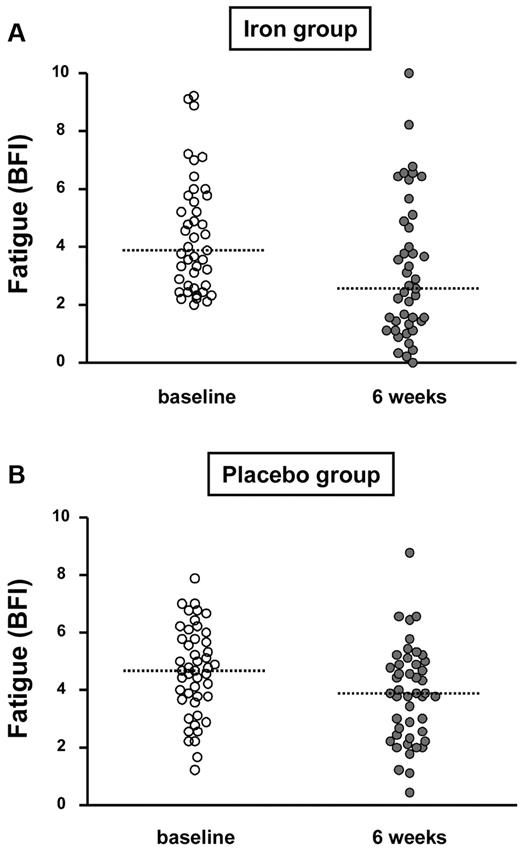
Fatigue at baseline and after 6 weeks in the iron-treated and the placebo-treated group (dotted lines correspond to medians).
Fatigue at baseline and after 6 weeks in the iron-treated and the placebo-treated group (dotted lines correspond to medians).
Change in fatigue after 6 weeks
Fatigue decreased in both study groups during the first 6 weeks after treatment initiation as assessed by the BFI score (Figure 2). After 6 weeks, median fatigue decreased by 1.1 in the iron group and by 0.7 in the placebo group (P = .07; Table 2).
Change of fatigue and laboratory parameters 6 weeks after treatment initiation with intravenous iron or intravenous placebo
| . | Iron group . | Placebo group . | P . |
|---|---|---|---|
| Change in fatigue (BFI) | |||
| n = 90, ITT all patients randomized | −1.1 (−2.2, −0.3) | −0.7 (−1.3, 0.0) | .07 |
| n = 86, ITT according to protocol* | −1.1 (−2.2, −0.4) | −0.8 (−1.4, 0.0) | .08 |
| Fatigue improved(SPI), n (%) | 28/43 (65%) | 19/47 (40%) | .02 |
| Fatigue slightly better | 12 (28%) | 9 (19%) | |
| Fatigue much better | 12 (28%) | 6 (13%) | |
| Fatigue completely resolved | 4 (9%) | 4 (8%) | |
| Change in laboratory parameters | |||
| Serum ferritin concentration, ng/mL | 98 (74, 113) | 1 (−7, 5) | < .001 |
| Transferrin saturation, % | 9 (0, 23) | 2 (−5, 9) | .006 |
| Haemoglobin, g/L | 1 ± 7 | 0 ± 6 | ns |
| C-reactive protein, mg/L | 0 (0, 0) | 0 (0, 0) | ns |
| . | Iron group . | Placebo group . | P . |
|---|---|---|---|
| Change in fatigue (BFI) | |||
| n = 90, ITT all patients randomized | −1.1 (−2.2, −0.3) | −0.7 (−1.3, 0.0) | .07 |
| n = 86, ITT according to protocol* | −1.1 (−2.2, −0.4) | −0.8 (−1.4, 0.0) | .08 |
| Fatigue improved(SPI), n (%) | 28/43 (65%) | 19/47 (40%) | .02 |
| Fatigue slightly better | 12 (28%) | 9 (19%) | |
| Fatigue much better | 12 (28%) | 6 (13%) | |
| Fatigue completely resolved | 4 (9%) | 4 (8%) | |
| Change in laboratory parameters | |||
| Serum ferritin concentration, ng/mL | 98 (74, 113) | 1 (−7, 5) | < .001 |
| Transferrin saturation, % | 9 (0, 23) | 2 (−5, 9) | .006 |
| Haemoglobin, g/L | 1 ± 7 | 0 ± 6 | ns |
| C-reactive protein, mg/L | 0 (0, 0) | 0 (0, 0) | ns |
Results are presented as means ± 1 SD, median values (quartiles Q1, Q3), or number of patients (%). P values were calculated with the t test, the Mann Whitney U test, or the χ2 test.
ns indicates not significant and no trend (P > .10); and ITT, intention-to-treat analysis.
Analysis included all patients treated at least once and with at least 1 postbaseline evaluation.
Improvement in fatigue 6 weeks after the start of treatment, as evaluated by the SPI, was reported by 65% of iron-treated and 40% of placebo-treated patients (P = .02; Table 2). The most prominent difference between the 2 study groups was observed in those patients who judged their fatigue to be “much better” after 6 weeks of treatment. These patients comprised 28% of the iron group but only 13% of the placebo group (Table 2).
Change in iron status after 6 weeks
In iron-treated patients, a significant increase in serum ferritin concentration (98 ng/mL vs 1 ng/mL; P < .001) and transferrin saturation (9% vs 2%; P = .006) was observed during the 6 weeks after treatment initiation compared with placebo-treated patients (Table 2). In contrast, hemoglobin levels remained normal and constant in both study groups during this period. As an inflammation marker, C-reactive protein remained stable in both groups throughout the study period.
Efficacy of iron treatment in subgroups
The World Health Organization (WHO) has defined a cutoff serum ferritin concentration of 15 ng/mL to identify iron deficiency.24 Therefore, in an additional analysis, the WHO criterion was applied to the present study. The efficacy of iron was evaluated in the subgroups of patients with serum ferritin concentration ≤ 15 ng/mL (n = 34) and > 15 ng/mL (n = 56) at baseline. Further analysis was performed in patients with transferrin saturation ≤ 20% and > 20% at baseline.
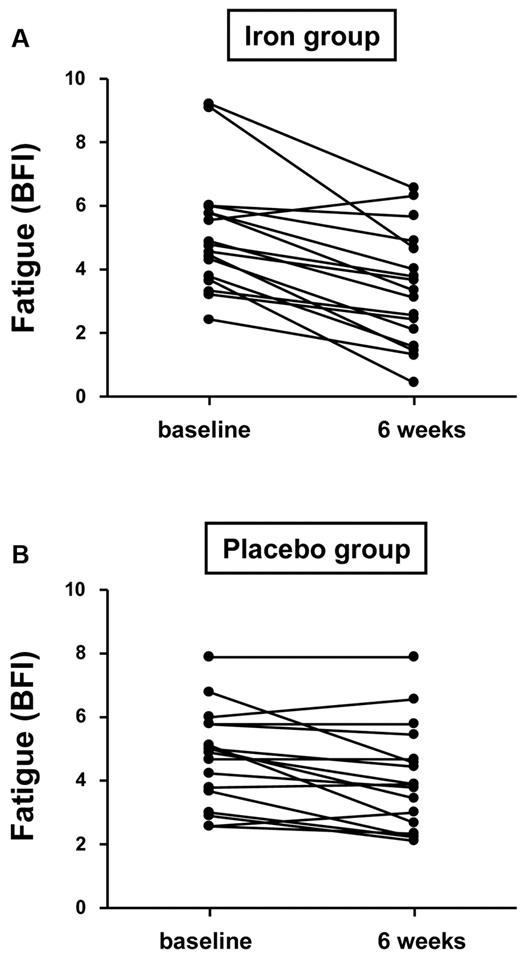
Among patients with a baseline serum ferritin concentration ≤ 15 ng/mL (n = 34), median fatigue according to the BFI score decreased by 1.8 in the iron group compared with 0.4 in the placebo group (P = .005; Figure 3 and Table 3). An improvement in fatigue, as assessed by the SPI, was reported by 82% of iron-treated patients compared with 47% of placebo-treated patients (P = .03; Table 3). No significant differences between the study groups in BFI or SPI score at 6 weeks were observed in patients with baseline serum ferritin > 15 ng/mL (Table 3).
Change of fatigue in patients with baseline ferritin ≤ 15 ng/mL depending on the administration of iron or placebo (P = .005).
Change of fatigue in patients with baseline ferritin ≤ 15 ng/mL depending on the administration of iron or placebo (P = .005).
Change in fatigue 6 weeks after treatment initiation with intravenous iron or intravenous placebo in dependence of the serum ferritin concentration at baseline
| . | Iron group . | Placebo group . | P . |
|---|---|---|---|
| Baseline ferritin ≤ 15 ng/mL (n = 34) | |||
| Change of fatigue (BFI), median | −1.8 (−2.5, −0.8) | −0.4 (−1.2, 0.0) | .005 |
| Fatigue improved (SPI), n (%) | 14/17 (82%) | 8/17 (47%) | .03 |
| Baseline ferritin > 15 ng/mL (n = 56) | |||
| Change of fatigue (BFI), median | −0.7 (−1.8, 0.0) | −0.8 (−1.4, 0.0) | ns |
| Fatigue improved (SPI), n (%) | 14/26 (54%) | 11/30 (37%) | ns |
| . | Iron group . | Placebo group . | P . |
|---|---|---|---|
| Baseline ferritin ≤ 15 ng/mL (n = 34) | |||
| Change of fatigue (BFI), median | −1.8 (−2.5, −0.8) | −0.4 (−1.2, 0.0) | .005 |
| Fatigue improved (SPI), n (%) | 14/17 (82%) | 8/17 (47%) | .03 |
| Baseline ferritin > 15 ng/mL (n = 56) | |||
| Change of fatigue (BFI), median | −0.7 (−1.8, 0.0) | −0.8 (−1.4, 0.0) | ns |
| Fatigue improved (SPI), n (%) | 14/26 (54%) | 11/30 (37%) | ns |
Results are presented as median values (quartiles Q1, Q3) or number of patients (%). P values were calculated with the Mann Whitney U test or the χ2 test.
ns indicates not significant and no trend (P > .10).
Similarly, in patients with transferrin saturation ≤ 20% at baseline, administration of iron resulted in a significantly greater reduction in fatigue than administration of placebo (BFI, P = .01; SPI, P = .002). However, patients with transferrin saturation > 20% experienced no significant reduction in fatigue (both questionnaires, P > .10).
Follow-up after 12 weeks
The decrease in fatigue (BFI) between baseline and 12 weeks was 1.3 in the iron group and 0.9 in the placebo group (P > .1), and improvement in fatigue (SPI) was reported by 63% of iron-treated patients and by 34% of placebo-treated patients (P = .006). Improvements in serum ferritin concentration and transferrin saturation in iron-treated compared with placebo-treated patients remained significant after 12 weeks (P < .001 and P = .006, respectively; Table 4).
Change in fatigue and serum iron status 12 weeks after treatment initiation with intravenous iron or intravenous placebo
| . | Iron group . | Placebo group . | P . |
|---|---|---|---|
| Total study population (n = 90) | |||
| Change of fatigue (BFI), median | −1.3 (−2.4, −0.5) | −0.9 (−2.2, 0.0) | ns |
| Fatigue improved (SPI), n (%) | 27/43 (63%) | 16/47 (34%) | .006 |
| Change of ferritin concentration, ng/mL | 81 (49, 100) | −1 (−7, 4) | < .001 |
| Change of transferrin saturation, % | 7 (0, 17) | 1 (5, 6) | .006 |
| Population with baseline ferritin ≤ 15 ng/mL (n = 34) | |||
| Change of fatigue (BFI), median | −2.3 (−3.2, 0.9) | −0.7 (−1.3, 0.1) | .03 |
| Fatigue improved (SPI), n (%) | 14/17 (82%) | 6/17 (35%) | .005 |
| . | Iron group . | Placebo group . | P . |
|---|---|---|---|
| Total study population (n = 90) | |||
| Change of fatigue (BFI), median | −1.3 (−2.4, −0.5) | −0.9 (−2.2, 0.0) | ns |
| Fatigue improved (SPI), n (%) | 27/43 (63%) | 16/47 (34%) | .006 |
| Change of ferritin concentration, ng/mL | 81 (49, 100) | −1 (−7, 4) | < .001 |
| Change of transferrin saturation, % | 7 (0, 17) | 1 (5, 6) | .006 |
| Population with baseline ferritin ≤ 15 ng/mL (n = 34) | |||
| Change of fatigue (BFI), median | −2.3 (−3.2, 0.9) | −0.7 (−1.3, 0.1) | .03 |
| Fatigue improved (SPI), n (%) | 14/17 (82%) | 6/17 (35%) | .005 |
Results are presented as median values (quartiles Q1, Q3) or number of patients (%). P values were calculated with the Mann Whitney U test or the χ2 test.
ns indicates not significant and no trend (P > .10).
Among patients with a baseline serum ferritin concentration ≤ 15 ng/mL, the median decrease in fatigue score between baseline and 12 weeks was 2.3 and 0.7 in the iron and the placebo groups, respectively (P = .03). Improvement in fatigue was reported by 82% of iron-treated patients and by 35% of placebo-treated patients (P = .005; Table 4).
Adverse events
Evaluation of adverse events was performed throughout the study period and included 89 patients who each received at least one dose of study medication. The study groups did not differ significantly in the number of patients reporting adverse events or in the number of adverse events per patient (Table 5). One serious adverse event was observed in the iron group (appendicitis) and one in the placebo group (traffic accident). Neither was classified as being drug associated.
Adverse events
| . | Iron group . | Placebo group . | P . |
|---|---|---|---|
| All adverse events | |||
| Number of patients reporting events, n (%) | 23 (53%) | 31 (66%) | ns |
| Total number of adverse events, n | 52 | 53 | ns |
| Number of serious adverse events, n | 1 | 1 | |
| Appendicitis | 1 | 0 | |
| Traffic accident | 0 | 1 | |
| Drug-associated adverse events | |||
| Number of patients reporting events, n | 9 (21%) | 3 (7%) | .05 |
| Total number of adverse events, n | 17 | 4 | ns |
| Nausea | 6 | 1 | |
| Chills | 4 | 0 | |
| Headache | 3 | 1 | |
| Dizziness | 1 | 1 | |
| Chest pain | 1 | 0 | |
| Dysaesthesia | 1 | 0 | |
| Dysgeusia | 1 | 0 | |
| Diarrhea | 0 | 1 |
| . | Iron group . | Placebo group . | P . |
|---|---|---|---|
| All adverse events | |||
| Number of patients reporting events, n (%) | 23 (53%) | 31 (66%) | ns |
| Total number of adverse events, n | 52 | 53 | ns |
| Number of serious adverse events, n | 1 | 1 | |
| Appendicitis | 1 | 0 | |
| Traffic accident | 0 | 1 | |
| Drug-associated adverse events | |||
| Number of patients reporting events, n | 9 (21%) | 3 (7%) | .05 |
| Total number of adverse events, n | 17 | 4 | ns |
| Nausea | 6 | 1 | |
| Chills | 4 | 0 | |
| Headache | 3 | 1 | |
| Dizziness | 1 | 1 | |
| Chest pain | 1 | 0 | |
| Dysaesthesia | 1 | 0 | |
| Dysgeusia | 1 | 0 | |
| Diarrhea | 0 | 1 |
P values were calculated with the χ2 test or the Mann Whitney U test (number of events per patient).
ns indicates not significant and no trend (P > .10).
Seventeen drug-associated adverse events were reported among 9 patients treated with intravenous iron, whereas 4 drug-associated adverse events were reported among 3 placebo-treated patients (Table 5). The difference between the study groups was significant regarding the number of patients reporting drug-associated adverse events (P = .05). However, none of these drug-associated events was considered serious—the most frequently observed were nausea, chills, and headache (Table 5). The occurrence of these drug-associated adverse events was limited to the period of drug administration.
Discussion
This randomized, double-blinded, placebo-controlled study investigated for the first time the efficacy and safety of intravenous iron therapy in the treatment of fatigue in premenopausal nonanemic (hemoglobin ≥ 120 g/L) women with low serum ferritin concentration ( ≤ 50 ng/mL). A significant effect of iron (compared with placebo) on fatigue was observed exclusively in patients with substantially depleted iron stores, as indicated by a serum ferritin concentration ≤ 15 ng/mL at baseline. More than 80% of these patients reported improved fatigue 6 and 12 weeks after treatment initiation, as well as decreases in the severity of fatigue to less than half of the initial value at study completion. These are the first results providing evidence that intravenous supplementation of iron can improve fatigue symptoms in iron-deficient, nonanemic premenopausal women.
In the present study, serum ferritin concentration ≤ 15 ng/mL or transferrin saturation ≤ 20% (with serum ferritin concentration ≤ 50 ng/mL) were predictive for a significant benefit from intravenous iron therapy. Of these criteria, serum ferritin concentration ≤ 15 ng/mL is probably more suitable for use in general practice. However, the size of the study population does not allow definitive determination of a cutoff serum ferritin concentration below which patients benefit from iron therapy.
A total dose of 800 mg of intravenous iron administered over 2 weeks resulted in a marked increase in serum ferritin concentration (98 ng/mL), which indicated sufficient replenishment of body iron stores. Iron administration, however, did not influence hemoglobin concentration, which was in the normal range at baseline and remained constant during the observation period in both iron-treated and placebo-treated patients. Therefore, the fatigue-reducing effects of iron therapy reflect the nonhematological functions of iron. Iron is an essential component of a large number of human metabolic enzymes, including ribonucleotide reductase, NADH dehydrogenase, succinate dehydrogenase, and cytochrome c reductase/oxidase. These enzymes catalyze essential biochemical reactions such as the formation of deoxyribonucleotides and aerobic oxidation of carbohydrates and fatty acids in the mitochondrial citric acid cycle and the respiratory chain.25-27
The results of this study are in agreement with previous studies indicating a beneficial effect of oral iron therapy in comparable patient groups.11-13,18 Oral iron treatment, however, can be accompanied by gastrointestinal side effects and usually requires administration over several months because intestinal iron absorption is low6 ; both of these factors affect patients' adherence to therapy. In contrast, intravenous iron can efficaciously replenish iron stores with a good safety and tolerability profile.14 In the present study, drug-associated adverse events were observed more frequently in iron-treated (21%) than placebo-treated (7%) patients. However, these events (mainly nausea, chills, and headache) were not serious and occurrence was limited to the period of administration.
Improvement in fatigue after 6 weeks was reported by 40% of placebo-treated patients. Further evaluation of this strong placebo effect showed a significant correlation between baseline fatigue and decrease in fatigue (r = 0.38, P = .009): patients with high initial fatigue showed a strong response to placebo. This highlights the importance of the emotional component of fatigue in patients who considered themselves to be severely fatigued.21,22 This observation may be accurate for the iron-deficient, otherwise healthy study population but not necessarily for other populations, such as patients suffering from fatigue as a result of cancer.
It is always recommended to carefully search for somatic, psychological, and/or social causes of fatigue. Even if a low serum ferritin value is found, a serious cause of iron deficiency such as gastrointestinal bleeding, malabsorption syndrome, or gynecological diseases must be considered even in premenopausal women.
In conclusion, in the present study, intravenous administration of 800 mg of iron improved fatigue in iron-deficient, nonanemic women with a good safety and tolerability profile. Response to iron was bound to the degree of depletion of iron stores, as indicated by a serum ferritin concentration ≤ 15 ng/mL before treatment. Investigation in a larger population is needed to confirm the conditions under which patients benefit from iron therapy.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Beat Schaub for his invaluable assistance in the development of this study and of the SPI questionnaire and Archimed Medical Communication AG (Zofingen, Switzerland) for the drafting of this manuscript, which was funded by Vifor Pharma and done under the direct guidance of the authors.
This study was sponsored by Vifor Pharma (Villars-sur-Glâne, Switzerland).
Authorship
Contribution: P.-A.K., E.B., C.B., J.F., and G.S. planned and initiated the study; P.-A.K. and C.B. recruited the patients; P.-A.K., E.B., and G.S. analyzed the data; P.-A.K. and G.S. wrote the manuscript; and all authors provided critical revision of the manuscript.
Conflict-of-interest disclosure: This study was funded by Vifor Pharma (Villars-sur-Glâne, Switzerland). The sponsor of the study was involved in the trial design and was responsible for data collection and storage. The authors had full access to all data and were responsible for the analysis and interpretation of the data presented in this publication. C.B. is a consulting expert for Vifor Pharma in the field of obstetrics and gynecology. The remaining authors declare no competing financial interests.
Correspondence: Dr P.-A. Krayenbuehl, Division of Internal Medicine, University Hospital, CH-8091 Zurich, Switzerland; e-mail: pierrea.krayenbuehl@usz.ch.