Abstract
Despite increasing knowledge on the mechanisms of invariant natural killer T (iNKT)–cell development in the thymus, the function of recent thymic emigrant (RTE) iNKT cells remains largely unexplored, principally because of a lack of bona fide markers to distinguish RTE from long-lived iNKT cells. Whether the recently described IL-17–producing iNKT cell subset is part of RTE has notably not been addressed. In the present study, we show that neuropilin-1 (Nrp-1), a transmembrane receptor mainly found on T-regulatory (Treg) cells in the murine immune system, is specifically expressed on RTE iNKT cells in naive mice. We used the Nrp-1 marker to discriminate RTE from mature iNKT cells and compare their functions. We show that RTE iNKT cells proliferate more than mature iNKT cells after in vitro activation; that, unlike mature iNKT cells, most RTE iNKT cells fail to rapidly produce IFN-γ and IL-4 after in vivo activation; and, most importantly, that IL-17–producing iNKT cells in lymphoid organs of naive mice are contained within the RTE iNKT cell pool. Our results establish an accurate marker of RTE iNKT cells and reveal that continuous thymic output is required for pro-inflammatory IL-17 secretion, a key function of adult iNKT cells.
Introduction
Invariant natural killer T (iNKT) cells are innate-like T lymphocytes expressing a semi-invariant TCR that recognizes endogenous and exogenous glycolipid antigens presented by the MHC class I-like molecule CD1d.1,2 Most iNKT cells are characterized by an activated memory phenotype (CD44high, CD69+), the expression of NK cell markers (CD122, NK1.1), and the rapid secretion of both IFN-γ and IL-4 after stimulation with antigens such as α-galactosylceramide (α-GalCer).3 Invariant NKT cells develop in the thymus, where double-positive (DP) thymocytes expressing the canonical Vα14Jα18 TCR rearrangement are positively selected by CD1d-expressing DP cells.4-6 Selected iNKT cells undergo several steps of maturation characterized by distinct phenotypes and functions: stage I (NK1.1− CD44low) and stage II (NK1.1− CD44high) iNKT cells proliferate intensely and secrete mostly IL-4, whereas stage III (NK1.1+ CD44high) iNKT cells are resting, secrete both IL-4 and IFN-γ, and are long-term thymus residents.7-10 Thymic export of iNKT cells to the periphery occurs at stage II and most of the exported cells quickly mature into NK1.1+ iNKT cells.7,9 However, some iNKT cells remain NK1.1− late after thymic export.11
Recently, NK1.1− iNKT cells have been shown to be a potent source of the pro-inflammatory cytokine IL-17.12-16 IL-17–producing iNKT cells are therefore part of a broad family of innate IL-17 producers that constitute an efficient first line of defense in the response to cellular stress, tissue injury, and pathogens.17 Some aspects of IL-17–producing iNKT-cell development have been unraveled,18 but whether these cells are part of the recent thymic emigrant (RTE) or of the long-lived NK1.1− iNKT cell subsets has remained controversial. The controversy partially stems from the current unavailability of surface markers with which to accurately discriminate RTE from mature iNKT cells and analyze their functions separately.
Neuropilin-1 (Nrp-1) is a transmembrane receptor for class 3 semaphorins19,20 and vascular endothelium growth factor isoforms.21 It is expressed in a wide range of tissues and mediates diverse cellular functions such as migration, adhesion, proliferation, and apoptosis.22,23 In the murine immune system, Nrp-1 is a marker of Foxp3+ T-regulatory (Treg) cells24 and is involved in their suppressive activity through homotypic interactions with Nrp-1 at the surface of dendritic cells25 and activation of latent TGF-β1.26 Nrp-1 is also expressed on immature thymocytes, but its function in these cells is less well characterized.27,28 The expression of Nrp-1 in other murine T-cell subsets has not been investigated to date.
In the present study, we identified a population of Nrp-1+ iNKT cells present in the thymus and peripheral lymphoid organs of naive mice. By in situ labeling of thymocytes and adult thymectomy, we showed that Nrp-1+ iNKT cells correspond to RTE iNKT cells. Using the Nrp-1 marker, we compared the functions of RTE and mature iNKT cells in vivo and in vitro. Most importantly, RTE iNKT cells in lymphoid organs comprised the pro-inflammatory IL-17–producing subset. We conclude from these experiments that continuous export of RTE iNKT cells makes a substantial contribution to innate IL-17 producers in lymphoid organs of adult mice.
Methods
Mice
C57BL/6 mice were purchased from Janvier. Mice were kept in specific pathogen-free conditions at our animal facility, and experimental studies were performed in accordance with the French Institutional Committee.
Cell preparation
Single-cell suspensions of spleen, thymus, and peripheral lymph nodes (pLNs; pooled inguinal, brachial, and axillary lymph nodes) were obtained by mechanical disruption and passing through a 40-μm cell strainer. Liver mononuclear cells were obtained by mechanical disruption, followed by centrifugation in a 35% Percoll (Sigma-Aldrich) solution. Red blood cells were removed from spleen and liver cell suspensions by hypotonic shock with ammonium chloride buffer. Viable cells were counted on a hemacytometer using Trypan blue exclusion, and the absolute numbers of lymphocyte subsets were calculated after FACS analysis.
Flow cytometry
Cell surface staining was performed in PBS buffer containing 2% (vol/vol) FCS and 0.01% (wt/vol) NaN3 on ice (FACS buffer). For intracellular cytokine staining, surface-labeled cells were fixed for 5 minutes in 4% (wt/vol) paraformaldehyde (in PBS) at room temperature, and stained for 30 minutes at room temperature in FACS buffer supplemented with 0.5% (wt/vol) saponin (Sigma-Aldrich). Nuclear Ki67 staining was done using the fixation and permeabilization reagents from a Foxp3 staining kit (eBioscience) following the manufacturer's instructions. BrdU incorporation was analyzed using the BrdU flow kit from BD Biosciences following the manufacturer's instructions.
Before cell-surface staining, surface Fc receptors were saturated by incubation with ascites-purified antibody to CD16/CD32 (2.4G2) for 10 minutes on ice. The following antibodies were used: Alexa Fluor 488 anti-Ki67 (B56), Pacific blue anti-CD3ϵ (500A2), PerCP and Pacific blue anti-CD4 (RM4-5), FITC and PerCP-Cy5.5 anti-CD69 (H1.2F3), PE anti-NK1.1 (PK136), PerCP-Cy5.5 anti-CD19 (6D5), FITC anti-TCRβ (H57-597), PerCP-Cy5.5 anti-CD25 (PC61), PE anti–GM-CSF (MP1-22E9; all purchased from BD Biosciences), and Pacific blue anti–IFN-γ (XMG1.2), PE-Cy7 anti–IL-4 (BVD6-24G2), PE anti–IL-17 (eBio 17B7), PE-Cy7 anti-CD44 (IM7), PerCP-Cy5.5 anti-NK1.1 (PK136), allophycocyanin–Alexa Fluor 750 anti-TCRβ (H57-597), PE anti–IL-13 (eBio13A), PerCP-eFluor710 anti–TNF-α (MP6-XT22), purchased from eBioscience. The empty and PBS57-loaded allophycocyanin- and PE-labeled mouse CD1d tetramers were obtained from the National Institutes of Health Tetramer Core Facility. The following antibodies were used for Nrp-1 expression analysis: polyclonal goat anti–rat Nrp-1 (AF566; R&D Systems); FITC, PE, and PE-Cy7 donkey anti–goat IgG (Santa Cruz Biotechnology); and Alexa Fluor 647 donkey anti–goat IgG (Invitrogen).
Data were acquired on a FACSCanto II flow cytometer (BD Biosciences) using FACSDiva Version 6.1 software (BD Biosciences) and were analyzed with FlowJo Version 7.6 software (TreeStar).
iNKT cell enrichment and cell sorting
Where indicated, cell suspensions were enriched in iNKT cells by depletion using antibodies directed against CD8α (53-6.7), CD62L (MEL-14), and CD19 (6D5), all purchased from BioLegend, and magnetic beads coupled to anti–rat IgG antibodies, purchased either from Invitrogen or from Ademtech, and following the manufacturer's instructions. For the enrichment of thymocyte suspensions, only anti-CD8α antibody was used for depletion.
For cell sorting, iNKT cell–enriched spleen cell suspensions were stained with PE CD1d Tet, FITC anti-TCRβ, and anti–Nrp-1, followed by Alexa Fluor 647 anti–goat IgG. Cells were then sorted on a FACSAria II (BD Biosciences) cell sorter.
BrdU treatment
Mice were injected IP with 2 mg of BrdU (Sigma-Aldrich) in 200 μL of PBS and killed 1 hour later for the analysis of proliferating thymocytes.
Cell culture and in vitro activation of iNKT cells
Total spleen cells (50 × 106 cells/mL) were labeled with 5μM CFSE (Sigma-Aldrich) in PBS for 10 minutes at 37°C, and rinsed twice with cold PBS with 2% FCS. CFSE-labeled splenocytes (2 × 106 cells/mL/well) were then cultured for 3 days in 24-well plates in lymphocyte culture medium (RPMI 1640, 10% [vol/vol] FCS, 10mM HEPES, 50μM 2-ME, 2mM L-glutamine, 100 U/mL of penicillin, and 100 μg/mL of streptomycin). Recombinant human IL-7 and IL-15 (R&D Systems) were used at 20 ng/mL. α-GalCer (Alexis Biochemicals) was used at 100 ng/mL. FACS-sorted Nrp-1− iNKT cells (2 × 104) were activated by anti-CD3ϵ (clone 145-2C11, generously provided by J. Diana, Inserm U561, Paris, France) and anti-CD28 (clone 37.51; BioLegend) antibodies that had been previously coated at 5 μg/mL in 50 μL of PBS for 2 hours at 37°C.
For functional assays, sorted Nrp-1− and Nrp-1+ iNKT cells (2 × 104) were activated by irradiated (30 Gy) spleen cells (1 × 105) and 100 ng/mL of α-GalCer for 3 days. Total spleen cells (5 × 105) from sham-thymectomized and thymectomized animals were cultured in the presence of 100 ng/mL of α-GalCer for 48 hours. Culture supernatants were collected and stored at −80°C until analysis. To determine cell proliferation, [3H]-thymidine (1 μCi/well; Amersham) was added for the last 12 hours of culture and the cell-associated radioactivity was read by a microscintillation β counter (Wallac).
To quantify and phenotype cytokine-producing iNKT cells in vitro, iNKT-enriched cell suspensions (5 × 105) were cultured in lymphocyte culture medium supplemented with 10nM phorbol 12-myristate 13-acetate (PMA), 1μM ionomycin, and 5 μg/mL of brefeldin A (all from Sigma-Aldrich) for 3 hours. Cells were then recovered and washed once in PBS plus 2% FCS before staining.
All cell cultures were at 37°C in a humidified atmosphere containing 5% CO2.
Quantification of cytokine secretion
IFN-γ and IL-4 concentrations in culture supernatants were measured by flow cytometry using the CBA Mouse Th1/Th2 Cytokine Kit (BD Biosciences) or by ELISA (R&D Systems) following the manufacturer's instructions. IL-17 concentration was measured by ELISA (R&D Systems), as described previously.16
In vivo activation of iNKT cells
For long-term activation of iNKT cells, mice were injected IP with 2 μg of α-GalCer in 200 μL of PBS. For short-term activation of iNKT cells and intracellular cytokine staining, mice were injected IP with 250 μg of brefeldin A in 200 μL of PBS (unless otherwise stated), followed 30 minutes later by IP injection of 2 μg of α-GalCer in 200 μL of PBS. Mice were killed after 2 hours for spleen cell recovery and analysis.
Intrathymic FITC injection
Five-week-old mice were deeply anesthetized by IP injection of a mixture of ketamine (10 mg/kg) and xylazine (1 mg/kg) in 200 μL of PBS. The shaved skin above the rib cage was cut open with fine scissors and the rib cage was opened with a 5-mm-long incision of the sternum to gain access to the thymus. Both thymic lobes were injected with 10 μL of a 2 mg/mL FITC (Sigma-Aldrich) solution in PBS. The wound was closed by sewing the 2 flaps of skin together with 2-3 suture points, and the animals were left to recover on their flanks in a heated cage. Control animals were left untreated.
Adult mouse thymectomy
Eight-week-old mice were deeply anesthetized and their rib cages opened as described in “Intrathymic FITC injection.” The 2 thymic lobes were then carefully removed with an aspirator. The wound was closed and the animals were left to recover. Control (sham-thymectomized) animals were anesthetized and had their rib cages opened. After 4 weeks, the animals were killed for analysis. At that point, the completeness of thymectomy was checked and mice with thymus remnants were excluded from the study.
Statistical analyses
Data are expressed as means ± SEM and were compared using the statistical tests indicated in the figure legends using Prism 5.0 software (GraphPad). P < .05 was considered significant.
Results
Nrp-1 is expressed by immature iNKT cells
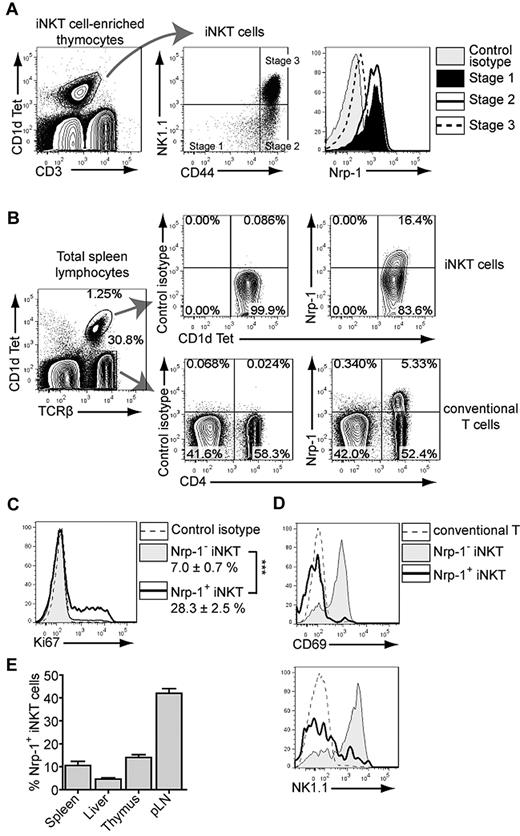
In the thymus, Nrp-1 is not expressed in mature single-positive cells except for Foxp3+ Treg precursors.27-29 However, among immature cortical double-negative (DN) and DP thymocytes, Nrp-1 is highly expressed on stages corresponding to the β-selection and pre-TCR-mediated expansion phases.28 Accordingly, in short-pulse BrdU experiments, we found that blastic proliferating (FSChigh BrdU+) thymocytes all expressed high levels of Nrp-1 compared with smaller nonproliferating cells, which expressed low levels of Nrp-1 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The iNKT cell lineage diverges from conventional αβ T cells at the DP stage.5,30,31 During their positive selection, Vα14+ DP cells receive signals resulting from cognate TCR engagement and signaling lymphocytic activating molecule (Slam)/Slam homotypic interactions,32 which initiate a proliferation wave mainly affecting stage I and stage II iNKT precursors33 but not long-term resident stage III iNKT cells.8 We found Nrp-1 to be highly expressed in stage I and stage II, but only dimly on stage III thymic iNKT cells (Figure 1A). Therefore, in non-Treg thymocytes, Nrp-1 expression was restricted to immature proliferating cells including NK1.1− iNKT cells.
Nrp-1 is expressed in immature thymic and peripheral iNKT cells in naive mice. (A) Nrp-1 expression in thymic iNKT cells. Thymocytes from 7-week-old mice were enriched in iNKT cells by depletion of CD8+ cells, and the resulting cell suspensions were stained for FACS analysis of Nrp-1 expression in thymic iNKT-cell stages. iNKT cells were defined as CD3int CD1d Tet+ (left dot plot) and stage I (CD44low NK1.1−), stage II (CD44highNK1.1−), and stage III (CD44high NK1.1+) iNKT cells were gated as shown on the middle dot plot. The right histogram plot is representative of Nrp-1 expression in stage I (full black histogram), stage II (thick black line), and stage III (dotted line) iNKT cells overlaid on control isotype staining of total iNKT cells (shaded gray histogram). (B) Representative FACS analysis of Nrp-1 expression in splenic TCRβ+ CD1d Tet+ iNKT cells and TCRβ+ CD1d Tet− conventional T cells of 8-week-old naive mice. Note the subset of iNKT cells expressing Nrp-1 at the same levels as Treg cells (CD4+ Nrp-1+ conventional T cells). (C) Intranuclear staining for the proliferation-associated marker Ki67 in splenic Nrp-1− (shaded gray) and Nrp-1+ (thick black line) iNKT cells overlaid on control isotype staining (dotted line) of total iNKT cells. The percentage of Ki67+ cells in both subsets is indicated (n = 5, ***P < .001 by paired Student t test). (D) Representative histogram overlays showing CD69 and NK1.1 expression in splenic conventional T cells (dotted line), Nrp-1− (shaded gray), and Nrp-1+ (thick black line) iNKT cells. (E) Relative proportions of Nrp-1+ iNKT cells in spleen, liver, thymus, and pLN iNKT cells of 8-week-old naive mice (n = 4).
Nrp-1 is expressed in immature thymic and peripheral iNKT cells in naive mice. (A) Nrp-1 expression in thymic iNKT cells. Thymocytes from 7-week-old mice were enriched in iNKT cells by depletion of CD8+ cells, and the resulting cell suspensions were stained for FACS analysis of Nrp-1 expression in thymic iNKT-cell stages. iNKT cells were defined as CD3int CD1d Tet+ (left dot plot) and stage I (CD44low NK1.1−), stage II (CD44highNK1.1−), and stage III (CD44high NK1.1+) iNKT cells were gated as shown on the middle dot plot. The right histogram plot is representative of Nrp-1 expression in stage I (full black histogram), stage II (thick black line), and stage III (dotted line) iNKT cells overlaid on control isotype staining of total iNKT cells (shaded gray histogram). (B) Representative FACS analysis of Nrp-1 expression in splenic TCRβ+ CD1d Tet+ iNKT cells and TCRβ+ CD1d Tet− conventional T cells of 8-week-old naive mice. Note the subset of iNKT cells expressing Nrp-1 at the same levels as Treg cells (CD4+ Nrp-1+ conventional T cells). (C) Intranuclear staining for the proliferation-associated marker Ki67 in splenic Nrp-1− (shaded gray) and Nrp-1+ (thick black line) iNKT cells overlaid on control isotype staining (dotted line) of total iNKT cells. The percentage of Ki67+ cells in both subsets is indicated (n = 5, ***P < .001 by paired Student t test). (D) Representative histogram overlays showing CD69 and NK1.1 expression in splenic conventional T cells (dotted line), Nrp-1− (shaded gray), and Nrp-1+ (thick black line) iNKT cells. (E) Relative proportions of Nrp-1+ iNKT cells in spleen, liver, thymus, and pLN iNKT cells of 8-week-old naive mice (n = 4).
Because stage II iNKT cells are the main subset of cells exported to the periphery,7,9 we analyzed Nrp-1 expression in iNKT cells in the periphery. In the spleens of naive adult mice, a subset of iNKT cells expressed Nrp-1 at levels similar to Treg cells (Figure 1B). Comparing Nrp-1− and Nrp-1+ iNKT cells, a higher percentage of splenic Nrp-1+ iNKT cells expressed the proliferation-associated marker Ki67 (Figure 1C), suggesting that Nrp-1 expression also correlated with proliferation in peripheral iNKT cells. Moreover, splenic Nrp-1+ iNKT cells were mostly CD69− and NK1.1− (Figure 1D), an immature phenotype characteristic of thymic stage II iNKT cells. We also detected a subset of Nrp-1+ iNKT cells bearing the same immature phenotype (CD69− NK1.1−) in other peripheral lymphoid organs of adult naive mice: the liver and pLNs. The relative abundance of Nrp-1+ iNKT cells in 8-week-old naive mice was low in the liver (5%), intermediate in the spleen and thymus (10%-15%), and high in the pLNs (40%; Figure 1E). Therefore, in the peripheral lymphoid organs of naive mice, Nrp-1 expression characterizes a subset of iNKT cells that display a phenotype very similar to thymic stage II iNKT cells.
Peripheral Nrp-1+ iNKT cells are RTE
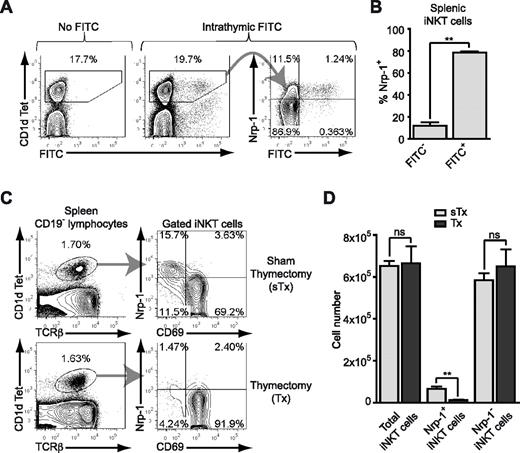
The majority of RTE iNKT cells in the spleen and liver bear the NK1.1− phenotype.7,9 However, this also characterizes a distinct subset of iNKT cells that remains unaffected by adult thymectomy.11 Using in situ labeling of thymocytes with FITC, we showed that Nrp-1 was highly expressed on almost all RTE iNKT cells in the spleen (Figure 2A-B). In contrast, Nrp-1 was not expressed in RTE conventional CD4+ and CD8+ T cells (supplemental Figure 2A), but marked RTE CD25high Treg cells (supplemental Figure 2B), which is consistent with its expression pattern in conventional thymocytes. Therefore, high expression of Nrp-1 was a specific characteristic of RTE iNKT cells. Given the percentage of randomly labeled thymocytes (30% on average) and the relative proportion of FITC+ cells in splenic Nrp-1+ iNKT cells 36 hours after intrathymic FITC injection (10% in average), we calculated that 36-hour RTE iNKT cells accounted for approximately one-third of the pool of Nrp-1+ iNKT cells in the spleen.
Nrp-1 is a marker of RTE iNKT cells. (A-B) Five-week-old mice were intrathymically injected with FITC and their spleens recovered after 36 hours. Splenocytes were enriched in iNKT cells and Nrp-1 expression was analyzed in iNKT cells by FACS. (A) Representative analysis of Nrp-1 and FITC expression in splenic CD1d Tet+ iNKT cells of control (No FITC) and treated (Intrathymic FITC) animals. (B) Nrp-1 expression by FITC− and FITC+ splenic iNKT cells from treated animals (n = 4, pooled data from 2 distinct experiments, **P < .01 by Student paired t test). (C-D) Eight-week-old mice were thymectomized (Tx) or sham-thymectomized (sTx) and killed 4 weeks later for analysis. (C) Representative FACS analysis of Nrp-1 and CD69 expression in splenic CD1d Tet+ CD19− iNKT cells of sTx and Tx mice. (D) Absolute numbers of total, Nrp-1+, and Nrp-1− iNKT cells in the spleens of sTx (light gray) and Tx (dark gray) mice (n = 4 for sTx and n = 5 for Tx, pooled data from 2 distinct experiments, ns: not significant, **P < .01 by unpaired Student t test).
Nrp-1 is a marker of RTE iNKT cells. (A-B) Five-week-old mice were intrathymically injected with FITC and their spleens recovered after 36 hours. Splenocytes were enriched in iNKT cells and Nrp-1 expression was analyzed in iNKT cells by FACS. (A) Representative analysis of Nrp-1 and FITC expression in splenic CD1d Tet+ iNKT cells of control (No FITC) and treated (Intrathymic FITC) animals. (B) Nrp-1 expression by FITC− and FITC+ splenic iNKT cells from treated animals (n = 4, pooled data from 2 distinct experiments, **P < .01 by Student paired t test). (C-D) Eight-week-old mice were thymectomized (Tx) or sham-thymectomized (sTx) and killed 4 weeks later for analysis. (C) Representative FACS analysis of Nrp-1 and CD69 expression in splenic CD1d Tet+ CD19− iNKT cells of sTx and Tx mice. (D) Absolute numbers of total, Nrp-1+, and Nrp-1− iNKT cells in the spleens of sTx (light gray) and Tx (dark gray) mice (n = 4 for sTx and n = 5 for Tx, pooled data from 2 distinct experiments, ns: not significant, **P < .01 by unpaired Student t test).
To further understand the contribution of thymic export to the Nrp-1+ iNKT cell subset in adult mice, we thymectomized (or sham-thymectomized as a control) 8-week-old mice. Four weeks after thymectomy, although the relative percentage of iNKT cells among spleen cell lymphocytes was unchanged, we observed a close to complete disappearance of the immature CD69−Nrp-1+ iNKT cell subset from the spleens of thymectomized animals (Figure 2C). Because the total number of spleen cells was left unchanged by thymectomy, this was reflected by a dramatic and specific decrease in splenic Nrp-1+ but not Nrp-1− iNKT total cell numbers after thymectomy (Figure 2D). At adult age, continuous thymic output thus entirely contributed to the Nrp-1+ iNKT cell pool. These results revealed Nrp-1 to be a bona fide marker of RTE iNKT cells in naive adult mice, and enabled us to use the Nrp-1 marker to study functional characteristics of RTE iNKT cells.
RTE proliferate more than mature iNKT cells in vitro
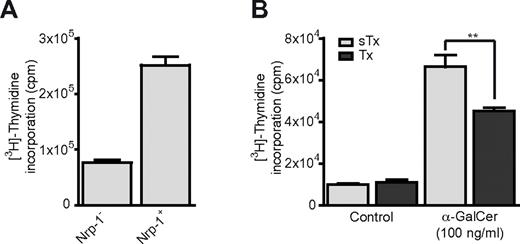
The response of iNKT cells to antigen stimulation is characterized by strong proliferation that is maximal 2-3 days after initial challenge.34 Three days after in vitro activation with α-GalCer, sorted splenic Nrp-1+ iNKT cells proliferated 3 times more than Nrp-1− iNKT cells (Figure 3A). To further analyze the contribution of RTE iNKT cells to the proliferative response of peripheral iNKT cells after antigen stimulation, we assessed the proliferation of total spleen cells from sham-thymectomized (sTx) and thymectomized (Tx) adult mice after a 2-day culture with α-GalCer. Spleen cells from Tx mice displayed significantly reduced proliferation in this assay (Figure 3B). Therefore, RTE iNKT cells have enhanced proliferative capacity after antigen stimulation and actively participate in the expansion of peripheral iNKT cells after in vitro α-GalCer challenge.
Nrp-1+ RTE iNKT cells proliferate more than Nrp-1− mature iNKT cells after in vitro activation. (A) Proliferation of FACS-sorted splenic Nrp-1− and Nrp-1+ iNKT cells after 3 days of in vitro activation with irradiated spleen cells in lymphocyte culture medium alone (Control) or supplemented with α-GalCer. Representative data of 2 distinct experiments. (B) Proliferation of total spleen cells from sham-thymectomized (sTx, light gray) and thymectomized (Tx, dark gray) mice after 2 days of in vitro activation with α-GalCer (n = 3 for sTx and n = 4 for Tx, **P < .01 by unpaired Student t test).
Nrp-1+ RTE iNKT cells proliferate more than Nrp-1− mature iNKT cells after in vitro activation. (A) Proliferation of FACS-sorted splenic Nrp-1− and Nrp-1+ iNKT cells after 3 days of in vitro activation with irradiated spleen cells in lymphocyte culture medium alone (Control) or supplemented with α-GalCer. Representative data of 2 distinct experiments. (B) Proliferation of total spleen cells from sham-thymectomized (sTx, light gray) and thymectomized (Tx, dark gray) mice after 2 days of in vitro activation with α-GalCer (n = 3 for sTx and n = 4 for Tx, **P < .01 by unpaired Student t test).
Nrp-1 is induced on proliferating iNKT cells after TCR activation
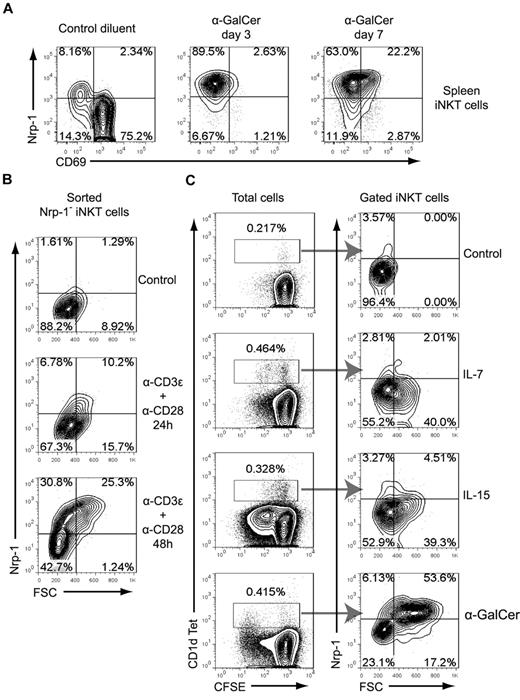
In vivo challenge with α-GalCer resulted in the majority of expanded splenic iNKT cells expressing Nrp-1 in combination with low CD69 expression at days 3 and 7 after IP injection (Figure 4A). This could have resulted from the superior proliferative response observed for Nrp-1+ iNKT cells in vitro and from de novo expression of Nrp-1 on mature iNKT cells after antigen stimulation. To address the latter hypothesis, we analyzed Nrp-1 expression after in vitro TCR activation of sorted mature splenic Nrp-1− iNKT cells. In this assay, Nrp-1 expression was induced on activated iNKT cells in a time-dependent manner (Figure 4B). Engagement of the TCR seemed to be required for the induction of Nrp-1 expression in proliferating mature iNKT cells in vitro because the cytokines IL-7 and IL-15 induced iNKT-cell proliferation (as described previously35 ) without Nrp-1 expression, whereas α-GalCer induced high expression of Nrp-1 in proliferating blastic iNKT cells (Figure 4C). Therefore, peripheral accumulation of Nrp-1+ iNKT cells after antigen stimulation in vivo may result from superior expansion of Nrp-1+ RTE iNKT cells and TCR-induced Nrp-1 expression in expanding iNKT cells.
Nrp-1 expression is induced on iNKT cells after TCR activation. (A) Mice were injected IP with α-GalCer or control diluent, and CD69 and Nrp-1 expression on gated TCRβ+ CD1d Tet+ spleen iNKT cells was analyzed after 3 and 7 days. Representative data of 1 mouse of 2 per time point in 1 of 2 experiments are shown. (B) FACS-sorted spleen Nrp-1− iNKT cells were cultivated in lymphocyte culture medium alone (Control) or activated with anti-CD3ϵ and anti-CD28, and Nrp-1 expression on activated iNKT cells was analyzed by FACS at 24 and 48 hours. Representative data of 1 of 3 experiments are shown. (C) Analysis of Nrp-1 expression on CD1d Tet+ cells after in vitro culture of CFSE-labeled spleen cells in lymphocyte culture medium alone (Control) or supplemented with IL-7, IL-15, or α-GalCer for 72 hours. These data are representative of 2 distinct experiments performed in duplicate wells.
Nrp-1 expression is induced on iNKT cells after TCR activation. (A) Mice were injected IP with α-GalCer or control diluent, and CD69 and Nrp-1 expression on gated TCRβ+ CD1d Tet+ spleen iNKT cells was analyzed after 3 and 7 days. Representative data of 1 mouse of 2 per time point in 1 of 2 experiments are shown. (B) FACS-sorted spleen Nrp-1− iNKT cells were cultivated in lymphocyte culture medium alone (Control) or activated with anti-CD3ϵ and anti-CD28, and Nrp-1 expression on activated iNKT cells was analyzed by FACS at 24 and 48 hours. Representative data of 1 of 3 experiments are shown. (C) Analysis of Nrp-1 expression on CD1d Tet+ cells after in vitro culture of CFSE-labeled spleen cells in lymphocyte culture medium alone (Control) or supplemented with IL-7, IL-15, or α-GalCer for 72 hours. These data are representative of 2 distinct experiments performed in duplicate wells.
Nrp-1+ RTE iNKT cells are biased toward IL-4 secretion after in vitro activation but contain few rapid IFN-γ and IL-4 producers in vivo
The innate effector properties of iNKT cells notably rely on their ability to rapidly and massively produce both IFN-γ and IL-4 after antigen encounter. First we analyzed IFN-γ and IL-4 production by in vitro–activated sorted splenic Nrp-1− and Nrp-1+ iNKT cells. Whereas mature Nrp-1− iNKT cells produced high amounts of both IFN-γ and IL-4, Nrp-1+ RTE iNKT cells produced 10 times less IFN-γ and twice as much IL-4, thus exhibiting a marked bias toward IL-4 production in vitro (Figure 5A). When we analyzed the in vivo production of these cytokines by intracellular staining 2 hours after IP α-GalCer injection, we found that most mature splenic Nrp-1− iNKT cells produced both IFN-γ and IL-4, but Nrp-1+ RTE iNKT cells were mostly negative for any of the 2 cytokines analyzed (Figure 5B). In particular, only 25%-30% of Nrp-1+ iNKT cells were positive for IL-4 in combination or not with IFN-γ. In an in vitro time-course experiment, Nrp-1− iNKT cell numbers continuously decreased in the first 72 hours and IL-4 secretion reached its maximum between 24 and 48 hours after activation, whereas Nrp-1+ iNKT cells started proliferating extensively after 24 hours and continuously secreted IL-4 (supplemental Figure 3). These results suggested that, in contrast to the rapid, proliferation-independent IL-4 production observed for mature Nrp-1− iNKT cells, the abundant IL-4 secretion by Nrp-1+ iNKT cells in response to antigen stimulation was slower and required cell proliferation.
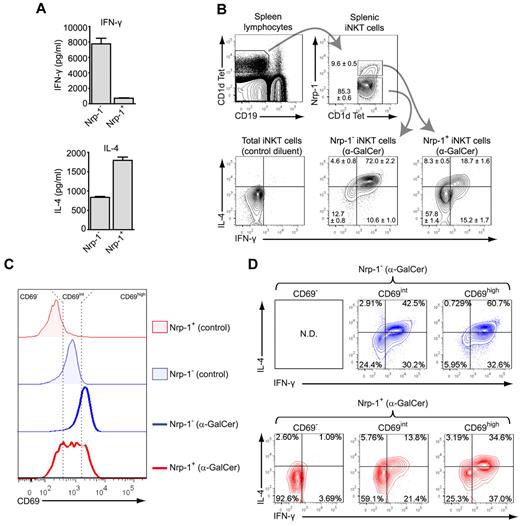
Nrp-1+ RTE iNKT cells are biased toward IL-4 secretion after in vitro activation but contain few rapid IFN-γ and IL-4 producers. (A) IFN-γ (top) and IL-4 (bottom) concentrations in culture supernatants of FACS-sorted splenic Nrp-1− and Nrp-1+ iNKT cells after 3 days of in vitro activation with irradiated spleen cells and α-GalCer. (B) Representative analysis of IFN-γ and IL-4 production in gated splenic Nrp-1− and Nrp-1+ iNKT cells 2 hours after IP injection of α-GalCer in 8-week-old mice. The mean ± SEM percentage of cells in defined gates or quadrants is indicated in FACS dot plots (n = 4, pooled data from 2 distinct experiments). (C-D) Mice were injected IP with 2 μg of α-GalCer or control diluent without prior injection of brefeldin A and killed 2 hours later for analysis. (C) CD69 expression in splenic Nrp-1− (blue) and Nrp-1+ (red) TCRβ+ CD1d Tet+ iNKT cells from control (shaded) and treated (bold line) animals. Three levels of CD69 expression (CD69−, CD69int, and CD69high) were determined, as indicated by the dotted lines. (D) Intracellular staining for IFN-γ and IL-4 in Nrp-1− (blue) and Nrp-1+ (red) iNKT cell subsets separated according to CD69 expression levels. These data are representative of 3 distinct experiments. ND indicates not detected.
Nrp-1+ RTE iNKT cells are biased toward IL-4 secretion after in vitro activation but contain few rapid IFN-γ and IL-4 producers. (A) IFN-γ (top) and IL-4 (bottom) concentrations in culture supernatants of FACS-sorted splenic Nrp-1− and Nrp-1+ iNKT cells after 3 days of in vitro activation with irradiated spleen cells and α-GalCer. (B) Representative analysis of IFN-γ and IL-4 production in gated splenic Nrp-1− and Nrp-1+ iNKT cells 2 hours after IP injection of α-GalCer in 8-week-old mice. The mean ± SEM percentage of cells in defined gates or quadrants is indicated in FACS dot plots (n = 4, pooled data from 2 distinct experiments). (C-D) Mice were injected IP with 2 μg of α-GalCer or control diluent without prior injection of brefeldin A and killed 2 hours later for analysis. (C) CD69 expression in splenic Nrp-1− (blue) and Nrp-1+ (red) TCRβ+ CD1d Tet+ iNKT cells from control (shaded) and treated (bold line) animals. Three levels of CD69 expression (CD69−, CD69int, and CD69high) were determined, as indicated by the dotted lines. (D) Intracellular staining for IFN-γ and IL-4 in Nrp-1− (blue) and Nrp-1+ (red) iNKT cell subsets separated according to CD69 expression levels. These data are representative of 3 distinct experiments. ND indicates not detected.
Consistent with the poor capacity of Nrp-1+ iNKT cells to rapidly produce IFN-γ and IL-4, adult thymectomized mice had as many splenic IFN-γ+ and IL-4+ iNKT cells as control mice after in vitro short-term activation with PMA and ionomycin (supplemental Figure 4).
Another hallmark of iNKT cell activation after in vivo antigen stimulation is the rapid up-regulation of surface CD69. Nrp-1+ iNKT cells, which were mostly CD69− in control animals, readily up-regulated CD69 2 hours after α-GalCer injection, although to a lesser extent than Nrp-1− iNKT cells (Figure 5C). CD69 up-regulation was positively correlated with increased percentages of IFN-γ+ and/or IL-4+ cells in both Nrp-1− and Nrp-1+ subsets (Figure 5D). In particular, those Nrp-1+ iNKT cells that did not up-regulate CD69 after α-GalCer treatment failed to produce IFN-γ and IL-4 (Figure 5D), whereas CD69int and CD69high subsets displayed nonnegligible percentages of IL-4+ and IFN-γ+ cells, showing that impaired rapid production of IFN-γ and IL-4 by Nrp-1+ iNKT cells after in vivo antigen challenge was at least partly due to a delay in activation.
IL-17–producing iNKT cells are RTE
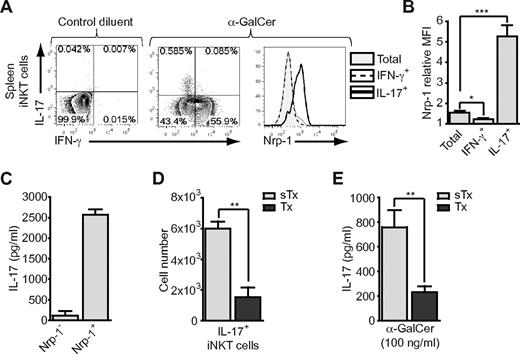
Within activated Nrp-1+ iNKT cells with high expression levels of CD69 (ie, similar to activated mature Nrp-1− iNKT cells), a relatively high percentage of cells were negative for either IFN-γ or IL-4 expression (Figure 5D), suggesting that these cells could be producing other cytokines. In a similar in vivo assay, we analyzed known iNKT cell–produced cytokines in IFN-γ−IL-4− (DN) and non-DN subsets within Nrp-1− and Nrp-1+ iNKT cells (supplemental Figure 5A).We detected IL-13– and TNF-α–producing cells in DN and non-DN cells of both populations, whereas GM-CSF production was restricted to non-DN cells in both populations (supplemental Figure 5B-D). Strikingly, the pro-inflammatory cytokine IL-17 was detected almost exclusively in the Nrp-1+ population, principally in DN cells (supplemental Figure 5E). IL-17–producing iNKT cells have been extensively described in the peripheral lymphoid organs and tissues of young adult mice,12-16,18,36,37 but whether these cells belong to RTE or to mature iNKT cells remains unknown. In contrast to IFN-γ–producing iNKT cells that were mostly Nrp-1−, IL-17–producing splenic iNKT cells expressed Nrp-1 (Figure 6A-B). Also consistent with our previous observations, only a small proportion of IL-17–producing iNKT cells coproduced IL-4 (supplemental Figure 6A-B). However, IL-17 production was not exclusive, because almost 60% of IL-17+ iNKT cells were also positive for TNF-α (supplemental Figure 6C-D). After in vitro activation with α-GalCer, sorted splenic Nrp-1+ iNKT cells produced high amounts of IL-17 in the supernatant, whereas production by Nrp-1− iNKT cells was close to null (Figure 6C). Therefore, in naive adult mice, splenic IL-17–producing iNKT cells expressed the RTE marker Nrp-1. Accordingly, the spleens of adult thymectomized mice contained 4 times fewer IL-17–producing iNKT cells than control mice (Figure 6D). This numeral defect was accompanied by a sharp reduction in IL-17 concentration in the supernatant of α-GalCer–stimulated spleen cells from thymectomized mice compared with control animals (Figure 6E), thereby confirming that most splenic IL-17–producing iNKT cells were RTE.
Splenic IL-17–producing iNKT cells are Nrp-1+ RTE iNKT cells. (A-B) Mice were treated as in Figure 5B and iNKT-enriched spleen cell suspensions were prepared for FACS analysis of IFN-γ and IL-17 production in iNKT cells. (A) Representative dot-plot analyses of IFN-γ and IL-17 expression in splenic iNKT cells of mice treated either with control diluent (left) or α-GalCer (middle), and histogram overlay showing Nrp-1 expression in total (shaded gray), IFN-γ+ (dotted line), and IL-17+ (thick black line) iNKT cells. (B) Nrp-1 relative MFI of total, IFN-γ+, and IL-17+ iNKT cells (n = 4, pooled data from 2 distinct experiments, *P < .05, ***P < .001 by unpaired Student t test). (C) IL-17 concentration in culture supernatants of FACS-sorted splenic Nrp-1− and Nrp-1+ iNKT cells after 3 days of in vitro activation with irradiated spleen cells and α-GalCer. (D) Absolute numbers of IL-17+ splenic iNKT cells in sham-thymectomized (sTx, light gray) and thymectomized (Tx, dark gray) mice assessed by FACS analysis after in vitro activation of iNKT-enriched cell suspensions with PMA and ionomycin (n = 3 for sTx and n = 4 for Tx, **P < .01 by unpaired Student t test). (E) IL-17 concentration in culture supernatant of total spleen cells from sham-thymectomized (sTx, light gray) and thymectomized (Tx, dark gray) mice after 2 days of in vitro activation with α-GalCer (n = 3 for sTx and n = 4 for Tx, **P < .01 by unpaired Student t test).
Splenic IL-17–producing iNKT cells are Nrp-1+ RTE iNKT cells. (A-B) Mice were treated as in Figure 5B and iNKT-enriched spleen cell suspensions were prepared for FACS analysis of IFN-γ and IL-17 production in iNKT cells. (A) Representative dot-plot analyses of IFN-γ and IL-17 expression in splenic iNKT cells of mice treated either with control diluent (left) or α-GalCer (middle), and histogram overlay showing Nrp-1 expression in total (shaded gray), IFN-γ+ (dotted line), and IL-17+ (thick black line) iNKT cells. (B) Nrp-1 relative MFI of total, IFN-γ+, and IL-17+ iNKT cells (n = 4, pooled data from 2 distinct experiments, *P < .05, ***P < .001 by unpaired Student t test). (C) IL-17 concentration in culture supernatants of FACS-sorted splenic Nrp-1− and Nrp-1+ iNKT cells after 3 days of in vitro activation with irradiated spleen cells and α-GalCer. (D) Absolute numbers of IL-17+ splenic iNKT cells in sham-thymectomized (sTx, light gray) and thymectomized (Tx, dark gray) mice assessed by FACS analysis after in vitro activation of iNKT-enriched cell suspensions with PMA and ionomycin (n = 3 for sTx and n = 4 for Tx, **P < .01 by unpaired Student t test). (E) IL-17 concentration in culture supernatant of total spleen cells from sham-thymectomized (sTx, light gray) and thymectomized (Tx, dark gray) mice after 2 days of in vitro activation with α-GalCer (n = 3 for sTx and n = 4 for Tx, **P < .01 by unpaired Student t test).
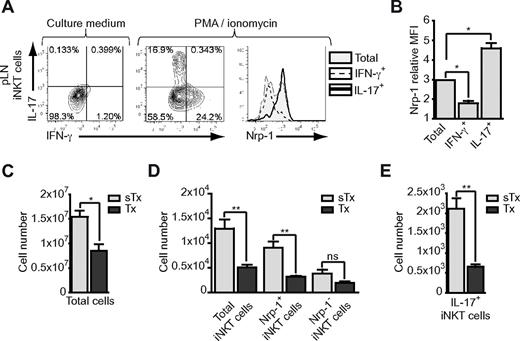
In skin-draining pLNs, iNKT cells are found in small numbers but are highly enriched in NK1.1− IL-17 producers15,36 as well as in Nrp-1+ iNKT cells (Figure 1E). Similar to what was observed in the spleen, IL-17–producing iNKT cells in pLNs expressed high levels of Nrp-1, whereas IFN-γ–producing cells were mostly Nrp-1− (Figure 7A-B). Because pLNs are mostly populated by naive T cells that are continuously exported from the thymus, adult thymectomy reduced the numbers of total cells in pLNs by approximately 2-fold (Figure 7C). As expected, this reduction affected exclusively naive CD44low but not memory phenotype CD44high conventional T cells (data not shown). After adult thymectomy, the numbers of total, Nrp-1+, and Nrp-1− iNKT cells were reduced 2.5-, 2.9-, and 2-fold, respectively (Figure 7D), reflecting a selective impact of thymus removal on Nrp-1+ iNKT cells numbers in pLNs. Moreover, intracellular staining after ex vivo activation of iNKT-enriched pLNs cell suspensions revealed a 3.2-fold reduction in IL-17–producing iNKT cell numbers in thymectomized mice (Figure 7E). Our experiments involving splenic and pLN-homing iNKT cells revealed that the majority of IL-17–producing iNKT cells in naive adult mice were part of the RTE subset rather than the long-lived NK1.1− subset.
IL-17–producing iNKT cells in pLNs are Nrp-1+ RTE iNKT cells. (A-B) Eight-week-old mice iNKT-enriched pLN cell suspensions were activated with PMA and ionomycin in the presence of brefeldin A and prepared for FACS analysis of IFN-γ and IL-17 production in iNKT cells. (A) Representative dot-plot analyses of IFN-γ and IL-17 expression in resting (left) and activated (middle) pLN iNKT cells, and histogram overlay (right) showing Nrp-1 expression in total (shaded gray), IFN-γ+ (dotted line), and IL-17+ (thick black line) pLN iNKT cells. (B) Nrp-1 relative MFI of total, IFN-γ+, and IL-17+ pLN iNKT cells (pooled data from 3 distinct experiments, *P < .05 by unpaired Student t test). (C-E) Absolute numbers of pLN total cells (C); total, Nrp-1−, and Nrp-1+ iNKT cells (D); and IL-17+ iNKT cells (E) in sham-thymectomized (sTx, light gray) and thymectomized (Tx, dark gray) mice (n = 3 for sTx and n = 4 for Tx, ns indicates not significant, *P < .05, **P < .01 by unpaired Student t test).
IL-17–producing iNKT cells in pLNs are Nrp-1+ RTE iNKT cells. (A-B) Eight-week-old mice iNKT-enriched pLN cell suspensions were activated with PMA and ionomycin in the presence of brefeldin A and prepared for FACS analysis of IFN-γ and IL-17 production in iNKT cells. (A) Representative dot-plot analyses of IFN-γ and IL-17 expression in resting (left) and activated (middle) pLN iNKT cells, and histogram overlay (right) showing Nrp-1 expression in total (shaded gray), IFN-γ+ (dotted line), and IL-17+ (thick black line) pLN iNKT cells. (B) Nrp-1 relative MFI of total, IFN-γ+, and IL-17+ pLN iNKT cells (pooled data from 3 distinct experiments, *P < .05 by unpaired Student t test). (C-E) Absolute numbers of pLN total cells (C); total, Nrp-1−, and Nrp-1+ iNKT cells (D); and IL-17+ iNKT cells (E) in sham-thymectomized (sTx, light gray) and thymectomized (Tx, dark gray) mice (n = 3 for sTx and n = 4 for Tx, ns indicates not significant, *P < .05, **P < .01 by unpaired Student t test).
Discussion
In the present study, we have shown that Nrp-1 expression defines a subset of immature iNKT cells in the thymus and peripheral lymphoid organs of naive mice. In immature thymocytes and thymic iNKT cells, Nrp-1 expression was correlated with proliferation. Furthermore, TCR-induced, but not IL-7– or IL-15–induced, proliferation of peripheral iNKT cells was associated with Nrp-1 de novo expression. We and others have reported Nrp-1 expression on activated human T cells29,38 and immature proliferating murine thymocytes.28,39 Therefore, Nrp-1 might be a general marker of TCR- and pre-TCR activation, and its presence on RTE iNKT cells, but not on naive and RTE conventional T cells, is likely because of the unconventional selection and development processes of iNKT cells, which require TCR-triggered proliferative expansion shortly before egress. Previous studies have shown that RTE iNKT cells share the thymic stage II phenotype (CD44highNK1.1−).7,9 Moreover, the very early activation marker CD69 has been described as an age-related maturation marker of splenic iNKT cells40 and was therefore postulated to be absent on RTE iNKT cells. Our results unequivocally reveal Nrp-1 as an accurate specific marker of RTE iNKT cells and show that the characteristic phenotype of these cells is indeed Nrp-1+NK1.1−CD69−. Furthermore, because Nrp-1 is expressed on ∼ 30% of splenic NK1.1− iNKT cells (data not shown), we can gather that RTE account for approximately one-third of total NK1.1− iNKT cells in the spleen, the remaining two-thirds being long-lived mature cells. This is in agreement with the study by McNab et al, which showed that adult thymectomy caused a 25%-50% reduction in splenic NK1.1− iNKT cell proportions.11
Based on the differential expression of Nrp-1 on RTE iNKT cells, we have been able to analyze for the first time the response of RTE iNKT cells to antigen stimulation both in vitro and in vivo. We have shown that RTE iNKT cells respond to antigen stimulation in vitro by proliferating vigorously and secreting high amounts of IL-4 and very low amounts of IFN-γ. The rapid effector functions of iNKT cells rely on the presence of high levels of IL-4 and IFN-γ mRNAs in resting mature iNKT cells.41,42 Thymic stage II iNKT cells possess abundant mRNA for IL-4 but not for IFN-γ,41 which is likely the reason for their strong bias for IL-4 production in vitro.7,15 Our in vitro data suggest that RTE iNKT cells, which retain this bias, might also possess mRNA for IL-4 but not IFN-γ in the resting state. Therefore, the poor capacity of RTE iNKT cells to rapidly produce IFN-γ and IL-4 after in vivo antigen challenge may not be because of the absence of cytokine mRNA in these cells. The in vitro time-course experiment and the incomplete up-regulation of CD69 in vivo suggest that the response of RTE iNKT cells to TCR triggering may be delayed compared with mature iNKT cells. Strong antigen stimulation of iNKT cells is known to induce anergy dependent on the long-lasting expression of programmed death 1 on primed iNKT cells.43,44 Signals involved in the positive selection of iNKT cells may trigger the same anergy program in expanding thymic stage II and then RTE iNKT cells. In support of this hypothesis, we found higher levels of programmed death 1 expression on Nrp-1+ RTE iNKT cells from naive mice (data not shown), which could account for the relative anergy of RTE iNKT cells in response to antigen stimulation in vivo.
Interestingly, many RTE iNKT cells that expressed high levels of CD69 after in vivo activation still did not produce IFN-γ or IL-4, which suggests that some RTE iNKT cells may exclusively produce nonclassic cytokines after stimulation. Our results show that IL-13 and TNF-α can be produced by RTE iNKT cells independently of IFN-γ and IL-4 shortly after α-GalCer stimulation. Furthermore, we have shown that Nrp-1+ RTE iNKT cells are potent and almost exclusive producers of the pro-inflammatory cytokine IL-17 in vivo and in vitro. Because we used brefeldin A, which inhibits protein trafficking to the cell membrane, to detect IL-17–producing cells, we have not been able to match IL-17 production to high CD69 up-regulation in activated Nrp-1+ RTE iNKT cells. However, most iNKT cells that produced IL-17 after stimulation did not simultaneously produce IFN-γ or IL-4, as described previously.36 Together with the phenotypic observation that IL-17–producing iNKT cells express the RTE marker Nrp-1, our analyses of adult thymectomized mice clearly showed that continuous thymic output is required to maintain the pool of IL-17–producing iNKT cells in lymphoid organs. Three nonmutually exclusive hypotheses might explain this unexpected observation: (1) IL-17–producing iNKT cells are only transitorily present in lymphoid organs after their egress from the thymus and preferentially home to nonlymphoid tissues, (2) the capacity to produce IL-17 is only transitory and is lost upon maturation, and/or (3) most IL-17–producing iNKT cells are short-lived in lymphoid organs. In support of the first hypothesis, IL-17–producing iNKT cells have been shown to express tissue homing receptors such as CCR6 and CD10336 and have been found in tissues such as the lungs16,37 and the skin.36 Moreover, we have previously shown that IL-17–producing iNKT cells represent a distinct and definitive lineage of iNKT cells that expresses the transcription factor ROR-γT and cannot give rise to IL-4 and IFN-γ producers upon transfer into fetal thymic organ cultures.18 Although this observation seems to rule out the second hypothesis, not all ROR-γT+ iNKT cells produce IL-17 after stimulation,18,36 so IL-17 production capacity could be limited to the most recently exported ROR-γT+ iNKT cells also expressing the RTE marker Nrp-1. Finally, whereas long-term survival and homeostatic renewal of mature iNKT cells in lymphoid organs is crucially dependent on IL-15,35 neither IL-17–producing iNKT cells36 nor RTE iNKT cells express the IL-15 receptor subunit CD122 (data not shown). The survival of IL-17–producing iNKT cells might depend on one or several cytokines that are not as abundant as IL-15 in the lymphoid organs of naive mice, which would result in a short half-life of these cells in the spleen and pLNs.
Our study also raises the question of Nrp-1 function in immature thymic and RTE iNKT cells. Nrp-1 has been shown to bind and enhance the intracellular signaling of TGF-β1,26,45 a cytokine crucially involved in iNKT-cell development and maturation.46-48 Nrp-1 expression might increase the effects of TGF-β1 in thymic immature iNKT cells and thus positively regulate their development. Increased sensitivity of Nrp-1+ iNKT cells to TGF-β1 might also regulate the acquisition of specific tissue-homing receptors (eg, CD103) on IL-17–producing iNKT cells, as suggested by Doisne et al,36 or it may facilitate their survival in nonlymphoid tissues. Moreover, Nrp-1 mediates long-term interactions between Treg cells and immature dendritic cells through Nrp-1/Nrp-1 trans interactions.25 A recent in vivo imaging study revealed the importance of cognate stable interactions between iNKT cells and macrophages for the initiation of immune responses to glycolipids in the lymph nodes.49 Nrp-1, which is highly expressed by lymph node–resident iNKT cells and may be expressed by macrophages,50 might play a pro-adhesive role in this context.
In conclusion, based on the characteristic Nrp-1 marker, we have identified important functional features of RTE iNKT cells. Remarkably, pro-inflammatory IL-17–producing iNKT cells in lymphoid organs are continuously renewed by thymic output in adult mice. These data therefore reveal the crucial role of thymic function in innate IL-17–mediated immune responses and provide the basis for a thorough analysis of the role of RTE iNKT cells in animal models of inflammation, infection, and autoimmunity. Our results also reveal the need for a more detailed understanding of Nrp-1 function in non-Treg T cells.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Lehuen for sharing unpublished data and for helpful discussions; A. Napolitano and P. Dellabona for sharing unpublished data and helpful information; J. Mégret and C. Garcia for cell sorting; M. McHeyzer-Williams for critically reviewing the manuscript; and the National Institutes of Health Tetramer Core Facility (Bethesda, MD) for providing CD1d tetramer reagents.
This work was supported in part by grants from Institut National du Cancer, Cancéropole Ile de France, Fondation de France, and Association pour la Recherche sur le Cancer, and by fellowships from Ministère de la Recherche et de l'Enseignement Supérieur, Association pour la Recherche sur le Cancer (to P.M.), and La Ligue contre le Cancer (to A.R.).
Authorship
Contribution: P.M. designed, performed, and analyzed the experiments, interpreted the data, and wrote the manuscript; A.R., B.M., and S.D. performed and analyzed the experiments; A.H. and M.L.d.M. designed the experiments, interpreted the data, and edited the manuscript; and M.-T.R. and O.H. designed the experiments, interpreted the data, edited the manuscript, and supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Olivier Hermine, CNRS UMR 8147, Hôpital Necker–Enfants Malades, Bâtiment Sèvres Porte S2 5ème étage, 149 rue de Sèvres, 75743 Paris Cedex 15, France; e-mail: ohermine@gmail.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal