Abstract
Hematopoietic stem cells (HSCs) engage in complex bidirectional signals with the hematopoietic microenvironment (HM), and there is emerging evidence that leukemia stem cells (LSCs) may use similar interactions. Using a syngeneic retroviral model of MLL-AF9 induced acute myeloid leukemia (AML), we have identified 2 different stages of leukemia progression, propagated by “pre-LSCs” and established leukemia (LSCs) and compared the homing properties of these distinctive entities to that of normal HSCs. The homing and microlocalization of pre-LSCs was most similar to long-term HSCs and was dependent on cell-intrinsic Wnt signaling. In contrast, the homing of established LSCs was most similar to that of committed myeloid progenitors and distinct from HSCs. Although osteoblast-derived Dickkopf-1, a potent Wnt inhibitor known to impair HSC function, dramatically impaired normal HSC localization within the bone marrow, it did not affect pre-LSCs, LSC homing, or AML development. Mechanistically, cell-intrinsic Wnt activation was observed in human and murine AML samples, explaining the independence of MLL-AF9 LSCs from niche-derived Wnt signals. These data identify differential engagement of HM associated with leukemic progression and identify an LSC niche that is physically distinct and independent of the constraints of Wnt signaling that apply to normal HSCs.
Introduction
Hematopoietic stem cells (HSCs) are supported by the bone marrow hematopoietic microenvironment to maintain long-term self-renewal.1-3 Leukemia stem cells (LSCs) possess the ability to initiate, maintain, and serially propagate leukemia in vivo. Furthermore, LSCs infiltrate the bone marrow4,5 and interfere with the normal HSC-microenvironment homeostasis.6 Available data indicate that LSCs also interact with the hematopoietic microenvironment to maintain self-renewal and to mitigate the effects of cytotoxic chemotherapy.5,7-9 Thus, disruption of LSC-niche interactions may have therapeutic value, as observed by an enhanced sensitivity to cytotoxic chemotherapy after leukemia mobilization.8-10
Leukemia is the consequence of stepwise genetic alterations that confer both proliferative and survival advantage, as well as self-renewal to the malignant cells.11 A recognized early stage of LSC development is the pre-LSC stage composed of immortalized hematopoietic stem and progenitor cells that give rise to leukemia in vivo with variable latency, presumably because of the gradual accumulation of additional genetic hits.12,13 In contrast, LSCs (derived from mice with established leukemia) give rise to fully penetrant, short-onset leukemia in secondary recipients.14 Biologically, pre-LSCs and LSCs are distinctive in their relative pace of disease onset and leukemogenic potential. However, it is not known whether this distinction corresponds to disparate requirements for cell-extrinsic signaling from the bone marrow microenvironment or whether a potential pre-LSC or LSC niche would overlap with that of normal HSCs.
A spectrum of signaling pathways have been demonstrated to regulate the interactions of HSCs with the bone marrow microenvironment.7,15 However, the cellular and molecular components of the LSC microenvironment remain poorly understood. Dysregulation of the canonical Wnt signaling pathway is known to constrain HSC function in vivo.16,17 Furthermore, canonical Wnt signaling is activated in some acute myeloid leukemia (AML) LSCs, and targeted genetic deletion of the downstream Wnt effector β-catenin inhibits leukemogenesis.12,13 Moreover, cell-extrinsic inhibition of Wnt signaling through ectopic DKK1 expression impairs leukemia cell proliferation in vitro.18
We used a syngeneic murine model of MLL-AF9–induced AML to determine the localization of pre-LSCs and LSCs within the bone marrow hematopoietic microenvironment at different stages of leukemic progression and analyzed the effect of cell-intrinsic and cell-extrinsic alterations of Wnt signaling on pre-LSC and LSC niche requirements.
Methods
Flow cytometry
Cells were stained with a lineage cocktail of biotin-labeled antimouse antibodies (Ter119, CD3, CD4, CD8, B220, Mac1, and Gr1; BD Biosciences PharMingen). Lineage-positive cells were depleted with Dynabeads (Invitrogen). Lineage-depleted cells were stained with c-kit (2B8), Sca-1 (D7), CD34 (RAM34), Flk2 (A2F10.1), FcγRII/III (93), and streptavidin-allophycocyanin-Cy7. The following gating strategies were used: HSCs, lineagelowc-kithighSca1+CD34−Flk2−; LKS, lineagelowc-kithighSca1+; granulocyte-macrophage progenitors (GMPs), lineagelowc-kithighSca1−CD34+FcγR+. LSCs were stained with a lineage cocktail of rat antimouse antibodies (CD3, CD4, CD8, Ter119, B220, CD19, Gr1, IL7R, and Sca1; Invitrogen) and counterstained with c-kit (2B8), CD34 (RAM34), and FcγRII/III (93) and goat antirat phycoerythrin-Cy5.5. LSCs were defined as GFP+lineagelowc-kithighSca1−CD34lowFcγR+ (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). For in vivo homing experiments, LSCs were obtained from secondary transplant recipients with established leukemia. For phenotyping studies, CD3 (145–2c11), Gr1 (RB6–8C5), Mac1 (M1/70), and B220 (RA3–6B2) were used.
In vivo, live mouse imaging
HSC, GMP, or LSC-enriched fractions were isolated by fluorescence-activated cell sorter (FACSAria; BD Biosciences). Pre-LSCs were harvested from early passage methylcellulose cultures. A total of 1 to 5 × 104 cells were labeled with a lipophilic dye (Vybrant DiD, Invitrogen) and injected into a lethally irradiated recipient mouse (9.5 Gy, 2 fractions, 2.3 kb collagen1α-GFP [Coll-GFP] or Dickkopf-1 [Dkk1]/Coll-GFP). Mice were anesthetized, and the entire exposed calvarium was imaged. Layered images were acquired every 6-10 μm. The shortest distance was measured from the DiD-labeled cell to osteoblast on each focal plane, and 3-dimensional distance was calculated. For mixing studies, LSCs were stained with either DiD or DiI (Invitrogen) and then mixed together in equal proportion before injection into a lethally irradiated recipient mouse. Recipient mice were examined 2 hours after injection for homing or 48 hours after injection for mixing/proliferation studies. Dkk1 and Coll-GFP and mice have been described.1,17 Dkk1 and Coll-GFP mice have been backcrossed onto C57Bl/6 background. β-Cateninfl/fl mice have previously been described.12
Human patient data
Gene expression data from patients with MLL-AF9–associated AML were obtained in collaboration with the German-Austrian AML Study Group and compared with published data sets of purified human CD34+ cells (n = 10)19,20 using the class comparison analysis tool. All human data were collected with written, informed consent in accordance with the Declaration of Helsinki. Enrichment of gene sets was assessed using the pathway comparison tool of the BRB Array Tool package.21 Unsupervised, hierarchical clustering analysis was performed based on well-measured genes of the canonical WNT pathway (http://cgap.nci.nih.gov/Pathways/BioCarta/h_wntPathway). Differentially expressed genes within the WNT pathway were chosen based on P value (cutoff P < .05), and the predicted net effects on WNT signaling were determined from published literature.
Generation of AML, LSCs, and pre-LSCs
pMSCV-MLL-AF9-IRES-GFP and the generation of AML using retrovirus22 from purified GMP or 5-fluorouracil primed bone marrow have been described.14 LSCs were purified from leukemic mice as previously described.14 To generate pre-LSCs, LKS or GMP populations were fluorescence-activated cell sorter purified and transduced with pMSCV-MLL-AF9-IRES-GFP or simultaneously transduced with pMSCV-HoxA9-IRES-GFP plus pMSCV-Meis1a-IRES-puro. Single GFP+ cells were then sorted into 96-well format in methylcellulose (M3234; StemCell Technologies) supplemented with IL-3 (10 ng/mL) and expanded in vitro as previously described.12 For in vivo leukemogenesis, pre-LSCs were injected 48 hours after transduction without in vitro expansion. For the β-cateninfl/fl and β-catenin−/− experiments, a retrovirally induced cre was used as previously described.12
A detailed explanation of the source of cells, oncogene, and relative level of β-catenin is provided in supplemental Table 1. For engraftment studies, AML samples were injected intravenously into sublethally irradiated recipient mice. After 24 hours or 7 days, the recipient mice were harvested and the percentage engraftment (24 hours) and relative proliferation (7 days) were measured by flow cytometry (GFP+ cells).
Statistical analysis
GraphPad Prism Version 5.0a (GraphPad Software) was used to analyze results and create graphs. All comparisons represent 2-tailed unpaired t test analysis unless otherwise specified. Flow cytometry data were analyzed with FlowJo Version 9.2 software (TreeStar).
Western blot
Whole-cell protein extracts for immunoprecipitation were prepared using lysis buffer (20mM Tris-HCl, 150mMNaCl, 1mM EDTA, 10% glycerol and 1% Triton X-100, Complete mini protease inhibitor cocktail, and phos-stop mini-tablets; Roche Diagnostics). For detection of protein, rabbit anti–mouse β-catenin (1:1000, 9562; Cell Signaling) and rabbit anti–mouse β-actin (1:5000, AC-15; Sigma-Aldrich) were used.
Quantitative RT-PCR
RNA was isolated from purified LSCs (RNeasy Mini Kit; QIAGEN). Complementary DNA was synthesized (TaqMan reagents), and relative transcript quantification was performed with SYBR Green reagents with the use of Applied Biosystems 7500 Real-Time PCR system and SDS Version 2.0.5 software (Applied Biosystems). Gene expression was compared with the Gapdh housekeeping gene.
Results
Distinct in vivo homing properties of HSCs, committed myeloid progenitors, pre-LSCs, and LSCs
LSCs are defined functionally by the ability to initiate, maintain, and serially propagate leukemia in vivo.23 The immunophenotype of established MLL-AF9 LSCs is similar to that of preleukemic LSCs. However, we and others have observed that pre-LSCs have distinct biologic properties compared with their LSC counterparts, including a markedly prolonged disease latency in secondary transplant models of disease.12,13 We hypothesized that pre-LSCs and LSCs may have differential requirements for interactions with the hematopoietic microenvironment. To test this hypothesis, MLL-AF9–induced AML was generated, for which pre-LSC and LSC populations have been identified (supplemental Table 1). We first purified MLL-AF9 pre-LSCs (GMPs transduced with MLL-AF9 and expanded in vitro) and MLL-AF9 LSCs, and labeled these populations with the lipophilic dye DiD. DiD-labeled pre-LSCs and LSCs were injected them into lethally irradiated coll-GFP osteoblast reporter mice to compare their homing pattern using in vivo 3-dimensional imaging.1 The homing of LSCs isolated from mice with established MLL-AF9 leukemia was distinct from that of pre-LSCs, with pre-LSCs homing very closely to bone marrow osteoblastic cells and LSCs homing further away from the osteolineage cells (Figure 1A-B). These results were similar to the differences between normal HSCs and more differentiated progenitors previously described.1 We therefore compared these data to the homing of HSCs (linlowc-kithighSca1+CD34−Flk2−) and GMPs (lineagelowc-kithighSca1−CD34+FcγR+) and found that pre-LSC homing was even closer to osteoblastic cells than HSCs (P < .01), whereas established LSCs localized similarly to GMP (P = .26) (Figure 1C). Individual LSCs were reimaged after 48 hours and found to be able to proliferate in vivo, giving rise to large, clonal leukemic clusters not seen with normal HSCs (Figure 1D), confirming that the imaging technique was detecting viable, and engrafting LSCs with in vivo proliferative capacity. These data may suggest that pre-LSCs may require close contact with osteoblasts for initiation of leukemia in vivo, whereas LSCs from established leukemia may be independent of these constraints.
Discrete patterns of homing of leukemic and pre-LSCs compared with normal stem and progenitor cells. (A) In vivo imaging of HSCs, GMPs, pre-LSCs (MLL-AF9 transduced GMPs, expanded in vitro), and LSCs (MLL-AF9 transduced GMPs, in vivo expanded, from leukemic mice) homing to the bone marrow cavity of lethally irradiated osteoblast reporter recipient mice. Red with white outline represents injected HSCs, GMPs, pre-LSCs, or LSCs stained ex vivo with Vybrant DiD dye; green, osteoblasts; and blue, bone matrix. White bar represents 10 μm. The shortest, 3-dimensional distance from each HSC, GMP, pre-LSC, or LSC to the closest osteoblast was measured for each cell. (B) Pre-LSCs (MLL-AF9 GMPs expanded in vitro) homed 8.7 μm (median; range, 0.5-42 μm) from osteoblasts compared with LSCs that homed 23.7 μm (median; range, 0-79.2 μm) from osteoblasts (P < .01). Individual data point represents an individual cell. n = 3 or 4 biologic replicates. (C) HSCs homed 12.8 μm (median; range 0-42.1 μm) from osteoblasts compared with GMPs that homed 21.0 μm (median; range, 1.0-89.2 μm) from osteoblasts (P = .03). n = 3 biologic replicates per condition. (D) LSCs (MLL-AF9–transduced GMPs, expanded in vivo from leukemic mice) proliferate and form clusters in vivo. LSCs were stained with either Vybrant DiD (Invitrogen, red) or DiI (Invitrogen, white) and mixed in equal proportions before injection into lethally irradiated recipient mice. After 48 hours, clusters were observed. In all cases, clusters were made of a single color (red or blue), confirming clonal LSC expansion in vivo. All images were acquired using a 25× water immersion objective (NA 0.9) on a custom-made confocal/two-photon microscope and acquisition software (1). Image analysis was performed with ImageJ and measurements were obtained using Photoshop CS4 extender.
Discrete patterns of homing of leukemic and pre-LSCs compared with normal stem and progenitor cells. (A) In vivo imaging of HSCs, GMPs, pre-LSCs (MLL-AF9 transduced GMPs, expanded in vitro), and LSCs (MLL-AF9 transduced GMPs, in vivo expanded, from leukemic mice) homing to the bone marrow cavity of lethally irradiated osteoblast reporter recipient mice. Red with white outline represents injected HSCs, GMPs, pre-LSCs, or LSCs stained ex vivo with Vybrant DiD dye; green, osteoblasts; and blue, bone matrix. White bar represents 10 μm. The shortest, 3-dimensional distance from each HSC, GMP, pre-LSC, or LSC to the closest osteoblast was measured for each cell. (B) Pre-LSCs (MLL-AF9 GMPs expanded in vitro) homed 8.7 μm (median; range, 0.5-42 μm) from osteoblasts compared with LSCs that homed 23.7 μm (median; range, 0-79.2 μm) from osteoblasts (P < .01). Individual data point represents an individual cell. n = 3 or 4 biologic replicates. (C) HSCs homed 12.8 μm (median; range 0-42.1 μm) from osteoblasts compared with GMPs that homed 21.0 μm (median; range, 1.0-89.2 μm) from osteoblasts (P = .03). n = 3 biologic replicates per condition. (D) LSCs (MLL-AF9–transduced GMPs, expanded in vivo from leukemic mice) proliferate and form clusters in vivo. LSCs were stained with either Vybrant DiD (Invitrogen, red) or DiI (Invitrogen, white) and mixed in equal proportions before injection into lethally irradiated recipient mice. After 48 hours, clusters were observed. In all cases, clusters were made of a single color (red or blue), confirming clonal LSC expansion in vivo. All images were acquired using a 25× water immersion objective (NA 0.9) on a custom-made confocal/two-photon microscope and acquisition software (1). Image analysis was performed with ImageJ and measurements were obtained using Photoshop CS4 extender.
Homing of pre-LSCs is specified by Wnt-β-catenin activation
To investigate whether disease latency may reflect a distinct relationship with the microanatomic site in the marrow environment, the homing of HoxA9-Meis1a–transduced LKS and GMP clones were analyzed. We have previously demonstrated that HoxA9 and Meis1a, key downstream targets of MLL-AF9, are sufficient to transform LKS but not GMP cell populations and that this differential effect is determined by the presence or absence, respectively, of constitutive Wnt-β-catenin.12 HoxA9-Meis1a-LKS and HoxA9-Meis1a-GMP were DiD labeled and injected into lethally irradiated Coll-GFP mice. Similar to the homing seen in MLL-AF9-GMP pre-LSCs, HoxA9-Meis1a-LKS homed closely to osteoblasts, whereas HoxA9-Meis1a-GMP homed further from osteoblasts, which is similar to normal GMP (Figure 2). This effect was β-catenin dependent, as expression of a constitutively active β-catenin construct (Xenopus NH3-terminal mutant of β-catenin that is sufficient for full leukemic transformation of HoxA9-Meis1a-GMP in vivo12 ) changed the homing pattern of HoxA9-Meis1a-GMP to that of HoxA9-Meis1a-LKS. These data suggest that β-catenin activation in HoxA9-Meis1a pre-LSCs may confer leukemogenic activity in part by enabling closer interaction with osteoblastic cells in the niche.
Homing of pre-LSCs is specified by Wnt signaling. (A) HoxA9-Meis1a-LKS+ pre-LSCs, characterized by active β-catenin signaling, homed 11 μm (median; range, 0-40.4 μm) from osteoblasts (n = 4 biologic replicates). In contrast, HoxA9-Meis1a-GMP (without active β-catenin signaling) pre-LSCs homed 17.6 μm (median; range, 0-56 μm) from osteoblasts (P < .01; n = 4 biologic replicates). HoxA9-Meis1a-GMP that expressed a constitutively active β-catenin homed 8 μm (median; range, 0.5-28 μm, n = 2 biologic replicates) from osteoblasts. P < .01, compared with HoxA9-Meis1a-GMP. (B) β-Catenin−/− MLL-AF9-GMP pre-LSCs homed 20 μm (median; range, 4-44 μm) from osteoblasts compared with control (β-cateninfl/fl) MLL-AF9-GMP pre-LSCs, which homed 10 μm (median; range, 4-26 μm) from osteoblasts (P < .01; n = 2 or 3 biologic replicates).
Homing of pre-LSCs is specified by Wnt signaling. (A) HoxA9-Meis1a-LKS+ pre-LSCs, characterized by active β-catenin signaling, homed 11 μm (median; range, 0-40.4 μm) from osteoblasts (n = 4 biologic replicates). In contrast, HoxA9-Meis1a-GMP (without active β-catenin signaling) pre-LSCs homed 17.6 μm (median; range, 0-56 μm) from osteoblasts (P < .01; n = 4 biologic replicates). HoxA9-Meis1a-GMP that expressed a constitutively active β-catenin homed 8 μm (median; range, 0.5-28 μm, n = 2 biologic replicates) from osteoblasts. P < .01, compared with HoxA9-Meis1a-GMP. (B) β-Catenin−/− MLL-AF9-GMP pre-LSCs homed 20 μm (median; range, 4-44 μm) from osteoblasts compared with control (β-cateninfl/fl) MLL-AF9-GMP pre-LSCs, which homed 10 μm (median; range, 4-26 μm) from osteoblasts (P < .01; n = 2 or 3 biologic replicates).
Homing of pre-LSCs is dependent on Wnt-β-catenin activation
To determine whether the homing of LSCs was dependent on Wnt-β-catenin activation, we investigated the homing of MLL-AF9-GMP pre-LSCs derived from β-cateninfl/fl mice, with or without cre-mediated deletion of β-catenin (β-catenin−/−). In consonance with the finding that β-catenin activation enabled closer interaction with osteoblastic cells in the niche, β-catenin−/− MLL-AF9-GMP pre-LSCs homed further from osteoblastic cells than β-cateninfl/fl MLL-AF9-GMP pre-LSCs (Figure 2B). These data confirm that β-catenin signaling is required for close interactions with osteoblastic cells in vivo. The difference in homing between β-cateninfl/fl and β-catenin−/− MLL-AF9-GMP pre-LSCs was not explained by differences in CXCR4, α-4 or α-5 integrin surface expression (supplemental Figure 2).
MLL-AF9–induced AML is resistant to endosteal Dkk1 in vivo
It has previously been demonstrated that MLL-AF9 pre-LSCs require β-catenin for the leukemogenesis12,13 and that osteoblastic cell-mediated modulation of Wnt signaling affects HSC function.16,17 However, the role of cell-extrinsic signaling from osteoblastic cells on pre-LSCs or LSCs has not been established. Therefore, to determine the dependence of MLL-AF9 pre-LSCs on osteoblastic cell signaling, AML development in lethally irradiated transgenic mice expressing the Wnt inhibitor Dkk1 in osteoblastic cells was monitored.17 Dkk1 and wild-type (WT) mice developed AML with similar disease latency when primary disease was generated from transformation of GMP or 5-fluorouracil-treated whole bone marrow (Figure 3A; and data not shown). There were no differences in peripheral blood blast morphology, immunophenotype, or pattern of infiltration into the bone marrow (supplemental Figure 3; and data not shown). To test whether cell-extrinsic Dkk1 expression could affect the self-renewal capacity of LSCs in vivo, serial transplantation of AML was performed through 3 generations of irradiated WT or Dkk1 recipient mice, and then disease initiation in quaternary recipient mice was monitored. No differences were seen in disease penetrance or latency between Dkk1 or WT controls (Figure 3B). Together, these data demonstrate that MLL-AF9 AML is resistant to the effects of Dkk1 expressed from osteoblastic cells in the bone marrow microenvironment.
LSCs are resistant to the effects of endosteal Dkk1 expression. (A) Kaplan-Meier survival curve demonstrating similar survival of WT recipients (solid line; median survival, 61 days, n = 6) compared with Dkk1 recipient mice (dashed line; median survival, 60 days, P = .36, n = 8) after injection with MLL-AF9-GMP–derived pre-LSCs. (B) Kaplan-Meier survival curve of recipient mice after the fourth round of leukemia transplantation. There was no difference in the survival of mice receiving AML that had been passaged through 4 generations of Dkk1 recipients (dashed line; median survival, 16 days, n = 5) to mice receiving AML that had been passaged through 4 generations of WT recipients (solid line; median survival, 16 days, P = .13, n = 5).
LSCs are resistant to the effects of endosteal Dkk1 expression. (A) Kaplan-Meier survival curve demonstrating similar survival of WT recipients (solid line; median survival, 61 days, n = 6) compared with Dkk1 recipient mice (dashed line; median survival, 60 days, P = .36, n = 8) after injection with MLL-AF9-GMP–derived pre-LSCs. (B) Kaplan-Meier survival curve of recipient mice after the fourth round of leukemia transplantation. There was no difference in the survival of mice receiving AML that had been passaged through 4 generations of Dkk1 recipients (dashed line; median survival, 16 days, n = 5) to mice receiving AML that had been passaged through 4 generations of WT recipients (solid line; median survival, 16 days, P = .13, n = 5).
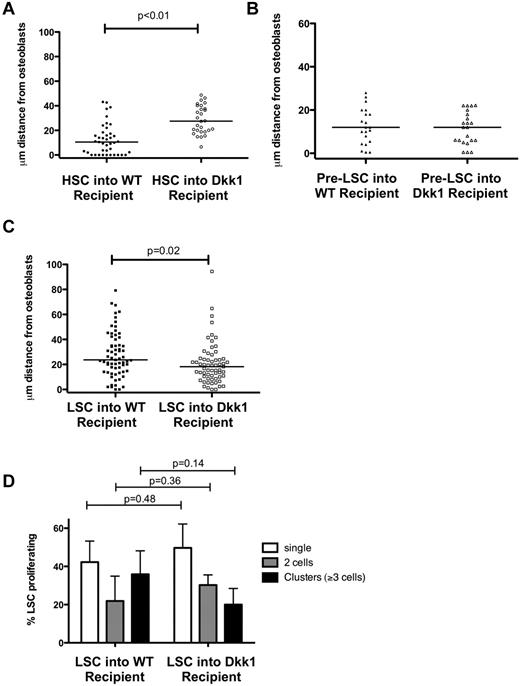
Pre-LSC and LSC homing and early engraftment are not affected by endosteal Dkk1
To understand the differences between the effects of Dkk1 on the homing of normal and malignant cell populations, HSCs, pre-LSCs, and LSCs were injected into Coll-GFP and Coll-GFP/ Dkk1 transgenic recipient mice. HSCs injected in Coll-GFP/ Dkk1 recipient mice displayed dramatically defective homing to the osteoblastic cells in the niche (Figure 4A), indicating that osteoblastic cell-derived Dkk1 affects not only the function, but also the localization of HSCs. Pre-LSCs (HoxA9-Meis1a LKS+) showed similar homing patterns in Dkk1 and WT recipients (Figure 4B). Surprisingly, LSCs localized slightly closer to osteoblastic cells in Dkk1 transgenic than in WT recipients (Figures 1B, 4C). The in vivo proliferation of LSCs was determined by counting the number and size of observed cell clusters 48 hours after injection, and no differences in proliferation of LSCs in Coll-GFP versus Coll-GFP/Dkk1 recipients were observed (Figure 4D). To validate these findings, the engraftment and proliferation of established leukemia were measured 24 hours and 7 days after injection. No differences were seen in the percentage of viable GFP+ leukemia cells in WT or Dkk1 recipients at both time points after injection (supplemental Figure 4). Together, these data demonstrate that both pre-LSCs and LSCs are resistant to the effects of endosteal-Dkk1 in vivo.
Dkk1 impairs the homing of HSCs, but not pre-LSCs and LSCs. (A) HSCs homed 10.5 μm (median) from osteoblasts in WT recipient mice and 27.6 μm from osteoblasts in Dkk1-transgenic mice (P < .01) (n = 3 biologic replicates). (B) Pre-LSCs homed 12.5 μm (median; range, 0.5-28 μm) from osteoblasts in WT and 11.5 μm (median; range, 0.5-22.2 μm) in Dkk1-transgenic mice (P = not significant; n = 2 biologic replicates). (C) LSCs homed slightly more closely to osteoblasts in Dkk1-transgenic mice (median, 18.2 μm; range, 0-94.4 μm) compared with WT recipient mice (median, 23.7 μm; range, 0-79.2 μm; n = 3 biologic replicates). (D) Proliferation of individual LSCs in vivo was measured 48 hours after injection. Each LSC was identified as composed of single cells, doublets, or clusters (≥ 3 cells together) and expressed relative to the total number of cells per mouse (n = 3 for WT and Dkk1). LSC proliferation in vivo was similar between WT and Dkk1 recipients (P = .36 for doublets, P = .14 for clusters).
Dkk1 impairs the homing of HSCs, but not pre-LSCs and LSCs. (A) HSCs homed 10.5 μm (median) from osteoblasts in WT recipient mice and 27.6 μm from osteoblasts in Dkk1-transgenic mice (P < .01) (n = 3 biologic replicates). (B) Pre-LSCs homed 12.5 μm (median; range, 0.5-28 μm) from osteoblasts in WT and 11.5 μm (median; range, 0.5-22.2 μm) in Dkk1-transgenic mice (P = not significant; n = 2 biologic replicates). (C) LSCs homed slightly more closely to osteoblasts in Dkk1-transgenic mice (median, 18.2 μm; range, 0-94.4 μm) compared with WT recipient mice (median, 23.7 μm; range, 0-79.2 μm; n = 3 biologic replicates). (D) Proliferation of individual LSCs in vivo was measured 48 hours after injection. Each LSC was identified as composed of single cells, doublets, or clusters (≥ 3 cells together) and expressed relative to the total number of cells per mouse (n = 3 for WT and Dkk1). LSC proliferation in vivo was similar between WT and Dkk1 recipients (P = .36 for doublets, P = .14 for clusters).
Cell-intrinsic activation of Wnt signaling in MLL-AF9 AML
To determine whether the MLL-AF9 fusion oncogene could be responsible for niche-independent activation of WNT signaling within the leukemia cell, gene expression data from patients with AML with t(9;11) (n = 20) were analyzed and compared with normal HSC-enriched CD34+ cells (n = 10). Differential expression of members of the WNT signaling pathway was observed (Goeman global test, P < .001). Unsupervised hierarchical clustering analysis distinguished t(9;11) AML samples from CD34+ samples based on differential gene expression of WNT pathway members (Figure 5A). To address the net effect on WNT signaling, the predicted effect on signaling for differentially expressed genes was examined (P < .05). Five of 7 genes overexpressed in t(9;11) AML were predicted to cause WNT pathway activation, including β-catenin, the central positive regulator of the pathway. In contrast, 6 of 7 genes overexpressed in CD34+ cells were predicted to lead to WNT pathway inhibition (Figure 5B). Collectively, these data suggest that the canonical WNT signaling pathway is activated by MLL-AF9, associated with t(9;11) in human AML.
MLL-AF9 AML exhibits cell-intrinsic Wnt activation. (A) Wnt signaling is enhanced in AML caused by the MLL-AF9 fusion oncogene. A comparison of gene expression analysis was performed on CD34+ patient cells (n = 10, enriched for HSC activity) and leukemia cells from patients with MLL-AF9–associated AML (n = 20). Unsupervised hierarchical clustering analysis of differentially expressed genes between CD34+ and AML samples. (B) Genes overexpressed in CD34+ were associated with inhibition of Wnt pathway, whereas genes overexpressed in AML were associated with activation of Wnt pathway. (C) Western blot of MLL-AF9 AML whole bone marrow compared with WT control bone marrow. (D) Western blot of representative AML samples derived from WT or Dkk1 recipient mice showing no difference in β-catenin stabilization between WT or Dkk1 recipient. (E) Real-time quantitative PCR on RNA extracted from LSCs. Analysis of gene expression of candidate Wnt/β-catenin target genes Cyclin D1 (Ccnd1), cMyc, and Axin2. Ccnd1 expression was higher in Dkk1 recipients than WT controls (P = .02); however, there were no significant differences between expression of cMyc or Axin2. The expression of canonical Wnt targets Tcf7 and Lef1 was below the detection of the assay.
MLL-AF9 AML exhibits cell-intrinsic Wnt activation. (A) Wnt signaling is enhanced in AML caused by the MLL-AF9 fusion oncogene. A comparison of gene expression analysis was performed on CD34+ patient cells (n = 10, enriched for HSC activity) and leukemia cells from patients with MLL-AF9–associated AML (n = 20). Unsupervised hierarchical clustering analysis of differentially expressed genes between CD34+ and AML samples. (B) Genes overexpressed in CD34+ were associated with inhibition of Wnt pathway, whereas genes overexpressed in AML were associated with activation of Wnt pathway. (C) Western blot of MLL-AF9 AML whole bone marrow compared with WT control bone marrow. (D) Western blot of representative AML samples derived from WT or Dkk1 recipient mice showing no difference in β-catenin stabilization between WT or Dkk1 recipient. (E) Real-time quantitative PCR on RNA extracted from LSCs. Analysis of gene expression of candidate Wnt/β-catenin target genes Cyclin D1 (Ccnd1), cMyc, and Axin2. Ccnd1 expression was higher in Dkk1 recipients than WT controls (P = .02); however, there were no significant differences between expression of cMyc or Axin2. The expression of canonical Wnt targets Tcf7 and Lef1 was below the detection of the assay.
Supporting these observations, increased stabilization of β-catenin, the critical downstream effector of Wnt signaling, was demonstrated in bone marrow from mice with AML compared with WT control mice (Figure 5C). There was no difference in levels of β-catenin stabilization between AML derived from WT or Dkk1 transgenic mice (Figure 5D; supplemental Figure 5). To determine whether osteoblastic cell-derived Dkk1 had any effect on Wnt activation in LSCs, real-time quantitative PCR was performed. There was no reduction in Wnt target gene expression in LSCs obtained from Dkk1 mice compared with WT (Figure 5E). Together, these data demonstrate that MLL-AF9 LSCs have substantial cell-intrinsic activation of Wnt signaling that is probably responsible for resistance to cell-extrinsic Wnt inhibition by Dkk1.
Discussion
These data identify a LSC niche that is both physically distinct and independent of the constraints of Wnt signaling that apply to normal HSCs. In the preleukemic setting, pre-LSCs are attracted to endosteal surfaces and home with apparent preference to osteoblastic cells. This suggests that these cells gain cell-extrinsic support from osteoblastic cells, which aids their survival and proliferation in vivo. With respect to cell-extrinsic Wnt signaling, it is probable that HSCs, pre-LSCs, and LSCs are affected by paracrine signals, such as the diffusion of soluble Wnt inhibitors (including Dkk1) or ligands rather than through direct cell-to-cell contact. Although the source of Dkk1 was from osteoblastic cells in this transgenic model, alternate niche components (such as mesenchymal stromal cells3 or endosteal macrophages24 ) could also conceivably regulate cell-extrinsic signaling in the niche. This is in contrast to known direct HSC-niche interactions via membrane-bound stem cell factor25 with the c-kit receptor and β-integrins with fibronectin.26 The close homing of pre-LSCs to osteoblastic cells would suggest that these cells require direct contact with osteoblasts, whereas LSCs from mice with established leukemia may use paracrine signaling pathways or may be independent of both.
The homing of pre-LSCs are consistent with xenograft transplantation models5 in which LSCs home to endosteal surfaces and use these sanctuaries to escape the toxic effects of chemotherapy. This pattern of homing also mimics that seen in HSC biology, a system where the requirements for bidirectional close interaction of HSCs and their microenvironment has been rigorously established. Importantly, there are a number of important caveats to this approach that should be recognized. First, a retroviral transduction, bone marrow transplantation system was used, leading to supra-physiologic oncogene expression and possible confounding by retroviral insertional mutagenesis. Second, the experiments used MLL-AF9 and related oncogenes to transform cells. MLL-AF9 was an ideal candidate for these studies because it is the product of one of the most frequent reciprocal translocations in patients with AML, it is associated with adverse prognosis in patients, and a highly-enriched LSCs population is readily isolated by flow cytometry. However, the purity of functional LSCs is still only 16%-25%, which may explain some of the variability in the distances between LSCs and osteoblast observed. In addition, we are currently pursuing similar approaches to determine whether the results found are generalizable to other oncogenic models of AML and related diseases. Finally, in vivo imaging represents a new and powerful tool to determine the juxtaposition between labeled LSCs and bone marrow components, such as osteoblastic cells; however, it is not yet able to provide definitive evidence of direct interactions between all putative components of the bone marrow hematopoietic microenvironment.
In pre-LSCs and HSCs, Wnt appears to be important in directing cell localization to the endosteum; however, in pre-LSCs, the Wnt cues are predominantly cell-intrinsic. HoxA9-Meis1a-GMP pre-LSCs, without activated Wnt signaling, do not home to endosteal surfaces and are unable to give rise to leukemia in vivo. The addition of constitutively active β-catenin to HoxA9-Meis1a-GMP (which is sufficient for leukemogenesis in vivo)12 caused pre-LSCs to home very closely to osteoblasts, suggesting that β-catenin activation in pre-LSCs may confer leukemogenic ability in part through enabling interactions with osteoblastic cell components of the niche. Wnt signaling has not been previously directly implicated in homing/engraftment. However, prostaglandin E2 has been shown to improve the engraftment of HSCs into the microenvironment and improve HSC function in a Wnt-dependent manner. Furthermore, Rac GTPases, key regulators of HSC engraftment, are critical for β-catenin signaling in other cell types.27-29
In contrast to the preleukemic models, we observed that established leukemia did not demonstrate the same requirement for close osteoblastic-LSC interactions. This suggests a gain of function in pathways conferring niche independence or reliance on alternative structures, such as vascular or perivascular components.4 These findings add dimension to the existing paradigm that LSCs selectively home to and engraft endosteal surfaces in xenograft models and suggest that LSCs may have distinctive zones within the microenvironment that foster their growth.5 In contrast, we have used a syngeneic leukemia model that precludes any potential impact of immunologic or cross-species incompatibility. Another important difference between the xenograft and syngenic models is that, in contrast to the pre-LSCs or human xenograft experiments, LSCs from established AML have a very high (∼ 1:4-6) leukemia initiating frequency.14 In LSCs, we observe in vivo proliferation of single LSC clones, supporting the interpretation that the observed population accurately represents LSCs in vivo. Interestingly, LSCs localized slightly closer to Dkk1-expressing osteoblastic cells, known to affect normal HSC function, than to WT osteoblastic cells. Leukemia cells have been described to generate an inhibitory niche for normal HSCs6 ; and although these data do not specifically examine HSC function, these results may suggest that the LSC niche itself might be one that normally would not support long-term HSC self-renewal.
These data provide new insights into the discrete patterns of homing and engraftment of leukemia initiating cells corresponding to the different stages of disease. Furthermore, these data demonstrate the independence of MLL-AF9 leukemia initiating cells on cell-extrinsic Wnt signals that constrain normal HSC function. Identification of the differential requirements for microenvironment interactions between LSCs and HSCs is fundamental to our biologic understanding of LSC self-renewal and may offer an opportunity to therapeutically target LSCs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Claudia Scholl and Stefan Fröhling, and members of the laboratories of D.G.G. and D.A.W. for technical advice and insightful comments.
S.W.L. was supported by the National Health and Medical Research Council of Australia, Australia/United States Fulbright Commission and Hematology Society of Australia and New Zealand. D.A.W. was supported by the National Institutes of Health (DK62757). D.T.S. was supported by the National Institutes of Health (HL44851). S.A.A. was supported by the National Institutes of Health (CA66996). Work performed in the laboratory of S.A.A. was also supported by the Leukemia & Lymphoma Society and the American Cancer Society. L.B. was supported in part by the Deutsche Forschungsgemeinschaft (Heisenberg-Stipendium BU 1339/3-1).
National Institutes of Health
Authorship
Contribution: S.W.L., C.L.C., Y.J.W., and C.R. designed experiments, performed experiments, and interpreted data; S.W.L. and C.L.C. wrote the manuscript; L.B. provided gene expression data and analysis; F.F., S.S., and S.M.S. performed experiments; and C.P.L., D.A.W., D.T.S., D.G.G., and S.A.A. designed experiments, reviewed the manuscript, and provided supervision.
Conflict-of-interest disclosure: D.T.S is a shareholder of Fate Therapeutics and consultant for Genzyme, Hospira and Fate Therapeutics. D.G.G. is now an employee of Merck. The remaining authors declare no competing financial interests.
Correspondence: David A. Williams, Division of Hematology/Oncology, Children's Hospital Boston and Dana-Farber Cancer Institute, Harvard Medical School and the Harvard Stem Cell Institute, 300 Longwood Ave, Karp 08125.3, Boston, MA 02115; e-mail: dawilliams@childrens.harvard.edu; Scott A. Armstrong, Division of Hematology/Oncology, Children's Hospital Boston and Dana-Farber Cancer Institute, Harvard Medical School and the Harvard Stem Cell Institute, 300 Longwood Ave, Karp 08211, Boston, MA 02115; e-mail: scott.armstrong@childrens.harvard.edu; and David T. Scadden, Department of Stem Cell and Regenerative Biology, Center for Regenerative Medicine, Massachusetts General Hospital, Harvard Medical School and the Harvard Stem Cell Institute, 185 Cambridge St, Boston, MA 02114; e-mail: dscadden@mgh.harvard.edu.
References
Author notes
S.W.L., Y.J.W., and C.L.C. contributed equally to this study.