Abstract
Type 17 programmed CD161hiCD8α+ T cells contribute to mucosal immunity to bacteria and yeast. In early life, microbial colonization induces proliferation of CD161hi cells that is dependent on their expression of a semi-invariant Vα7.2+ TCR. Although prevalent in adults, CD161hiCD8α+ cells exhibit weak proliferative and cytokine responses to TCR ligation. The mechanisms responsible for the dichotomous response of neonatal and adult CD161hi cells, and the signals that enable their effector function, have not been established. We describe acquired regulation of TCR signaling in adult memory CD161hiCD8α+ T cells that is absent in cord CD161hi cells and adult CD161lo cells. Regulated TCR signaling in CD161hi cells was due to profound alterations in TCR signaling pathway gene expression and could be overcome by costimulation through CD28 or innate cytokine receptors, which dictated the fate of their progeny. Costimulation with IL-1β during TCR ligation markedly increased proinflammatory IL-17 production, while IL-12–induced Tc1-like function and restored the response to TCR ligation without costimulation. CD161hi cells from umbilical cord blood and granulocyte colony stimulating factor-mobilized leukaphereses differed in frequency and function, suggesting future evaluation of the contribution of CD161hi cells in hematopoietic stem cell grafts to transplant outcomes is warranted.
Introduction
Conventional TCRαβ+ T cells undergo thymic selection, which in healthy individuals provides a diverse TCR repertoire capable of binding peptide antigens presented by polymorphic MHC molecules, without inducing autoimmunity.1 In contrast, subsets of innate-like TCRαβ+ cells express invariant or semi-invariant TCRs that respond to conserved ligands presented by ‘nonclassic’ nonpolymorphic MHC-like molecules.2-4 Subsets of memory T cells have been described within both the conventional and innate-like TCRαβ+ CD8α+ T-cell compartments that differ in TCR repertoire, homing, effector function, and the capacity to protect against specific pathogens.5-9
We identified a subset of human TCRαβ+ CD8α+ T cells characterized by expression of high levels of CD161 and costimulatory molecules, and showed these cells have an increased capacity for rapid ABCB1-mediated efflux of cytotoxic drugs and chemotherapy resistance.10 CD161hiCD8α+ memory T cells, in contrast to their CD161lo counterparts, were found by others to predominantly express TCRs composed of the invariant Vα7.2-Jα33 TCRα chain and a limited repertoire of TCRβ chains, and to be activated in the presence of microbes through recognition of the nonpolymorphic MHC class Ib molecule, MR1.4,11-15 CD161hi cells are prevalent in blood, and localize to extra-nodal tissues, including the liver and gastrointestinal mucosa, leading to their designation as mucosal-associated invariant T (MAIT) cells.10,11,14,16 CD161hiCD8α+ cells express high levels of RORγ, and in addition to IFN-γ, a subset secretes IL-17 in response to mitogen stimulation.16 Collectively, these results led to the hypothesis that CD161hiCD8α+ cells preferentially contribute to prevention of mucosal bacterial and yeast infection, in contrast to their CD161lo counterparts that control viral infections.4,11,12
CD161hi CD8α+ T cells are found at low frequency in umbilical cord blood (UCB) but their numbers increase rapidly after birth in response to gastrointestinal colonization.14,15,17 Accumulation of the homologous murine Vα19+ subset is dependent on MR1, suggesting that proliferation is induced by cognate semi-invariant TCR ligation mediated by MR1. In adults, the CD161hiCD8α+ subset is maintained at high frequency, but contains a very low fraction of Ki-67+ cells and proliferates and produces cytokines poorly to anti-CD3 monoclonal antibody (αCD3) stimulation.10,18 The mechanisms responsible for this seemingly dichotomous response of neonatal and adult CD161hi cells and the signals required to activate effector function in adult CD161hi T cells are unknown. Using gene expression profiling and functional assays of purified T-cell subsets, we show that TCR signaling and effector function of CD161hiCD8α+ T cells in adults is subject to regulation that is distinct from that in CD161loCD8α+ T cells and UCB CD161hi cells. TCR signaling could be augmented by costimulation through CD28 or innate cytokine receptors, which, depending on the costimulatory cytokines, could either markedly increase IL-17 production by the progeny of CD161hi cells or induce a predominantly Tc1-like response that was no longer constrained by TCR regulation. This regulation of CD161hiCD8α+ T cells might allow this large semi-invariant subset to distinguish pathogenic from commensal microbes, and suggests a potential role for dysregulation of TCR signaling in CD161hi cells in inflammatory diseases that have been linked to IL-17 production including autoimmunity and GVHD after allogeneic HSCT.16,19-25 We show there are profound differences in the frequency and regulation of CD161hi cells in different hematopoietic stem cell (HSC) graft sources, suggesting that their contribution to immunologic recovery and/or immune mediated pathology after HSC transplant (HSCT) warrants further study.
Methods
Blood samples
Peripheral blood from healthy donors and HSCT patients, and leukapheresis products from G-CSF–mobilized HSCT donors were obtained after written informed consent. UCB was obtained with maternal consent. Studies were performed according to guidelines of the Declaration of Helsinki with approval from the Institutional Review Board of the Fred Hutchinson Cancer Research Center.
Antibodies and cytokines
Monoclonal antibodies (mAbs) to CD3, CD8α, CD4, CD16, TcRγδ, TcRαβ, CD161, granzyme B, perforin, and IFN-γ were obtained from BD Biosciences; Vα24 antibody was from Coulter Immunotech; CD95, IL-17A, RORγ and Tbet antibodies were from eBioscience; and CD62L antibody was from Biolegend. Recombinant human IL-1β, IL-12 and IL-23 were obtained from R&D Systems; IL-18 was obtained from MBL International; and IL-2 was obtained from Novartis. IL-1β, IL-12, IL-18, and IL-23 were used at 10 ng/mL and IL-2 was used at 20 U/mL.
Immunophenotyping
Cells were surface labeled with antibodies for 20 minutes on ice. Acquisition was performed on an LSR-II (BD Biosciences) with analysis using FlowJo Version 8.5.3 software (TreeStar). Adult blood CD161hi/CD62L+, CD161hi/CD62L−, CD161lo/CD62L+, and CD161lo/CD62L− subsets were identified within the CD4−/CD16−/TcRγδ−/Vα24−/CD3+/CD8α+/CD95+ pool. For detection of intracellular antigens, cells were stained with live/dead Fixable Violet Stain (Invitrogen) followed by surface staining, fixation, permeabilization and intracellular staining.
Isolation of CD8α+ T-cell subsets
Effluxing CD161hi and noneffluxing CD161lo CD8α+ subsets in the adult blood CD62L+ and CD62L− compartments were enriched to > 98% purity, as described.10 Briefly, CD8+ cells were selected using CD8 Microbeads (Miltenyi), loaded with Rh123 (Sigma-Aldrich) and cultured for 30 minutes to allow Rh123 efflux, then labeled with fluorochrome-conjugated antibodies to CD4, CD16, TCRγδ, Vα24, CD8α, CD95, CD62L and CD161 before sort-purification on a FACS ARIA equipped with 405 nm, 488 nm and 633 nm lasers (BD Biosciences). CD161hi CD62L+ and CD62L− subsets were identified as CD62L+/Rh123lo/CD161hi and CD62L−/Rh123lo/CD161hi events, respectively, in the CD4−/CD16−/TcRγδ−/Vα24−/CD8α+/CD95+ population. CD161lo CD62L+ and CD62L− subsets were identified as CD62L+/Rh123hi/CD161int/neg and CD62L−/Rh123hi/CD161int/neg events, respectively, in the CD4−/CD16−/TcRγδ−/Vα24−/CD8α+/CD95+ population. Naive T cells (TN) were identified as CD4−/CD16−/TcRγδ−/Vα24−/CD8α+/CD95−/CD62L+ events. CD161hi cells in G-CSF-mobilized HSCT donors and UCB were identified as CD4−/CD16−/TCRγδ−/Vα24−/CD8α+/CD161hi events and were sort-purified from CD8+ cells isolated using CD8 Microbeads.
Proliferation assay
Subsets were cultured in 96 well plates at 1-2 × 104 cells/well for 3 days with plate-bound αCD3 1 μg/mL (OKT3, Ortho Biotech), with or without αCD28 5 μg/mL (Fred Hutchinson Cancer Research Center Shared Resources) or cytokines as indicated, then pulsed overnight with tritiated thymidine before harvesting and scintillation counting (Perkin Elmer).
Luminex cytokine bead array assay
Sort-purified memory CD8+ T-cell subsets were cultured for 18 hours at 4-6 × 104 cells per well in 96 well plates coated with αCD3 mAb (1 μg/mL), with or without αCD28 mAb (5 μg/mL); or in uncoated plates in medium supplemented or not with PMA 5 ng/mL (Sigma-Aldrich) and ionomycin 1 μg/mL (Sigma-Aldrich). Supernatant cytokine concentrations were assessed by multiplex cytokine bead assay according to the manufacturer's instructions (Luminex).
Intracellular cytokine flow cytometry
T cells were cultured alone or with PMA 5 ng/mL and ionomycin 1 μg/mL for 90 minutes before addition of brefeldin A 10 μg/mL and culture for 4 additional hours. Cytokines were detected by intracellular staining.
Transcriptional profiling
Complete methods are described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In brief, transcriptional profiling was performed using Illumina WG-6 Expression BeadChips Version 3.0 (Illumina). Differential gene expression was defined by a log2 ratio > 0.585 ( ± 1.5-fold) and false discovery rate (FDR) = 0.05 and signatures were analyzed using the Ingenuity Pathway Analysis application (Ingenuity Systems Inc), Partek Version 6.4 software (Partek Inc), R, TIGR's MultiExperimental Viewer (MeV), and by GSEA (Broad Institute, Massachusetts Institute of Technology).26-28 Probabilities associated with the shared transcriptional profiles were determined using the hypergeometric distribution. Data are available in Gene Expression Omnibus (GEO; accession number GSE23663).
Differentiation assays
Sort-purified subsets were stimulated with αCD3 (1 μg/mL), αCD28 (5 μg/mL) and indicated cytokines, and maintained for 14 days with IL-2 supplementation every 3-4 days, before assessment of cytokine secretion, immunophenotype and proliferation.
Statistical methods
Statistical analysis was performed using GraphPad Prism 5 (GraphPad). Data are shown as the mean ± SE. Two-tailed Mann-Whitney or paired t tests were used to compare 2 groups. Multiple conditions were compared using 1-way ANOVA.
Results
Adult CD161hiCD8α+ memory T cells acquire regulation of TCR signaling.
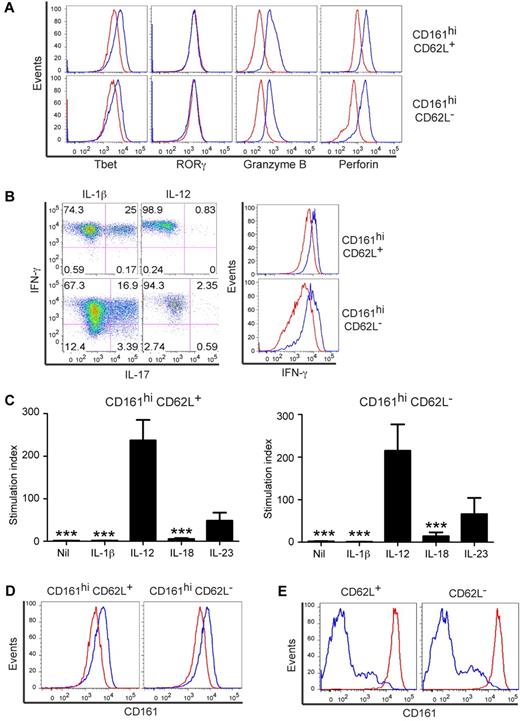
In contrast to conventional CD161lo memory cells, CD161hi memory cells in adult blood proliferate and produce cytokines poorly in response to αCD3.10,18 This led to the suggestion that they represented an anergic, terminally differentiated subset,18 although this seems inconsistent with our findings that CD161hi memory T cells are prevalent in both CD62L+ and CD62L− subsets of adult blood (Figure 1A), and express high levels of CD27, CD28, and bcl-2, and low levels of granzyme B and perforin.10 Naive CD161hiCD8α+ T cells are present in small numbers in UCB and proliferate rapidly in early life in response to microbial colonization to provide a larger frequency of CD161hi memory cells,11,14,15 suggesting that the response to TCR signaling may be regulated differently in naive and memory CD161hi cells. Therefore, we compared responses of sort-purified CD161hi and CD161lo memory T cells from adults and CD161hi naive T cells from UCB to αCD3. We separated adult CD161hi and CD161lo memory subsets by expression of CD62L because CD62L expression distinguishes CD8+ subsets with different proliferative and effector functions. Unlike CD161lo memory cells, CD161hi cells in both CD62L+ and CD62L− memory compartments did not proliferate to αCD3 stimulation (Figure 1B). In contrast, naive UCB CD161hi cells proliferated briskly to αCD3 (Figure 1C), indicating that the lack of proliferation by CD161hi cells to αCD3 is acquired during the transition from birth to early adulthood.
Acquired down-regulation of the response to TCR signaling in CD161hi CD62L+ and CD62L− memory CD8α+ T-cell subsets. (A) The FACS plot (left) is gated on CD95+ memory CD8α+ TcRαβ+ T cells and quadrants indicate high versus intermediate/absent expression of CD161. The graph (right) shows the percentages of CD161hi cells within the CD62L+ and CD62L− memory subsets. Horizontal bars represent the mean ± SE of experiments in 57 individuals. (B) Sort-purified adult memory subsets were cultured with αCD3 for 72 hours before assessment of proliferation by tritiated thymidine incorporation. Data are representative of the mean ± SE of 13 separate experiments. (C) UCB CD161hi cells were cultured alone (Nil) or with αCD3 for 72 hours before assessment of proliferation by tritiated thymidine incorporation. Data are representative of the mean ± SE of 4 separate experiments. (D-E) Sort-purified subsets were stimulated with αCD3 (D) or PMA/ionomycin (E) and cytokine concentrations were assessed in the supernatant 18 hours later. Data represent the mean ± SE of at least 4 separate experiments.
Acquired down-regulation of the response to TCR signaling in CD161hi CD62L+ and CD62L− memory CD8α+ T-cell subsets. (A) The FACS plot (left) is gated on CD95+ memory CD8α+ TcRαβ+ T cells and quadrants indicate high versus intermediate/absent expression of CD161. The graph (right) shows the percentages of CD161hi cells within the CD62L+ and CD62L− memory subsets. Horizontal bars represent the mean ± SE of experiments in 57 individuals. (B) Sort-purified adult memory subsets were cultured with αCD3 for 72 hours before assessment of proliferation by tritiated thymidine incorporation. Data are representative of the mean ± SE of 13 separate experiments. (C) UCB CD161hi cells were cultured alone (Nil) or with αCD3 for 72 hours before assessment of proliferation by tritiated thymidine incorporation. Data are representative of the mean ± SE of 4 separate experiments. (D-E) Sort-purified subsets were stimulated with αCD3 (D) or PMA/ionomycin (E) and cytokine concentrations were assessed in the supernatant 18 hours later. Data represent the mean ± SE of at least 4 separate experiments.
Too few CD161hi cells were obtained from UCB to analyze cytokine secretion; however, we evaluated cytokine secretion after αCD3 stimulation of adult CD161hi and CD161lo CD62L+ and CD62L− cells to determine whether the reduced proliferation was associated with lower cytokine production. CD161hi cells produced markedly lower levels of cytokines compared with CD161lo cells (Figure 1D). Minimal IL-17 secretion was detected from CD161hi or CD161lo cells after αCD3 stimulation (data not shown). To exclude the possibility that the low IFN-γ, IL-2, MIP-1α, and TNF-α production by CD161hi cells was because of polarized cytokine secretion, we evaluated cytokine secretion after PMA and ionomycin stimulation to bypass TCR signaling (Figure 1E). The CD161hi subsets secreted markedly higher levels of IL-17 than CD161lo subsets and less IL-2 than the CD161lo CD62L+ subset, but secretion of IFN-γ, MIP-1α, and TNF-α was equivalent to their CD161lo counterparts (Figure 1E). Thus, in addition to an acquired reduction in proliferation to TCR signaling, adult CD161hiCD8α+ memory T cells exhibit diminished effector function compared with conventional CD161lo memory cells.
The transcriptional profiles of semi-invariant CD161hi and conventional CD161lo memory subsets are distinct
To provide insight into the basis for the functional differences between semi-invariant CD161hi and conventional CD161lo memory cells, we segregated memory CD95+ Rh123 effluxing CD161hi cells into CD62L+ and CD62L− subsets, and compared their gene expression profiles with the conventional noneffluxing CD95+ CD161lo CD62L+ and CD62L− subsets, and with adult CD8α+ naive (TN) cells. Insufficient UCB or adult naive CD161hi cells were obtained for transcriptional analysis. The transcriptional profiles of CD95+ CD161hi and CD161lo subsets were remarkably distinct (Figure 2A-B; supplemental Methods). Compared with their CD161lo counterparts and TN cells, CD161hi subsets in both the CD62L+ and CD62L− memory compartments expressed significantly higher levels of ZBTB16, a transcription factor associated with the development of semi-invariant CD161hi T cells15 ; CXCR6 and CCR2, consistent with their proposed tissue homing properties; and type 17–associated genes, including RORC, RORA, IL23R, KLRB1, CCR6, and CCL20 (Figure 2C). More genes were differentially expressed between subpopulations distinguished by CD161 expression (CD161hi vs CD161lo CD62L+ cells, 992 genes; CD161hi vs CD161lo CD62L− cells, 715 genes), compared with those identified on the basis of CD62L expression (CD161hi CD62L+ vs CD161hi CD62L− cells, 265 genes; CD161lo CD62L+ vs CD161lo CD62L− cells, 302 genes). Thus, the expression profile of semi-invariant CD161hi cells is profoundly different from their CD161lo counterparts, and CD62L expression further defines transcriptionally distinct subsets within each of the CD161hi and CD161lo subsets.
Transcriptional profiling identifies 4 distinct memory CD8α+ T-cell subsets defined by expression of CD161 and CD62L. (A) Principal component analysis shows grouping of gene expression profiles of TN (orange), CD161hi CD62L+ (red), CD161hi CD62L− (green), CD161lo CD62L+ (blue), and CD161lo CD62L− (purple) subsets isolated from 6 healthy individuals. (B) Hierarchical cluster analysis of gene expression in sort-purified memory subsets compared with matched TN cells from 6 healthy individuals shows transcriptionally distinct memory subsets. Genes and subsets are clustered in columns and rows, respectively. All samples of each subset clustered together; therefore, only the names of the clustered subsets and not the individual samples are shown in the figure. Red profiles represent higher gene expression (maximum 32-fold) and green profiles represent lower gene expression (minimum 32-fold) compared with matched TN cells. (C) Volcano plots show differentially expressed transcriptional profiles of CD161hi CD62L+ compared with CD161lo CD62L+ cells (top left), CD161hi CD62L+ compared with TN cells (top right), CD161hi CD62L− compared with CD161lo CD62L− cells (bottom left), and CD161hi CD62L− compared with TN cells (bottom right) from 6 healthy individuals. Each point represents a single gene and/or probe, and type 17 associated genes are color-coded, as indicated in the legend. The numerator and denominator defining the mean log2 ratio of gene expression are listed first and second, respectively, in the volcano plot title; thus, genes expressed at higher levels in the numerator subset are depicted on the right of each plot.
Transcriptional profiling identifies 4 distinct memory CD8α+ T-cell subsets defined by expression of CD161 and CD62L. (A) Principal component analysis shows grouping of gene expression profiles of TN (orange), CD161hi CD62L+ (red), CD161hi CD62L− (green), CD161lo CD62L+ (blue), and CD161lo CD62L− (purple) subsets isolated from 6 healthy individuals. (B) Hierarchical cluster analysis of gene expression in sort-purified memory subsets compared with matched TN cells from 6 healthy individuals shows transcriptionally distinct memory subsets. Genes and subsets are clustered in columns and rows, respectively. All samples of each subset clustered together; therefore, only the names of the clustered subsets and not the individual samples are shown in the figure. Red profiles represent higher gene expression (maximum 32-fold) and green profiles represent lower gene expression (minimum 32-fold) compared with matched TN cells. (C) Volcano plots show differentially expressed transcriptional profiles of CD161hi CD62L+ compared with CD161lo CD62L+ cells (top left), CD161hi CD62L+ compared with TN cells (top right), CD161hi CD62L− compared with CD161lo CD62L− cells (bottom left), and CD161hi CD62L− compared with TN cells (bottom right) from 6 healthy individuals. Each point represents a single gene and/or probe, and type 17 associated genes are color-coded, as indicated in the legend. The numerator and denominator defining the mean log2 ratio of gene expression are listed first and second, respectively, in the volcano plot title; thus, genes expressed at higher levels in the numerator subset are depicted on the right of each plot.
TCR signaling is regulated at multiple levels in adult CD161hi CD62L+ and CD62L− cells and is distinct from that in anergic or exhausted T cells
We next performed transcriptional pathway analysis to provide insight into mechanisms that may regulate TCR signaling in CD161hi cells. Multiple genes encoding proteins that positively regulate TCR signaling including ITK, MAL, CD8A, CD8B, KRAS, PPP3CC, and IKBKE were expressed at lower levels in CD161hi subsets compared with CD161lo subsets (Figure 3A); and genes encoding negative regulators of TCR and cytokine signaling, including SOCS1, SOCS2, SOCS3, DUSP5, DUSP6, and SPRY1 were expressed at higher levels in CD161hi subsets compared with CD161lo subsets (Figure 3B). Differences in TCR signaling pathway gene expression between CD161hi and CD161lo subsets were greater for CD62L+ cells than for CD62L− cells, and genes encoding proteins in the proximal TCR signaling pathway were down-regulated more than those in the distal pathway.
The TCR signaling pathway is regulated at multiple levels in CD161hi CD62L+ and CD62L− cells. (A) A transcriptional pathway analysis of the TCR signaling pathway in CD161hi and CD161lo CD62L+ subsets from 6 healthy donors was created using Ingenuity Systems software (IPA 7). Green and red symbols represent significantly down-regulated or up-regulated genes, respectively, in CD161hi CD62L+ cells compared with their CD161lo counterparts. White, gray, and orange symbols are not differentially expressed between subsets. (B) Volcano plots showing differentially expressed transcriptional profiles of CD161hi CD62L+ (left) and CD161hi CD62L− (right) subsets compared with their CD161lo counterparts are shown from 6 healthy donors. Genes/probes are color-coded as indicated in the legend.
The TCR signaling pathway is regulated at multiple levels in CD161hi CD62L+ and CD62L− cells. (A) A transcriptional pathway analysis of the TCR signaling pathway in CD161hi and CD161lo CD62L+ subsets from 6 healthy donors was created using Ingenuity Systems software (IPA 7). Green and red symbols represent significantly down-regulated or up-regulated genes, respectively, in CD161hi CD62L+ cells compared with their CD161lo counterparts. White, gray, and orange symbols are not differentially expressed between subsets. (B) Volcano plots showing differentially expressed transcriptional profiles of CD161hi CD62L+ (left) and CD161hi CD62L− (right) subsets compared with their CD161lo counterparts are shown from 6 healthy donors. Genes/probes are color-coded as indicated in the legend.
Our previous studies showed that CD161hiCD8α+ T cells exhibited a surface phenotype and pattern of cytokine production that is distinct from those of “exhausted” and “anergic” T cells described in chronic viral infection and tolerance, respectively.10,29-31 However, it was possible that the reduced responsiveness of CD161hi cells to TCR stimulation might be mediated by similar transcriptional alterations observed in exhausted or anergic T cells. We established a differentially expressed gene profile between functional CD161lo cells in the CD62L+ and CD62L− subsets and their CD161hi counterparts, and compared up-regulated and down-regulated gene sets in CD161hi and CD161lo memory cells with published datasets of genes that were differentially expressed between exhausted and functional memory cells in chronic murine LCMV infection.31 Apart from LCK, which was down-regulated in exhausted cells and CD161hi CD62L+ cells, no TCR signaling pathway genes were similarly up-regulated or down-regulated in exhausted CD8+ T cells and CD161hi subsets, but not in CD161lo subsets. Gene set enrichment analysis (GSEA) found no enrichment of genes expressed in both exhausted compared with functional memory cells, and CD161hi subsets compared with CD161lo subsets.
We then generated a list of candidate genes reported to be up-regulated in T cells in models of anergy (supplemental Dataset) to determine whether there was enrichment of genes expressed in anergic cells in the dataset of genes expressed in CD161hi or CD161lo cells.29,32 We found an overrepresentation of anergy-associated genes in the genes expressed at higher levels in the CD161lo subsets (CD161loCD62L+, P = .03; CD161loCD62L−, P = .02) and not those expressed in the CD161hi subsets (CD161hiCD62L+, P = .29; CD161hiCD62L−, P = .27). While there are limitations of comparing datasets derived from murine models of T-cell exhaustion and anergy with human T-cell expression data, the findings suggest that the mechanisms for regulating TCR signaling in CD161hi T cells are not shared with these models of T-cell nonresponsiveness.
Costimulation through CD28 or with innate cytokines increases proliferation of memory CD161hiCD8α+ T cells and the frequency of cells that secrete IL-17
The polymicrobial specificity and high frequency of CD8α+CD161hi cells in healthy adults implies these cells have a key role in normal immunity, and the distinct regulation of TCR signaling suggests that additional signals from activated APC might be required to induce effector function of CD161hi cells. CD161hiCD8α+ cells are uniformly CD28+ and express higher levels of IL1RAP, IL12RB1, IL18RAP, IL18R1, and IL23R, compared with CD161lo cells (supplemental Dataset).10 Therefore, we assessed proliferation and cytokine production of sort-purified CD161hi and CD161lo CD62L+ and CD62L− cells after stimulation with αCD3 alone, αCD3 with αCD28 monoclonal antibodies (αCD3/αCD28), and αCD3 with IL-1β, IL-12, IL-18, or IL-23. Addition of αCD28 augmented proliferation of CD161hi cells and secretion of IFN-γ, IL-2, IL-17, MIP-1α, and TNF-α (Figure 4A-B), although the cytokine levels were lower than after PMA/ionomycin stimulation (Figure 1E). No proliferation of CD161hi subsets was seen in cultures with IL-1β, IL-12, IL-18, or IL-23 alone (data not shown), but the addition of IL-1β, IL-12, IL-18, or IL-23 to αCD3 induced substantial proliferation of CD161hi CD62L+ and CD62L− subsets (Figure 5A). Moreover, costimulation with IL-1β, IL-18, and IL-23 enhanced proliferation of CD161hi subsets to a greater extent than CD161lo subsets (Figure 5B).
Costimulation with αCD28 induces proliferation and cytokine secretion in CD161hi CD8α+ T cells. (A,B) Sort-purified subsets were cultured with αCD3 (white) or αCD3/αCD28 (black) before assessment of proliferation after 72 hours (A) and cytokine secretion after 18 hours (B). Data represent the mean ± SE of at least 5 experiments.
Costimulation with αCD28 induces proliferation and cytokine secretion in CD161hi CD8α+ T cells. (A,B) Sort-purified subsets were cultured with αCD3 (white) or αCD3/αCD28 (black) before assessment of proliferation after 72 hours (A) and cytokine secretion after 18 hours (B). Data represent the mean ± SE of at least 5 experiments.
Innate cytokines present during TCR signaling induce proliferation of CD161hi CD8+ T cells. (A) Sort-purified CD161hi CD62L+ (left) and CD62L− (right) cells were cultured with αCD3 with or without IL-1β, IL-12, IL-18, IL-23, or αCD28 for 72 hours before assessment of proliferation by tritiated thymidine incorporation. The broken lines represent the stimulation indices of CD161lo CD62L+ (left) and CD161lo CD62L− cells (right) obtained after stimulation with αCD3 alone. The P values (*P < .05, **P < .01) refer to the difference between the stimulation indices of cells stimulated with αCD3 in the presence of the indicated costimulation and cells stimulated with αCD3 alone (Nil). Data represent the mean ± SE of 4 separate experiments. (B) Costimulation with IL-1β, IL-18, and IL-23 has a more selective effect on CD161hi cells than on CD161lo cells. Sort-purified subsets were cultured with αCD3 with or without IL-1β, IL-12, IL-18, or IL-23 for 72 hours before assessment of proliferation. Data represent the mean ± SE of at least 4 separate experiments. (C) Sort-purified CD161hi CD62L+ and CD62L− cells were stimulated with PMA and ionomycin before intracellular staining with antibodies to IL-17. Data from 5 individuals are depicted. (D) CD161hi CD62L+ (left) and CD62L− cells (right) were stimulated with αCD3/αCD28 and IL-1β, IL-12, IL-18, or IL-23. After 14 days, the cells were stimulated with PMA/ionomycin and the fraction of IL-17 secreting cells was determined by intracellular cytokine flow cytometry. Data represent the mean ± SE of at least 5 separate experiments. P values were determined by 1-way ANOVA.
Innate cytokines present during TCR signaling induce proliferation of CD161hi CD8+ T cells. (A) Sort-purified CD161hi CD62L+ (left) and CD62L− (right) cells were cultured with αCD3 with or without IL-1β, IL-12, IL-18, IL-23, or αCD28 for 72 hours before assessment of proliferation by tritiated thymidine incorporation. The broken lines represent the stimulation indices of CD161lo CD62L+ (left) and CD161lo CD62L− cells (right) obtained after stimulation with αCD3 alone. The P values (*P < .05, **P < .01) refer to the difference between the stimulation indices of cells stimulated with αCD3 in the presence of the indicated costimulation and cells stimulated with αCD3 alone (Nil). Data represent the mean ± SE of 4 separate experiments. (B) Costimulation with IL-1β, IL-18, and IL-23 has a more selective effect on CD161hi cells than on CD161lo cells. Sort-purified subsets were cultured with αCD3 with or without IL-1β, IL-12, IL-18, or IL-23 for 72 hours before assessment of proliferation. Data represent the mean ± SE of at least 4 separate experiments. (C) Sort-purified CD161hi CD62L+ and CD62L− cells were stimulated with PMA and ionomycin before intracellular staining with antibodies to IL-17. Data from 5 individuals are depicted. (D) CD161hi CD62L+ (left) and CD62L− cells (right) were stimulated with αCD3/αCD28 and IL-1β, IL-12, IL-18, or IL-23. After 14 days, the cells were stimulated with PMA/ionomycin and the fraction of IL-17 secreting cells was determined by intracellular cytokine flow cytometry. Data represent the mean ± SE of at least 5 separate experiments. P values were determined by 1-way ANOVA.
We then examined whether culture with innate cytokines might affect subsequent IL-17 secretion by CD161hi cells. We stimulated sort-purified CD161hi CD62L+ and CD62L− cells with αCD3/αCD28 alone or in the presence of IL-1β, IL-12, IL-18, or IL-23 for 14 days, and then determined the frequency of cells that secreted IL-17 in response to PMA and ionomycin. Before culture, only 4.6 ± 1.5% and 8.3 ± 2.4% of CD161hi CD62L+ and CD62L− cells, respectively, secreted IL-17 to PMA/ionomycin stimulation (Figure 5C). The number of IL-17 secreting cells in both CD161hi subsets was markedly increased by culture with IL-1β (Figure 5D), while cultures stimulated in IL-12, IL-18, or IL-23 contained ∾ 3- to 4-fold lower IL-17 secreting cells. These findings demonstrate that specific innate cytokines provide signals that cooperate with TCR signaling to induce proliferation and enable effector function in CD161hiCD8α+ T cells.
Distinct innate cytokines dictate the programming of CD161hiCD8α+ cells and the response of their progeny to subsequent TCR signaling
The observation that CD161hiCD8α+ T cells that proliferated in the presence of IL-12 resulted in a lower frequency of cells capable of producing IL-17 raised the possibility that IL-12 might induce differentiation to Tc1-like cells. We stimulated sort-purified CD161hi subsets with αCD3/αCD28 and IL-12 or IL-1β and cultured them for 14 days before assessing their phenotype and function. Similar T-cell proliferation was observed during the 14-day culture period in all culture conditions (data not shown). The progeny of CD161hi cells stimulated in IL-1β maintained the phenotype of freshly isolated CD161hi cells, including low levels of Tbet, perforin, and granzyme B, and a high proportion produced both IL-17 and IFN-γ after PMA/ionomycin stimulation (Figure 6A-B). In contrast, cells stimulated in IL-12 expressed higher Tbet, perforin, granzyme B, and IFN-γ, but lost IL-17 secretion, consistent with a Tc1-like phenotype (Figure 6A-B). Despite the loss of IL-17 production, we did not observe a reduction in RORγ expression in CD161hi T cells cultured in IL-12 compared with those stimulated in IL-1β (Figure 6A).
Innate cytokines dictate the fate of CD161hi CD62L+ and CD62L− cells. (A) CD161hi CD62L+ (top) and CD62L− (bottom) cells were stimulated with αCD3/αCD28 and IL-1β (red) or IL-12 (blue) and cultured for 14 days before assessment of Tbet, RORγ, perforin, and granzyme B expression. Data represent at least 4 experiments. (B) CD161hi CD62L+ (top) and CD62L− (bottom) cells were stimulated and cultured for 14 days as in panel A before stimulation with PMA/ionomycin and assessment by cytokine flow cytometry (left panels). The IFN-γ MFI is shown (right panels) for cells cultured in IL-1β (red) or IL-12 (blue). Data represent at least 7 separate experiments. (C) CD161hi CD62L+ (left) and CD62L− (right) cells were stimulated with αCD3/αCD28 and innate cytokines, cultured for 14 days, and restimulated with αCD3 with or without αCD28 for 72 hours before assessment of tritiated thymidine incorporation. All conditions proliferated after stimulation on day 14 with αCD3/αCD28 (data not shown) and only those stimulated with αCD3 alone are shown. Data depict the mean ± SE of at least 5 experiments. ***P < .001 (1-way ANOVA) indicates a significant difference in proliferation of cells cultured in the indicated condition and those in IL-12. (D) Sort-purified subsets were stimulated with αCD3/αCD28 in the presence of IL-1β (red) or IL-12 (blue) and cultured for 14 days before assessment of CD161 expression. Data represent at least 5 experiments. (E) CD161hi (red) and CD161lo (blue) CD62L+ (left) and CD62L− (right) cells were stimulated with αCD3/αCD28 in the presence of IL-12 and cultured for 14 days before assessment of CD161 expression. Data represent 6 experiments.
Innate cytokines dictate the fate of CD161hi CD62L+ and CD62L− cells. (A) CD161hi CD62L+ (top) and CD62L− (bottom) cells were stimulated with αCD3/αCD28 and IL-1β (red) or IL-12 (blue) and cultured for 14 days before assessment of Tbet, RORγ, perforin, and granzyme B expression. Data represent at least 4 experiments. (B) CD161hi CD62L+ (top) and CD62L− (bottom) cells were stimulated and cultured for 14 days as in panel A before stimulation with PMA/ionomycin and assessment by cytokine flow cytometry (left panels). The IFN-γ MFI is shown (right panels) for cells cultured in IL-1β (red) or IL-12 (blue). Data represent at least 7 separate experiments. (C) CD161hi CD62L+ (left) and CD62L− (right) cells were stimulated with αCD3/αCD28 and innate cytokines, cultured for 14 days, and restimulated with αCD3 with or without αCD28 for 72 hours before assessment of tritiated thymidine incorporation. All conditions proliferated after stimulation on day 14 with αCD3/αCD28 (data not shown) and only those stimulated with αCD3 alone are shown. Data depict the mean ± SE of at least 5 experiments. ***P < .001 (1-way ANOVA) indicates a significant difference in proliferation of cells cultured in the indicated condition and those in IL-12. (D) Sort-purified subsets were stimulated with αCD3/αCD28 in the presence of IL-1β (red) or IL-12 (blue) and cultured for 14 days before assessment of CD161 expression. Data represent at least 5 experiments. (E) CD161hi (red) and CD161lo (blue) CD62L+ (left) and CD62L− (right) cells were stimulated with αCD3/αCD28 in the presence of IL-12 and cultured for 14 days before assessment of CD161 expression. Data represent 6 experiments.
We next evaluated whether CD161hi cells stimulated with αCD3/αCD28 and IL-12 could proliferate to αCD3 in the absence of costimulation, analogous to freshly isolated conventional CD161loCD8α+ T cells. Aliquots of CD161hi subsets were stimulated with αCD3/αCD28 and IL-1β, IL-12, IL-18, IL-23, or no innate cytokine and cultured for 14 days, then assayed for proliferation to αCD3 alone or with αCD28. After 14 days, CD161hi cells initially stimulated in IL-18 and IL-1β or without innate cytokines exhibited minimal proliferation to subsequent αCD3 stimulation unless costimulation was again provided, thus maintaining a similar functional phenotype to freshly isolated CD161hi cells (Figure 6C; and data not shown). In contrast, CD161hi cells initially stimulated in IL-12 briskly proliferated to αCD3 without costimulation, similar to freshly isolated CD161lo cells. The acquisition of responsiveness to αCD3 did not reflect outgrowth of contaminating CD161lo cells in the IL-12–stimulated cultures, because the CD161hi phenotype was retained on the progeny cells (Figure 6D), and CD161lo cells stimulated under the same conditions did not acquire CD161hi expression (Figure 6E). CD161hi cells initially stimulated in IL-23 also acquired the capacity to proliferate to αCD3 alone, but proliferation was less than that observed in cultures exposed to IL-12. The capacity of cells stimulated and cultured in each condition to subsequently proliferate to αCD3 in the absence of costimulation was inversely correlated with their ability to secrete IL-17 (Figures 5D, 6C). The data demonstrate that innate cytokines that enable effector function in CD161hi cells also dictate the fate of TCR signaling pathway regulation in their progeny.
CD161hi cells differ in frequency and in TCR signaling in UCB and G-CSF–mobilized leukapheresis products
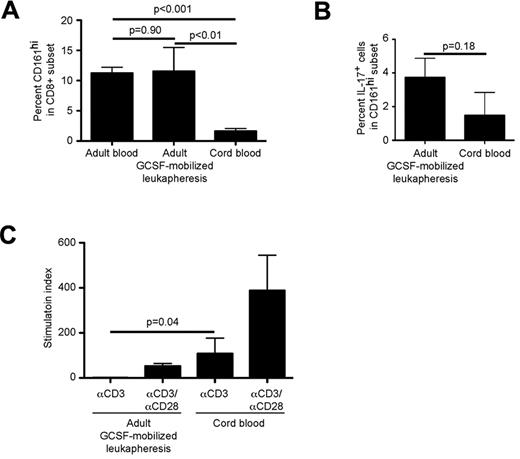
The composition of conventional T-cell subsets in different donor HSC graft sources can influence the incidence of postHSCT complications such as GVHD and infection. Mucosal disruption, microbial translocation and inflammation contribute to GVHD pathogenesis and infection after HSCT. Thus, the frequency and function of CD161hi CD8α+ T cells in different HSC graft sources, and their migration to the gastrointestinal tract and reconstitution after HSCT could influence clinical outcomes. We compared CD161hi cells in leukapheresis products obtained from adults who had received G-CSF for 4 days for HSC mobilization with their counterparts from UCB. CD161hi cells in G-CSF–mobilized blood were present at a similar frequency to CD161hi cells in blood from adults who had not received G-CSF, but in much higher frequency and absolute number compared with UCB (Figure 7A). A greater fraction of CD161hi cells from G-CSF–mobilized donors secreted IL-17 in response to PMA/ionomycin than from UCB, but the difference did not reach statistical significance (Figure 7B). The dependence of CD161hi cells on costimulation for cell proliferation also differed depending on the graft source. Whereas CD161hi cells from UCB proliferated to αCD3 alone, those from G-CSF-mobilized blood, like CD161hi cells in nonmobilized adult blood (Figure 1B), did not proliferate to αCD3 and were dependent on costimulation (Figure 7C), suggesting that G-CSF mobilization does not affect regulation of TCR signaling in CD161hi cells. These results reveal dramatic differences in the frequency and function of CD161hiCD8α T cells in different HSC graft sources.
CD161hi cells in UCB and G-CSF–mobilized leukapheresis products differ in frequency and in regulation of response to TCR signaling. (A) Percentages of CD161hi T cells in CD8α+ T cells in adult blood (n = 57), G-CSF–mobilized adult leukapheresis products (n = 5) and UCB units (n = 5) were evaluated by flow cytometry. Data represent the mean ± SE. (B) Immunomagnetically selected CD8α+ T cells from G-CSF–mobilized adult leukapheresis products (n = 5) and UCB units (n = 5) were stimulated with PMA/ionomycin for 5 hours followed by surface and intracellular labeling to identify IL-17 secreting CD161hi cells. (C) CD161hiCD8α+ T cells were isolated from G-CSF–mobilized leukapheresis products (n = 3) and UCB units (n = 4) and stimulated with αCD3 in the absence of costimulation followed by assessment of tritiated thymidine incorporation after 72 hours. Proliferation of CD161hi cells from G-CSF–mobilized leukapheresis was restored in the presence of αCD28 costimulation (data not shown). Data represent the mean ± SE.
CD161hi cells in UCB and G-CSF–mobilized leukapheresis products differ in frequency and in regulation of response to TCR signaling. (A) Percentages of CD161hi T cells in CD8α+ T cells in adult blood (n = 57), G-CSF–mobilized adult leukapheresis products (n = 5) and UCB units (n = 5) were evaluated by flow cytometry. Data represent the mean ± SE. (B) Immunomagnetically selected CD8α+ T cells from G-CSF–mobilized adult leukapheresis products (n = 5) and UCB units (n = 5) were stimulated with PMA/ionomycin for 5 hours followed by surface and intracellular labeling to identify IL-17 secreting CD161hi cells. (C) CD161hiCD8α+ T cells were isolated from G-CSF–mobilized leukapheresis products (n = 3) and UCB units (n = 4) and stimulated with αCD3 in the absence of costimulation followed by assessment of tritiated thymidine incorporation after 72 hours. Proliferation of CD161hi cells from G-CSF–mobilized leukapheresis was restored in the presence of αCD28 costimulation (data not shown). Data represent the mean ± SE.
Discussion
The recent findings that type 17–programmed CD161hi cells express semi-invariant TCRs and are activated by microbe-derived ligands presented by the nonclassic MHC-like molecule MR1, has provided insight into mechanisms of lymphocyte accumulation in the gastrointestinal tract in early life and maintenance of mucosal immunity.4,12,14,15 However, the factors that regulate proliferation and quiescence of CD161hi cells in neonates and adults, respectively, and their response to microbe-derived TCR ligands in the presence or absence of inflammatory signals have not been elucidated. Here, we reconcile apparently paradoxical observations from previous studies by uncovering a novel acquired mechanism of regulation of TCR signaling that is present in CD161hi cells in adult blood, but not in UCB, and identify innate cytokines that are permissive for effector function and dictate the subsequent response of CD161hi cells to signaling through the TCR.
Proliferation and accumulation of CD161hi cells in the first few months of life coincides with neonatal microbial colonization and is temporally associated with their transition from naive to memory phenotype.14 CD161hi cell accumulation does not occur in the absence of an interaction between the semi-invariant TCR, microbes and MR1,14,15 suggesting that TCR signaling is essential in this process. Consistent with this, we show that proliferation to αCD3 is robust in naive CD161hi cells from UCB. In contrast, memory CD161hi cells from adults did not respond to αCD3 stimulation, suggesting down-regulation of TCR signaling was acquired during or after the naive-memory transition. Although identification of the timing and nature of the signals that impose TCR signaling pathway regulation in CD161hi cells requires further study, our data show that TCR regulation is a physiologic mechanism that enables adult memory CD161hi cells to maintain quiescence in spite of persistent microbial colonization that stimulates proliferation of naive CD161hi T cells in neonates.
A previous study suggested that CD161hiCD8α+ T cells were a terminally differentiated subset that may result from chronic antigen stimulation.18 The observations that CD161hi cells are found at relatively high frequency in adults and express high levels of CD27, CD28, and CD127, and low levels of PD-1, provide compelling arguments against this hypothesis and suggest they have an important role in human immunity that extends beyond a contribution in neonatal life.10 The alterations in TCR signaling molecules in CD161hi cells that we identified through gene expression profiling are also not shared with those observed in anergic or exhausted T cells.29,31
Our microarray studies confirmed the expression of high levels of type 17–associated genes that was recently reported for CD161hiCD8α+ T cells,16 consistent with their putative role in mucosal immunity to bacteria and yeasts. In contrast to prior work, we describe profound differences between CD161hi and CD161lo cells in the expression of multiple genes that are crucial for TCR signaling, providing a potential explanation for the lack of response to αCD3, and highlighting the importance of TCR regulation in the normal homeostasis of memory CD161hi cells. Our data thus provides the first comprehensive gene expression profiling data that compares semi-invariant and conventional CD62L+ and CD62L− subsets, and will be critical to further studies of the distinct roles of CD161hi and CD161lo CD8α+ T cells in human immunity and after HSCT.
Regulation of TCR signaling in adult memory CD161hi cells is distinct from that of their CD161lo counterparts, and the microbial specificities of the CD161hi subsets may underlie this difference. Multiple species of bacteria and yeast colonize the gastrointestinal tract17 and activate semi-invariant TCRs on CD161hi cells. This promiscuous recognition of pathogens by CD161hi cells might mandate that they are more stringently regulated to prevent “off-target” effector function than CD161lo cells, which exhibit limited cross-reactivity for peptide antigens presented by classic MHC molecules.33 Moreover, in contrast to viruses that cause acute infections in humans and warrant complete elimination to minimize host injury, commensal bacteria and yeast provide tangible benefit to the host. Thus, regulation of the effector function of bacteria- and yeast-specific CD161hi cells may serve to limit responses only to settings where additional signals of inflammation resulting from a breach in mucosal barriers are provided.17
Our data provide insight into the requirements for eliciting effector function from memory CD161hiCD8α+ T cells. The CD161hi subsets express high levels of costimulatory and cytokine receptors, and we found that signals provided by αCD28, IL-1β, IL-12, IL-18, and IL-23 dramatically increased proliferation of CD161hi subsets to TCR ligation, and influenced the frequency of effector cells in the CD161hi subsets capable of secreting IL-17. In healthy individuals, the requirement for costimulatory signals in conjunction with TCR signaling may be essential to limit the activation of CD161hiCD8α+ T cells only to tissues in which pathogens have provoked an innate immune response that up-regulates CD28 ligands on APC and results in secretion of inflammatory cytokines. This could be especially important for limiting responses in the portal circulation where CD161hi cells might encounter their TCR ligand absorbed from the gastrointestinal tract in the absence of intact microbes, pathogen-associated molecular patterns or inflammation, and could provide a means by which CD161hi cells distinguish pathogenic infection from commensal colonization.
CD161hi T cells and dysregulation of IL-17 secretion have been linked to the pathogenesis of inflammatory disorders, such as Crohn's disease, rheumatoid arthritis, multiple sclerosis, chronic hepatitis C infection, and GVHD.16,19,22,24,34-36 Our data suggests a pathway in which an inciting inflammatory event could enable IL-1β and/or CD28 costimulation allowing proliferation and IL-17 secretion to be unleashed from the normally quiescent CD161hiCD8α+ population. Although IL-17–mediated inflammation might contribute to tissue injury, there is considerable controversy regarding the relative contributions of type 17 and type 1 effector cells to the pathogenesis of many inflammatory diseases.21,37 We found that CD161hi cells stimulated in the presence of IL-1β or IL-18 maintained their capacity to secrete IL-17 and retained down-regulation of TCR signaling, whereas those stimulated in IL-12 became Tc1-like cells that were no longer restrained by TCR regulation. Unrestrained effector function in Tc1-like CD161hi progeny induced by the widespread presentation by MR1 of the microbe-derived semi-invariant TCR ligand might further propagate or prolong tissue injury, thus providing both type 1 and type 17 driven mechanisms by which CD161hi cells could mediate tissue injury.
The high prevalence of CD161hiCD8α+ T cells in normal adult blood suggests that reconstitution of this subset after HSCT is likely to be important for restoring normal immune function. However, murine models of GVHD have implicated a role for IL-17–producing T cells and a small clinical study showed that T cells capable of producing IL-17 correlate with the development of acute GVHD, suggesting their recovery could also be pathogenic.22,34,38,39 Chemotherapy and radiation before HSCT induce the secretion of inflammatory cytokines and activation of APC that are critical for GVHD pathogenesis40 and might contribute to activation of CD161hi cells that encounter microbes as a consequence of mucosal injury and production of proinflammatory cytokines. The high efflux capacity of CD161hi memory T cells, which may have evolved to protect these cells from xenobiotics in hostile mucosal environments, might also protect them from immunosuppressive drugs, many of which are ABCB1 substrates.10,41 Very little is currently known about the reconstitution of CD161hiCD8α+ T cells after HSCT or whether this is altered by immunosuppression. As a prelude to such studies, we evaluated the frequency and function of this subset of T cells in HSC graft sources. We show that G-CSF–mobilized peripheral blood stem cell (PBSC) products contain a much higher frequency of CD161hi cells compared with UCB, which in conjunction with the higher absolute number of T cells in PBSC, would result in a markedly higher CD161hi cell dose compared with UCB. In contrast to CD161hiCD8α+ T cells in UCB, which are not dependent on costimulation after TCR ligation, CD161hiCD8α+ T cells from G-CSF–mobilized products remained unresponsive to αCD3 stimulation alone like CD161hiCD8+ T cells from normal blood. Studies are in progress to define the kinetics of reconstitution of this distinct subset of T cells after HSCT from different graft sources, determine their functional properties, and define a potential contribution to the pathogenesis of GVHD and protection from microbial infection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge assistance from Colette Chaney and Stephanie Crouch.
This work was supported by the Thomsen Family (C.J.T.); Leukemia & Lymphoma Society (C.J.T.); Fred Hutchinson Cancer Research Center Breast Cancer Research Program (C.J.T.); and National Institutes of Health grants CA18029, CA114536, AI53193, and AI086683-01 (S.R.R.); and P01 AI33484 and R01 HLO94260 (J.H.).
National Institutes of Health
Authorship
Contribution: C.J.T. designed and performed experiments, analyzed data, prepared figures, and wrote the paper; R.C.J. and H.M.S. performed experiments and analyzed data; C.D., L.T., S.H., and J.A.H. acquired samples and designed experiments; J.D. and R.B. analyzed data and prepared figures; and S.R.R. designed experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cameron J. Turtle, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: cturtle@fhcrc.org.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal