Abstract
Replacement therapy with factor IX (FIX) concentrates is the recommended treatment for patients with hemophilia B, an X-linked bleeding disorder occurring in 1:25 000 male births. N9-GP is a recombinant FIX molecule with a prolonged half-life which is obtained by site-directed glycoPEGylation where a 40-kDa polyethylene glycol molecule is attached to the activation peptide of FIX. This first human dose trial in patients with hemophilia B investigated the safety and pharmacokinetic properties of a single IV dose of N9-GP. Sixteen previously treated patients received one dose of their previous FIX product followed by one dose of N9-GP at the same dose level (25, 50, or 100 U/kg). None of the patients developed inhibitors. One patient developed transient hypersensitivity symptoms during administration of N9-GP and was excluded from pharmacokinetic analyses. In the remaining 15 patients, N9-GP was well-tolerated. The half-life was 93 hours, which was 5 times higher than the patient's previous product. The incremental recovery of N9-GP was 94% and 20% higher compared with recombinant and plasma-derived products, respectively. These results indicate that N9-GP has the potential to reduce dosing frequency while providing effective treatment of bleeding episodes with a single dose. The trial was registered at www.clinicaltrials.gov as NCT00956345.
Introduction
Hemophilia B is a recessive X-linked congenital bleeding disorder caused by mutations in the coagulation factor 9 (factor IX [FIX]) gene with an incidence of 1 in 25 000 male births.1
The mainstay of hemophilia B treatment is replacement therapy, where the missing or deficient FIX molecule is replaced by repeated injections of purified plasma-derived FIX (pdFIX) or recombinant FIX (rFIX) concentrates.2 The therapeutic aim is to prevent bleeding episodes, and to provide rapid and definitive treatment of bleeding episodes when they occur. Preventing bleeding episodes clearly reduces the risk of life threatening bleeding episodes (eg, intracranial hemorrhage)3 and bleeding episodes in joints, allowing joints, muscles and bones to develop normally. Routine prophylaxis with FIX concentrates has been shown to prevent frequent absence from school and work, and to secure a nearly normal lifestyle with a good quality of life.4,5
On-demand treatment is the current standard therapy for patients with mild or moderate hemophilia B who have a low bleeding tendency. These patients may not be so familiar with injecting themselves and some may need to go to a hospital to receive the treatment. A single effective dose, instead of multiple doses, would therefore be an advantage when treating a bleeding episode. For patients with severe hemophilia B who have a high bleeding frequency, the preferred treatment modality, if available, is routine prophylaxis with regular injections of FIX concentrates and supplemental treatment for bleeding episodes.6-10 According to national and international guidelines, the typical recommended dosing frequency of today's commercially available FIX concentrates is twice weekly to obtain a FIX activity level in plasma sufficient for prophylaxis.8,11,12 The need for frequent dosing, especially in pediatric patients, presents challenges in terms of repeated venous access that may necessitate the placement of indwelling catheters that is associated with frequent complications such as thromboses and infections.13,14 Furthermore, frequent dosing interrupts daily activities and therefore, lack of compliance with a frequent injection regimen is one of the most commonly cited reasons for failure of prophylaxis.15 Similar to patients with mild or moderate hemophilia B, patients with severe hemophilia B would also benefit from a single effective dose, instead of multiple doses, when they experience breakthrough bleeding episodes during prophylaxis.
A recombinant FIX product with a longer plasma half-life (t[1/2]) than today's commercially available FIX products would presumably require fewer injections and potentially result in better compliance with a prophylactic regimen as well as higher clinical efficacy because of shorter time intervals with FIX activity levels < 1%.16
N9-GP is a recombinant serum-free hemostatic protein that has been modified to prolong the t[1/2]. This is achieved by site-directed glycoPEGylation that makes it possible to attach a 40-kDa polyethylene glycol (PEG) molecule to the FIX activation peptide. On activation by FIX's physiologic activators, the activation peptide—with the attached PEG—is cleaved off thereby leaving the wild-type activated FIX (FIXa).
We report here the results of the first human dose trial conducted with N9-GP in male patients with hemophilia B and a FIX activity ≤ 2%. The safety results of a single dose of N9-GP at 3 different dose levels are presented as well as the enhanced pharmacokinetic properties of N9-GP compared with the patients' previous FIX products.
Methods
Patients
The patients were selected according to the Committee for Medicinal Products of Human Use (CHMP) draft guideline on the clinical investigation of pdFIX and rFIX products17 and recruited at 14 sites in 6 countries (Germany, France, Japan, Spain, United Kingdom, and United States). The patients were previously treated male patients with hemophilia B and a FIX activity ≤ 2% with at least 150 exposure days to a FIX product before their enrolment. Patients with a history of neutralizing Abs (inhibitors) against FIX and patients with increased risk of thromboembolic events were excluded. In addition, immunoincompetent patients with a CD4+ lymphocyte count below 200/μL were excluded. All patients provided written informed consent. The trial was approved by independent ethics committees and institutional review boards and was conducted in accordance with the Declaration of Helsinki18 and Good Clinical Practice.19 The trial was registered at www.clinicaltrials.gov as NCT00956345.
Trial design
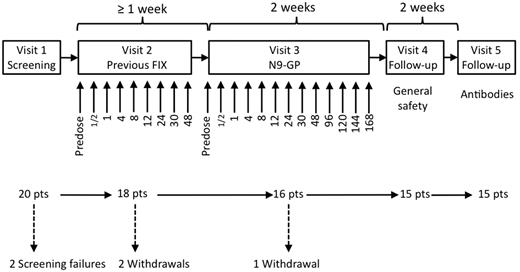
The trial was a first human dose trial designed as an open-label dose escalation trial with the purpose of evaluating safety and pharmacokinetic properties of 3 ascending doses of N9-GP in hemophilia B patients in a nonbleeding state. The doses were 25 U/kg, 50 U/kg, and 100 U/kg. Five patients were to complete each of the 3 dose cohorts. The trial consisted of 5 visits in total (Figure 1). At the first dosing visit (Visit 2) the patient received a single dose of their previous FIX product (pdFIX or rFIX) at the same dose level as the N9-GP dose that was to be administered at the following visit (Visit 3). A wash-out period of 7 days was required before dosing at Visits 2 and 3. If patients received FIX treatment within these 7 days, the visit was postponed accordingly. There was also a 7-day wash-out period before the Ab assessment at Visit 5 to avoid the interference of FIX activity on the Ab assays.
Trial design. The trial consisted of 5 visits. At Visit 2, patients were dosed with their previous FIX product and blood samples for pharmacokinetic assessments were taken at the time points (in hours) indicated below the arrows. At Visit 3, patients were dosed with N9-GP and blood samples for safety and pharmacokinetic assessments were taken at the time points (in hours) indicated below the arrows. There was a wash-out period of at least 7 days before Visits 2, 3, and 5.
Trial design. The trial consisted of 5 visits. At Visit 2, patients were dosed with their previous FIX product and blood samples for pharmacokinetic assessments were taken at the time points (in hours) indicated below the arrows. At Visit 3, patients were dosed with N9-GP and blood samples for safety and pharmacokinetic assessments were taken at the time points (in hours) indicated below the arrows. There was a wash-out period of at least 7 days before Visits 2, 3, and 5.
Dosing with N9-GP was staggered by 2 days in all cohorts to allow reporting of any serious or severe adverse events, before another patient was dosed with N9-GP. Furthermore, criteria were prespecified in the protocol and in a safety manual that was used to identify and mitigate any risks before progressing to a subsequent cohort. Dosing with N9-GP in the subsequent cohort did not occur before patients in the previous cohort had been treated and data were reviewed by a safety group in accordance with the protocol and safety manual.
Trial objectives and endpoints
The primary objective of the trial was to determine safety by evaluating adverse events, Ab formation including inhibitors against FIX and N9-GP, physical examination, vital signs, electrocardiogram (ECG), injection site reactions, urinalysis, and clinical laboratory assessments (hematology, biochemistry, troponin T, coagulation-related parameters (fibrinogen, antithrombin [AT], prothrombin fragment 1 and 2 [F1 + 2], D-dimers, activated partial thromboplastin time [aPTT], prothrombin time [PT], and thrombin antithrombin [TAT]). Secondary objectives were to evaluate the pharmacokinetic properties of N9-GP and to compare these with the pharmacokinetic properties of the patients' previous FIX product. For coagulation factors it is common practice to apply activity-based assays as a surrogate end point for the assessment of pharmacokinetic properties.20,21 Therefore, the pharmacokinetic parameters of N9-GP were based on FIX coagulation activity measurements.
The activity of N9-GP is expressed in units (U) and the activity of the patient's previous FIX product is expressed in international units (IU). A primary reference standard of N9-GP was used, in which the biologic activity was determined relative to the Ph. Eur. Human Coagulation Factor IX, batch 2. The biologic activity in the reference material was defined in a way that one N9-GP U was equal to 1 FIX IU. One international unit of FIX or 1 U of N9-GP activity is equivalent to the FIX activity in 1 mL of normal human plasma.
Blood samples for analyses of FIX activity of a patient's previous FIX product were drawn before dose and again at 30 minutes, 1, 4, 8, 12, 24, 30, and 48 hours after dose. Blood samples for analyses of FIX activity of N9-GP were drawn before dose and at 30 minutes, 1, 4, 8, 12, 24, 30, 48, 96, 120, 144, and 168 hours after dose. In addition, blood samples were drawn 2 and 4 weeks after dosing with N9-GP; however, if patients had received a FIX product after 168 hours, these values were excluded from the pharmacokinetic calculations. The actual time of the blood draws was used in the pharmacokinetic analyses.
The pharmacokinetic parameters included incremental recovery (IR30min) determined as the peak level recorded 30 minutes after administration and reported in (U/mL)/(U/kg), t[1/2], area under the plasma concentration curve from administration to infinity (AUC), plasma clearance (CL), FIX plasma activity 30 minutes after administration (C30min), and volume of distribution (Vz).
Analytical methods
The assay used for the determination of inhibitors against FIX was a Nijmegen modified Bethesda assay, which is a coagulation assay based on in vitro determination of activated partial thromboplastin time (aPTT) in human citrated plasma.22,23 Inhibitors were recognized by comparison of a cut-off point defined by inhibitor negative FIX deficient plasma and plasma pools. Inhibitors were to be defined as positive at Bethesda unit (BU) titers ≥ 0.6 BU according to the CHMP guideline.17
FIX activity was measured using a modification of the aPTT assay (instrument: Siemens BSC-XP; aPTT reagent: Trinity Auto aPTT). Plasma samples were diluted (1:2, 1:4, and 1:8) and mixed 1:1 with FIX-depleted plasma (Precision Biologic FIX-deficient plasma) containing < 0.01 IU/mL FIX activity and at least 0.75 IU/mL of the other coagulation factors. Thereafter, the aPTT reagent was added and the mixture was incubated. After incubation, calcium chloride was added and the time to clot formation, measured in seconds, was detected optically. FIX activity for the previous FIX product and N9-GP was measured with the same assay justifying the comparison between the pharmacokinetic properties of the patients' previous FIX and N9-GP.
Statistical methods
The aim of the trial was to evaluate the safety and pharmacokinetic properties of N9-GP. Such analyses are descriptive rather than based on a statistical approach. Thus, no formal power calculation was performed. To ensure an adequate evaluation of the safety and pharmacokinetic properties of N9-GP, a total of 15 patients (5 in each of the 3 cohorts) were to complete the trial. Safety evaluation was based on summary data and individual data and included all the patients exposed to N9-GP.
The pharmacokinetic parameters were calculated using standard noncompartmental methods. All pharmacokinetic endpoints for N9-GP were modeled by ANOVA on the log transformed parameter values with dose as an independent variable. All pharmacokinetic endpoints for the previous FIX treatment were modeled by a similar model. In addition, analyses were done separately for the 2 types of previous FIX treatments (pdFIX and rFIX). Therefore, the pharmacokinetic endpoints were also modeled by an analysis of variance on the log transformed parameter values with dose, type of FIX treatment and dose by type of FIX treatment interaction as independent variables. Estimates with 95% confidence intervals (CIs) were provided back-transformed to the original scale.
Dose linearity for N9-GP was evaluated based on AUC and C30min. This was done by analysis of covariance (analysis of covariance) on log transformed parameter values with log dose as a covariate. The slope estimate was provided with a 95% CI. Assuming adequate dose linearity, IR30, Vz, CL, and t[1/2] were also compared between the previous FIX treatment and N9-GP. These 4 parameters were chosen because they were considered to be independent of the dose level. The analyses were also done separately for the 2 types of previous FIX treatments. The comparison of N9-GP and the previous FIX treatment was performed using a mixed model with patient as a random effect and treatment (N9-GP, previous FIX), type of previous FIX treatment (pdFIX, rFIX) and treatment by type of previous FIX treatment interaction as independent variables.
Drug product
The rFIX part of N9-GP is synthesized in Chinese hamster ovary (CHO) cells, a mammalian cell line that is well characterized (shown to be free of known infectious agents), and has been used in the production of other recombinant proteins. The rFIX has an amino acid sequence identical to the currently marketed rFIX, BeneFIX.
GlycoPEGylation of rFIX is carried out enzymatically where terminal sialic acids on the N-glycan structures of rFIX are replaced with another sialic acid conjugated to a branched 40K-PEG. There are 2 possible PEGylation sites (Asn157 and Asn167), both situated on the activation peptide. However, N9-GP is mainly mono-PEGylated (∼ 80%). For the mono-PEGylated N9-GP, the distribution between the 2 possible PEGylation sites is approximately equal. The N9-GP drug product for this trial was supplied as a freeze-dried powder in single-use vials and was reconstituted in 4.2 mL of sterile water for intravenous injection.
Results
Patients
A total of 20 male patients with hemophilia B were screened (Figure 1). Two of these patients did not satisfy the screening criteria and a further 2 patients were withdrawn because of meeting a withdrawal criterion and because of noncompliance before dosing with N9-GP. One patient in the 25 U/kg dose cohort was withdrawn because of a treatment-related serious adverse event during dosing with N9-GP, leaving 15 patients who completed the trial. The median age was 30.0 years ranging from 21-55 years. Of the 16 patients who were exposed to N9-GP, 13 were white and 3 were Japanese. The median BMI was 25.2 kg/m2 (ranging from 18.4-29.3 kg/m2). All 16 patients exposed to N9-GP were included in the safety analysis set. The patient who was withdrawn during N9-GP administration had only received a small volume of N9-GP and was therefore excluded from the pharmacokinetic analysis set.
Safety results
Of the 16 patients exposed to N9-GP, a total of 11 treatment emergent adverse events were reported in 6 (37.5%) patients. Ten of these events in 5 patients were rated as moderate or mild and 3 were rated as probably or possibly related to N9-GP. These 3 events were fatigue (2 events in 1 patient) and myalgia. One serious adverse event following treatment was reported in one patient and was judged as being severe and probably related to N9-GP. The event was a hypersensitivity reaction which occurred during administration of N9-GP in a 25-year-old male patient who had no history of inhibitors. He had previously been treated with pdFIX products without any history of allergic reactions. No concomitant medication was taken at the time of the event. Immediately following administration of 0.6 mL of N9-GP, the patient experienced nausea with vertigo and reddening of the face resulting in immediate discontinuation of the injection. The patient experienced a cold sweat and paraesthesia of the face, legs, and arms and reported difficulty in swallowing and pressure over the thorax. Marked swelling of the whole face, hyperemia of conjunctiva and redness of the whole body were noted by the investigator. Blood pressure and pulse were normal at all times. After intravenous infusion of 500 mL of 0.9% NaCl, 250 mg of cortisone and 2 mg of clemastine, the rash and swelling resolved within 2.5 hours. The patient had fully recovered 7.5 hours after onset of the event. Analyses of the patient's pre- and postdose blood samples for FIX inhibitors, N9-GP binding Abs, IgE against N9-GP, IgE against rFIX, IgE against CHO cells, IgE against hamster epithelium, Ig against host cell proteins, and Ig against murine IgG were performed to investigate the cause of the allergic reaction. No Abs were detected in any of these tests. Furthermore, the patient had no sign of consumption of complement factors 24 hours after the allergic reaction, supporting the conclusion that no immune complexes were formed. After the event, the patient was treated with his previous pdFIX product without any complications.
Blood samples for Ab assessment were taken for all patients before N9-GP exposure and 4-5 weeks after exposure. None of the patients developed inhibitors (BU titer ≥ 0.6) after N9-GP exposure. The physical examination of the patients reflected the underlying disease. One patient was HIV positive and 12 patients were hepatitis C positive. Other safety-related parameters and blood samples analyzed before and after N9-GP exposure did not reveal any unexpected findings.
Pharmacokinetic results
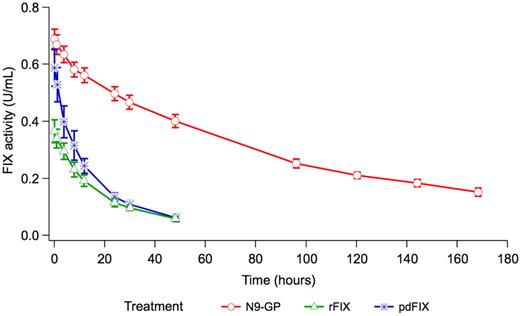
Before dosing at Visits 2 and 3, all the patients had FIX activity levels ≤ 2% indicating that all patients complied with the 7-day wash-out period. Selected pharmacokinetic parameters included IR30, C30min, t[1/2], CL, Vz, and AUC for N9-GP and the previous FIX product at all 3 dose levels (Table 1). Dose linearity for N9-GP was evaluated based on AUC and C30min. The slope estimate for AUC was 1.118 U × h/mL and the 95% CI was 0.906-1.330 U × h/mL. The slope estimate for C30 was 0.925 U/mL and the 95% CI was 0.711-1.139 U/mL. Based on these results, dose linearity could not be rejected; thus, dose linearity was assumed within the dose range investigated (25-100 U/kg). Approximately half (9/16) of the patients used pdFIX as their previous FIX product (3 in the 25 U/kg cohort, 4 in the 50 U/kg cohort, and 2 in the 100 U/kg cohort) and 7 used rFIX (3 in the 25 U/kg cohort, 1 in the 50 U/kg cohort, and 3 in the 100 U/kg cohort). The estimated means for IR30, Vz, t[1/2], and AUC (adjusted to a dose of 50 U/kg) were calculated for N9-GP and the previous FIX product and compared under the assumption of dose linearity (Table 2). The mean t[1/2] of N9-GP was 93 hours which was approximately 5 times longer compared with the patients' previous FIX product (P < .001), (Table 2, Figure 2). The mean incremental recovery of N9-GP was 0.0133 (U/mL)/(U/kg) which was 94% higher compared with rFIX (P < .001) and 20% higher compared with pdFIX (P = .083), (Table 2, Figure 2). The mean CL of N9-GP was 0.70 mL/h/kg which was approximately 10-fold slower compared with the patients' previous FIX products (P < .001), (Table 2, Figure 2). The Vz of N9-GP was 94.18 mL/kg which was approximately half of the patients' previous FIX product (P < .001), (Table 2). Lastly, AUC (adjusted to a dose of 50 U/kg) of N9-GP was ∼ 8-fold higher compared with pdFIX and 10-fold higher compared with rFIX (Table 2).
Pharmacokinetic parameters of N9-GP
| . | N9-GP . | ||
|---|---|---|---|
| 25 U/kg . | 50 U/kg . | 100 U/kg . | |
| No. of patients | 5 | 5 | 5 |
| AUC, U × h/mL | |||
| Mean (SD) | 33.04 (3.58) | 73.28 (23.05) | 158.75 (37.86) |
| Median | 32.62 | 68.25 | 146.68 |
| Min;Max | 28.23;38.04 | 50.61;111.04 | 126.80;224.24 |
| IR30, (U/mL)/(U/kg) | |||
| Mean (SD) | 0.0140 (0.0004) | 0.0139 (0.0044) | 0.0128 (0.0023) |
| Median | 0.0138 | 0.0158 | 0.0121 |
| Min;Max | 0.0137;0.0147 | 0.0081;0.0181 | 0.0111;0.0168 |
| t[1/2], h | |||
| Mean (SD) | 82.94 (18.15) | 96.25 (41.85) | 110.45 (17.48) |
| Median | 73.64 | 73.65 | 115.01 |
| Min;Max | 67.82;110.00 | 61.66;154.08 | 83.35;131.19 |
| CL, mL/h/kg | |||
| Mean (SD) | 0.76 (0.08) | 0.74 (0.21) | 0.65 (0.13) |
| Median | 0.77 | 0.73 | 0.68 |
| Min;Max | 0.66;0.89 | 0.45;0.99 | 0.45;0.79 |
| C30, U/mL | |||
| Mean (SD) | 0.35 (0.01) | 0.70 (0.22) | 1.28 (0.23) |
| Median | 0.35 | 0.79 | 1.21 |
| Min;Max | 0.34;0.37 | 0.40;0.90 | 1.11;1.68 |
| Vz, mL/kg | |||
| Mean (SD) | 90.13 (13.24) | 99.50 (47.42) | 101.96 (12.00) |
| Median | 86.63 | 77.51 | 107.95 |
| Min;Max | 73.58;104.30 | 68.36;181.26 | 84.41;113.12 |
| . | N9-GP . | ||
|---|---|---|---|
| 25 U/kg . | 50 U/kg . | 100 U/kg . | |
| No. of patients | 5 | 5 | 5 |
| AUC, U × h/mL | |||
| Mean (SD) | 33.04 (3.58) | 73.28 (23.05) | 158.75 (37.86) |
| Median | 32.62 | 68.25 | 146.68 |
| Min;Max | 28.23;38.04 | 50.61;111.04 | 126.80;224.24 |
| IR30, (U/mL)/(U/kg) | |||
| Mean (SD) | 0.0140 (0.0004) | 0.0139 (0.0044) | 0.0128 (0.0023) |
| Median | 0.0138 | 0.0158 | 0.0121 |
| Min;Max | 0.0137;0.0147 | 0.0081;0.0181 | 0.0111;0.0168 |
| t[1/2], h | |||
| Mean (SD) | 82.94 (18.15) | 96.25 (41.85) | 110.45 (17.48) |
| Median | 73.64 | 73.65 | 115.01 |
| Min;Max | 67.82;110.00 | 61.66;154.08 | 83.35;131.19 |
| CL, mL/h/kg | |||
| Mean (SD) | 0.76 (0.08) | 0.74 (0.21) | 0.65 (0.13) |
| Median | 0.77 | 0.73 | 0.68 |
| Min;Max | 0.66;0.89 | 0.45;0.99 | 0.45;0.79 |
| C30, U/mL | |||
| Mean (SD) | 0.35 (0.01) | 0.70 (0.22) | 1.28 (0.23) |
| Median | 0.35 | 0.79 | 1.21 |
| Min;Max | 0.34;0.37 | 0.40;0.90 | 1.11;1.68 |
| Vz, mL/kg | |||
| Mean (SD) | 90.13 (13.24) | 99.50 (47.42) | 101.96 (12.00) |
| Median | 86.63 | 77.51 | 107.95 |
| Min;Max | 73.58;104.30 | 68.36;181.26 | 84.41;113.12 |
AUC indicates area under the plasma activity curve from administration to infinity; IR30min, incremental recovery determined as the peak level recorded 30 minutes after administration; t[1/2], plasma half-life; CL, plasma clearance; C30min, FIX plasma activity 30 minutes after administration; and Vz, volume of distribution.
Comparison of pharmacokinetic parameters based on FIX activity
| . | N9-GP . | Previous FIX . | Treatment difference N9-GP/previous FIX ratio (95% CI), P . | ||
|---|---|---|---|---|---|
| N . | Estimate . | N . | Estimate . | ||
| pdFIX | |||||
| IR30, (U/mL)/(U/kg) | 8 | 0.0134 | 8 | 0.0112 | 1.20 (0.97-1.48), 0.083 |
| t[1/2], h | 8 | 92.67 | 8 | 17.79 | 5.21 (3.88-6.99), < .001 |
| CL, mL/h/kg | 8 | 0.71 | 8 | 5.48 | 0.13 (0.12-0.15), < .001 |
| Vz, mL/kg | 8 | 95.22 | 8 | 140.58 | 0.68 (0.49-0.93), 0.018 |
| AUC, U × h/mL* | 8 | 70.20 | 8 | 9.13 | 7.69 (6.92-8.55), < .001 |
| rFIX | |||||
| IR30, (U/mL)/(U/kg) | 7 | 0.0131 | 6 | 0.0068 | 1.94 (1.53-2.47), < .001 |
| t[1/2], h | 7 | 92.76 | 7 | 19.34 | 4.80 (3.50-6.57), < .001 |
| CL, mL/h/kg | 7 | 0.70 | 7 | 6.99 | 0.10 (0.09-0.11), < .001 |
| Vz, mL/kg | 7 | 93.16 | 7 | 194.98 | 0.48 (0.34-0.67), < .001 |
| AUC, U × h/mL* | 7 | 72.47 | 7 | 7.15 | 10.13 (9.04-11.35) < .001 |
| . | N9-GP . | Previous FIX . | Treatment difference N9-GP/previous FIX ratio (95% CI), P . | ||
|---|---|---|---|---|---|
| N . | Estimate . | N . | Estimate . | ||
| pdFIX | |||||
| IR30, (U/mL)/(U/kg) | 8 | 0.0134 | 8 | 0.0112 | 1.20 (0.97-1.48), 0.083 |
| t[1/2], h | 8 | 92.67 | 8 | 17.79 | 5.21 (3.88-6.99), < .001 |
| CL, mL/h/kg | 8 | 0.71 | 8 | 5.48 | 0.13 (0.12-0.15), < .001 |
| Vz, mL/kg | 8 | 95.22 | 8 | 140.58 | 0.68 (0.49-0.93), 0.018 |
| AUC, U × h/mL* | 8 | 70.20 | 8 | 9.13 | 7.69 (6.92-8.55), < .001 |
| rFIX | |||||
| IR30, (U/mL)/(U/kg) | 7 | 0.0131 | 6 | 0.0068 | 1.94 (1.53-2.47), < .001 |
| t[1/2], h | 7 | 92.76 | 7 | 19.34 | 4.80 (3.50-6.57), < .001 |
| CL, mL/h/kg | 7 | 0.70 | 7 | 6.99 | 0.10 (0.09-0.11), < .001 |
| Vz, mL/kg | 7 | 93.16 | 7 | 194.98 | 0.48 (0.34-0.67), < .001 |
| AUC, U × h/mL* | 7 | 72.47 | 7 | 7.15 | 10.13 (9.04-11.35) < .001 |
N indicates number of patients; IR30min, incremental recovery determined as the peak level recorded 30 minutes after administration; t[1/2], plasma half-life; CL, plasma clearance; Vz, volume of distribution; and AUC, area under the plasma activity curve.
AUC is adjusted to a dose of 50 U/kg under the assumption of dose linearity. The estimated means and 95% CI are presented for plasma-derived FIX (pdFIX), recombinant FIX (rFIX), and N9-GP. The treatment difference (ratio: N9-GP/previous FIX) and 95% CI are presented.
Estimated mean FIX activity profiles adjusted to a dose of 50 U/kg. rFIX indicates the FIX activity profile for patients' previous recombinant FIX product and pdFIX indicates the FIX activity profile for patients' previous plasma-derived FIX product, both measured in international units per milliliter. N9-GP indicates the FIX activity profile for N9-GP measured in units per milliliter. Vertical bars indicate the SEM.
Estimated mean FIX activity profiles adjusted to a dose of 50 U/kg. rFIX indicates the FIX activity profile for patients' previous recombinant FIX product and pdFIX indicates the FIX activity profile for patients' previous plasma-derived FIX product, both measured in international units per milliliter. N9-GP indicates the FIX activity profile for N9-GP measured in units per milliliter. Vertical bars indicate the SEM.
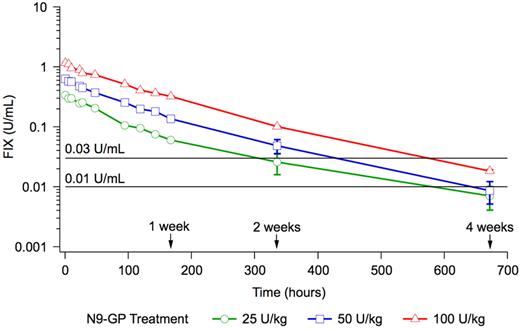
None of the patients received any FIX treatment until 168 hours after dosing with N9-GP. Two weeks after dosing, 4 patients had received FIX treatment as on-demand or prophylaxis and 4 weeks after dosing, 9 patients had received FIX treatment. FIX activity values from these patients were excluded from the posthoc pharmacokinetic evaluation after 2 or 4 weeks depending on when they received their FIX treatment. The time from administration of N9-GP until a FIX activity of 1% and 3% was estimated posthoc based on dose normalization to 50 U/kg, and excluding values from patients who received FIX treatment. The time to 1% FIX activity was 22.5 days (540 hours) and the time to 3% FIX activity was 16.2 days (389 hours; Figure 3).
Mean pharmacokinetic profiles on a log scale. The horizontal lines indicate a FIX activity of 1% (0.01 U/mL) and 3% (0.03 U/mL), respectively. Vertical bars indicate the SEM.
Mean pharmacokinetic profiles on a log scale. The horizontal lines indicate a FIX activity of 1% (0.01 U/mL) and 3% (0.03 U/mL), respectively. Vertical bars indicate the SEM.
Discussion
The N9-GP product is a recombinant serum-free hemostatic protein where the inactive section of the FIX molecule (the activation peptide) has a covalent attachment of a 40-kDa PEG molecule. On activation by FIX's physiologic activators, the activation peptide—with the attached PEG—is cleaved off thereby leaving the wild-type FIXa.
The rFIX part of N9-GP has an amino acid sequence identical to the currently marketed rFIX and is synthesized in CHO cells, a mammalian cell line that is well characterized and shown to be free of known infectious agents eliminating the risk of transmission of viral and other blood-borne diseases.
N9-GP was generally well tolerated; however, one patient experienced a serious adverse event that was judged as severe and probably related to N9-GP. The event was a hypersensitivity reaction with an onset soon after the injection of N9-GP was initiated leading to the withdrawal of the patient. The event was resolved after 7.5 hours and laboratory samples did not show any signs of inhibitor development. After the event, the patient was dosed several times with his previous pdFIX without any complications. Hypersensitivity reactions have been reported for all FIX products and have manifested as pruritus, rash, urticaria, hives, facial swelling, dizziness, hypotension, nausea, chest discomfort, cough, dyspnea, wheezing, flushing, generalized discomfort, and fatigue.24,25 Therefore, the event observed in the present trial was not unexpected. In a trial including 56 previously treated patients, Roth et al reported 4 episodes in 4 patients with signs or symptoms of allergic reactions to rFIX.26 In 3 of these 4 patients, symptoms occurred once or twice and did not recur despite continued treatment with rFIX.
PEGylation has become widely used as a posttranslational modification methodology for improving the efficacy and physicochemical properties of therapeutic proteins.27,28 PEG conjugation has been reported to increase the circulation time of drugs by interfering with renal clearance by glomerular filtration, protecting against enzymatic digestion, blocking interaction with clearance receptors, and reduce the generation of neutralizing Abs.29
The safety of N9-GP has been studied in a toxicology program in accordance with international guidelines without any PEG-related safety findings (unpublished data). The pre-clinical program also comprised a study demonstrating that urine is the primary route of excretion of the 40-kDa PEG used for N9-GP. As there are no specific biomarkers related to the potential retention of PEG, comprehensive surveillance of general safety was applied in the present trial to capture potential PEG-related safety issues, and the same level of surveillance will be applied in any future trials with N9-GP.
Since the first launch of a PEGylated drug in 1990, 11 PEGylated drugs have been marketed. The size of the attached PEG molecule varies among these drugs. Three of these use the 40-kDa PEG molecule also used for N9-GP and 1 of these 3 drugs (Cimzia; certolizumab pegol) which was launched in 2007/2008 is indicated for chronic treatment of rheumatoid arthritis and Crohn diseases. The PEG exposure over time resulting from chronic treatment with Cimzia is higher than the PEG exposure of a prophylaxis regimen with N9-GP and clinical trials and postmarketing experience have not revealed any PEG-related safety issues with Cimzia after 3.5 years of treatment.30,31 Hemophilia treatment has a lifetime perspective, and ongoing safety surveillance of PEGylated products is warranted.
Current management and prevention of bleeding episodes in patients with hemophilia B involves frequent and multiple injections of coagulation factors, because of their relatively short t[1/2].32 A typical dosing schedule is therefore inconvenient, making compliance difficult and impacts patient quality of life.32 In the present trial, the t[1/2] of N9-GP was 93 hours, which represents a 5-fold increase compared with the patients' previous FIX products (Table 2). The incremental recovery of N9-GP was 94% higher than for rFIX and 20% higher than for pdFIX (Table 2); however, only the difference between N9-GP and rFIX was statistically significant (P < .001). Based on these results, patients may be prophylactically covered with once-weekly—or even less frequent—dosing with N9-GP. A less frequent dose regimen may obviate the need for indwelling central venous catheters in many pediatric patients on prophylaxis who currently receive several weekly injections to avoid spontaneous bleeding episodes. Furthermore, N9-GP holds the potential to arrest a bleeding episode with one single injection which would be an advantage over current treatment with rFIX where ∼ 20% of bleeding episodes need more than one injection to obtain hemostasis.26 Patients who are treated using an on-demand regimen may find it attractive to shift to a prophylactic regimen if the interval between doses is sufficiently long and not too different from an on-demand regimen. The benefit of less frequent bleeding episodes in joints and the reduced risk of life-threatening bleeding episodes would most likely have a positive effect on these patients' quality of life.33,34
It cannot be stated with certainty whether the prophylactic effect of today's commercially available FIX products is only a result of FIX trough activity levels > 1% or also a result of the frequent FIX activity peak levels obtained with 2 or 3 weekly FIX doses. Therefore, the time from administration of N9-GP until a FIX activity of 1% was estimated posthoc, based on dose normalization to 50 U/kg. It could also be speculated that a prophylactic regimen with once-weekly or less frequent dosing may necessitate trough levels of FIX activity > 1%. Therefore, the time to a FIX activity of 3% was also estimated posthoc. The time to 1% FIX activity was 22.5 days (540 hours) and the time to 3% FIX activity was 16.2 days (389 hours). However, these results should be interpreted with caution. After 168 hours, the FIX activity was only measured 2 and 4 weeks after dose and only in patients who did not receive a FIX product before week 2 (N = 11) and week 4 (N = 6). Three of the patients in the trial were Japanese, and there were no apparent differences regarding safety or between the pharmacokinetic profiles of these patients, compared with the 12 white patients.
Several publications indicate that the IR30 of pdFIX is higher than for rFIX.9,35-39 In our trial, the estimated mean IR30 was 0.0112 (U/mL)/(U/kg) for pdFIX and 0.0068 (U/mL)/(U/kg) for rFIX, which confirms these observations (Table 2). Interestingly, the estimated IR30 of N9-GP was higher compared with both pdFIX and rFIX. In addition, the volume of distribution of N9-GP was approximately half compared with the patients' previous FIX product indicating that N9-GP is not as highly tissue bound compared with the patients' previous FIX products. The results also indicate that N9-GP primarily circulates in blood and is not further distributed to any great extent.
Pharmacokinetic data for new FIX products are generally accepted as important surrogate endpoints for efficacy. Therefore, the long t[1/2] and the high IR30 of N9-GP are of great clinical interest. A regimen involving frequent injections is known to be one of the main reasons for failure of prophylaxis,15 but the long t[1/2] of N9-GP may permit prophylaxis with fewer injections, thereby improving compliance and reducing the discomfort of the treatment. Furthermore, it is anticipated that a single dose of N9-GP may be sufficient to obtain and maintain hemostasis in the treatment of acute bleeding episodes. These anticipated benefits remain to be verified in additional clinical trials, but the long t[1/2] offers a theoretical possibility of profoundly modifying the current schedule of prophylaxis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Erik Andersen, medical writer at Novo Nordisk A/S, provided editorial support to this manuscript. Henning Friis Andersen, statistician at Novo Nordisk, and Helle Frimer-Larsen, statistician at Larix, provided the statistical analyses and programming. The trial was sponsored by Novo Nordisk A/S.
Investigators participating in the NN7999-3639 trial are thanked for their contributions. These were Wolfgang Miesbach, Hämophiliezentrum, J.-W.-Goethe Universität, Frankfurt, Germany; Robert Klamroth, Vivantes Klinikum am Friedrichshain, Klinik für Innere Medizin, Berlin, Germany; Johannes Oldenburg, Universitätsklinikum Bonn, Institut für Experimentelle Hämatologie und Transfusionsmedizin, Bonn, Germany; Tadashi Matsushita, Nagoya University Hospital, Department of Hematology, Aichi, Japan; Midori Shima, Nara Medical University Hospital, Department of Pediatrics, Nara, Japan; Satoshi Higasa, Hyogo College of Medicine Hospital, Division of Hematology, Hyogo, Japan; Mónica Martín Salces, Unidad de Coagulopatías, Servicio de Hematología, Hospital Universitario La Paz, Madrid, Spain; Edward Tuddenham, Royal Free Hospital, Hemophilia Center and Thrombosis Unit, London, United Kingdom; David Bevan, St Thomas Hospital, Center for Hemostasis & Thrombosis, London, United Kingdom; John C. Barrett, VCU Coagulation Program, Richmond, VA; and Michael Recht, Oregon Health & Science University, Portland, OR.
Authorship
Contribution: C.N. designed and performed research, collected data, interpreted data, and wrote and revised the manuscript; K.K. and J.M. designed research, interpreted data, and revised the manuscript; and A.T. and P.G. collected data, interpreted data, and revised the manuscript.
Conflict-of-interest disclosure: C.N. and A.T. received research support, lecture fees, and honoraria for consultancy from Novo Nordisk. P.G. has received financial support from Novo Nordisk to attend conferences as well as honoraria for lectures. K.K. was employed at Novo Nordisk A/S throughout the trial period and is still scientific and clinical advisor for Novo Nordisk A/S. J.M. is employed at Novo Nordisk A/S.
Correspondence: Claude Negrier, MD, Centre Régional de Traitement de l'Hémophilie, Université Claude Bernard Lyon1, Hôpital Edouard Herriot, Pavillon E, Place d'Arsonval, 69003, Lyon, France; e-mail: claude.negrier@chu-lyon.fr.