In this issue of Blood, Hsu et al1 and Dickinson et al2 independently report the presence of mutations in the region of GATA2 encoding the carboxy-terminal zinc-finger domain of the protein in a rare genetic disease associated with reduced production of marrow-derived immune cells susceptible to development of myelodysplastic syndrome and acute myeloid leukemia.
The central role of the GATA family of transcription factors in hematopoietic development has been well established in mice and in culture models of human hematopoiesis.3 The expansion of early cell compartments is under the control of GATA2. With maturation, this control switches to GATA1 for the erythroid/megakaryocytic lineage and to GATA3 for T cells. The GATA factor that controls the late phases of myelo-monocytic maturation has not been definitively established, but the recognition that GATA2 regulates phagocytosis by pulmonary alveolar macrophages4 and, in cooperation with PU.1, expression of c-Fms,5 the gene that encodes the receptor for macrophage colony-stimulating factor (M-CSF; also known as CSF1), and dendritic cell maturation,6 suggests that it may be GATA2.
Several inherited and acquired disorders involving deficient production of erythroid (dyserythropoietic anemia, congenital erythropoietic porphyria, X-linked thalassemia) and megakaryocytic (thrombocytopenia, X-linked gray platelet syndrome) cells have been associated with mutations either in the FOG1 or the DNA binding motif of the amino-terminal zinc finger of GATA1.7 These mutations are not associated with increased risk of transformation. The deletion of the amino-terminal region of GATA1 induces thrombocytopenia and, in association with chromosomal abnormalities present in Down syndrome, transient myeloproliferative disorders and megakaryocytic leukemia.8 By contrast, with the exception of a gain-of-function mutation described in one patient with chronic myelo-monocytic leukemia,9 mutations in the GATA2 gene have not been reported. It was conceived that given the “exclusive” role of GATA2 in stem cell regulation, loss of function GATA2 mutations would remain undetected because they would lead to lethality. However, quantitative alterations in GATA2 expression, possibly secondary to the primary lesions, have been described. For example, CD34pos cells from patients with aplastic anemia10 and blasts from acute myeloid leukemia (AML)11 are both characterized by reduced levels of GATA2. In addition, although hypomorphic GATA2 mutations do not induce a strong phenotype in mice,12 genome-wide analyses of transcriptional reprogramming of mouse models of AML have identified GATA2 as one of the few key transcription factors whose expression is reduced in these leukemic blasts.11 A preliminary report presented as a “late breaking” abstract at the 2010 American Society of Hematology annual meeting mapped mutations in the coding region of GATA2 in 4 patients with myelodysplatic syndrome (MDS) that did not report immunodeficiency.13 This report also provided an initial characterization of the biologic consequences of the mutations on the DNA binding properties of GATA2 that included reduced affinity for the c-Fms promoter.
The Holland laboratory has identified and characterized a rare case of genetic immunodeficiency distinguished by reduced levels of all the immune cells produced in the marrow such as monocytes, dendritic, natural killer, and B cells (that they call MonoMAC).14 The inheritance of the disease is autosomal dominant, but sporadic forms have also been described. The disease manifests itself with recurrent mycobacterial and other opportunistic fungal and papilloma virus infections. The marrow of these patients is hypocellular, with absence of multilymphoid progenitor cells and reduced levels of myeloid progenitor cells, and presents with dysplasia not only for the myeloid lineage but also for the erythroid and megakaryocytic lineage. The disease may eventually evolve into MDS and AML. Analysis of genes controlling hematopoietic stem cell development and maintenance, such as RUNX1, PU.1, CEBPA, and ERG, indicates that all were wild-type in MonoMAC patients.1 Based on the clue provided by the abstract presented at the American Society of Hematology annual meeting in 201013 and on the possible involvement of GATA2 in monocytedendritic cell development,4-6 Hsu et al guided a search for GATA2 lesions in 20 patients with MonoMAC syndrome.1 Astonishingly, all of the patients presented mutations that altered the domain of GATA2 encoding the carboxy-terminal zinc finger.
Exome sequencing is emerging as a powerful tool to identify genes mutated in human disorders. Dickinson et al applied this technique to identify mutations involved in a syndrome that they call DCML deficiency with a phenotype similar to MonoMAC.2 They sequenced the exomes of 4 patients with the familial form of the disease and identified exactly the same mutations found by Hsu and colleagues. Given the different geographic origin of the patients, it is unlikely that the patients analyzed in the 2 studies are related. Therefore, overall these 2 papers establish a strong correlation between the presence of mutations in the GATA2 gene and disease manifestation. The fact that these 2 studies independently identified the same gene also provides strong validation for the efficacy of the exome sequencing approach as tool for gene mutation discovery.
As far as a mechanism that could subsequently lead to development of MDS, it can be argued that hyperstimulation of a stem cell clone that produces hematopoietic progenitor cells with reduced expansion potential (after haploinsufficient reduction in levels and/or optimal activity of GATA2 as described in the present studies) may lead to stem cell exhaustion (see figure). MonoMAC/DCML patients described in these 2 studies have had to deal with an extended onslaught of infection from the immunodeficiency, yielding a highly stressed bone marrow with a low capacity for mounting an adequate response in the absence of additional acquired mutation(s).
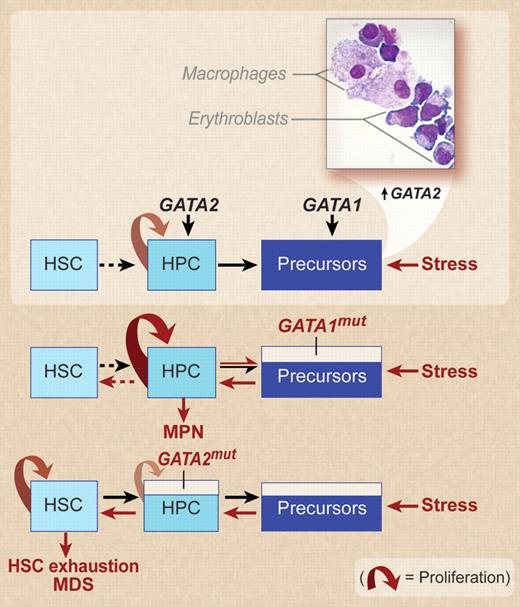
A model for the effect of stress on the hematopoietic compartments in the presence of wild-type GATA1 and GATA2 genes or in the presence of loss-of-function GATA1 and GATA2 mutations. In vitro models of stress erythropoiesis indicate that the stress signal increases the erythroid output by amplifying the hematopoietic progenitor cell compartments (HPCs) through increased levels of GATA2 expression.16 Surprisingly, in spite of the purity of CD34pos populations used to start the culture and the exclusively erythroid-permissive culture conditions used, macrophages are always detected in these cultures and erythroblastic-island–like structures (inset, kindly provided by Migliaccio laboratory) are frequently observed on smears prepared with erythroid cells expanded under stress conditions. In the presence of GATA1mut, which impairs the maturation potential of the precursor compartments, stress must induce an even greater expansion of the HPC compartment leading to the development of myeloproliferative neoplasms (MPNs)8,16 ; however, the amplification potential of the HPCs is sufficient to increase the precursor cell demand necessary to respond to stress, leaving the hematopoietic stem cell compartment (HSC) unstimulated. In contrast, the presence of a loss-of-function GATA2 mutation hampers HPC proliferation, reducing in turn the numbers of precursor cells generated by the HPCs. An effective response to the increased precursor cell demand induced by stress requires the generation of new HPCs from the HSC compartment, resulting, if the stress is prolonged, in HSC exhaustion and MDS. Professional illustration by Debra T. Dartez.
A model for the effect of stress on the hematopoietic compartments in the presence of wild-type GATA1 and GATA2 genes or in the presence of loss-of-function GATA1 and GATA2 mutations. In vitro models of stress erythropoiesis indicate that the stress signal increases the erythroid output by amplifying the hematopoietic progenitor cell compartments (HPCs) through increased levels of GATA2 expression.16 Surprisingly, in spite of the purity of CD34pos populations used to start the culture and the exclusively erythroid-permissive culture conditions used, macrophages are always detected in these cultures and erythroblastic-island–like structures (inset, kindly provided by Migliaccio laboratory) are frequently observed on smears prepared with erythroid cells expanded under stress conditions. In the presence of GATA1mut, which impairs the maturation potential of the precursor compartments, stress must induce an even greater expansion of the HPC compartment leading to the development of myeloproliferative neoplasms (MPNs)8,16 ; however, the amplification potential of the HPCs is sufficient to increase the precursor cell demand necessary to respond to stress, leaving the hematopoietic stem cell compartment (HSC) unstimulated. In contrast, the presence of a loss-of-function GATA2 mutation hampers HPC proliferation, reducing in turn the numbers of precursor cells generated by the HPCs. An effective response to the increased precursor cell demand induced by stress requires the generation of new HPCs from the HSC compartment, resulting, if the stress is prolonged, in HSC exhaustion and MDS. Professional illustration by Debra T. Dartez.
Of interest, the mutations associated with congenital anemia and thrombocytopenias are located in the amino-terminal zinc finger domain of GATA1 while those found in MonoMAC and in DCML deficiency are located in the carboxy-terminal zinc finger of GATA2. This suggests that, in spite of the stringent similarities of the primary structure of the 2 proteins, there must be subtle but important differences in the tertiary structure of GATA1 and GATA2 zinc fingers that determine the unique biologic functions of the 2 proteins. Related to this point, the 2 zinc fingers of all GATA proteins are critical for establishing proper DNA binding and protein interactions.15 As a result, the nonhaploinsufficient category of GATA2 mutations described in both studies (R398W and T354M) suggests that at least one, if not both, of these critical functions are likely altered. Structural modeling suggests how the point mutants can alter GATA2 interaction with DNA, either by loss of stabilizing hydrogen bonds or steric issues with large side chains.2 Related to this, a more extreme case is the del 340-381 mutation (again seen in patients in both studies), which removes a large portion of zinc finger 2. It will be of interest to quantitatively verify the predicted lower DNA binding affinity of these GATA2 variants. By analogy to GATA1, other regions of the GATA2 protein are likely associated with positive cofactors. Given that these GATA2 variants are expressed in the presence of a normal allele, dominant negative effects may be operant as a result of nonproductive interactions, a suggestion supported by the recent abstract linking the T354M mutation to MDS/AML.13
The work of Hsu et al and Dickinson et al breaks ground on numerous fronts, as they (1) identify a previously nonrecognized correlation between GATA2 and development of bone marrow derived immune cells, including macrophages (see figure); (2) suggest there are important functional differences between 2 factors (GATA1 and GATA2) whose biologic activity have typically been considered to be almost identical; and (3) establish a link between the stress response of donors with a genetically impaired ability to expand hematopoietic/stem progenitor cells (based on reduced GATA2 activity) and development of MDS. In this regard, it would be informative to evaluate whether hypomorphic GATA2 mice12 will develop a myelodyspastic-like phenotype when challenged with mycobacteria (or other stimuli) and would therefore represent an animal model for this mysterious disease.
In conclusion, these papers represent a satisfying conclusion to the long scientific journey that began with the recognition of a human genetic disease and ends with identification of the genetic lesion that caused the disease.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■