Bcr-Abl remains the prime target for CML therapy, but recent findings from Sheng and colleagues suggest that its inhibition may expose a new loophole in the cell death process.1 Their report defines a new role for the Bcr-Abl signaling complex in suppressing autophagy, a catabolic process that partially destroys a cell's content.2
The cell uses autophagy to reallocate its content to essential processes when no other source exists and to temporarily offset cell destruction during stressful or nutrient-lean times. Its activation can therefore relieve the cell of stress, such as the effects from inhibiting oncogene addiction via Bcr-Abl. Studies have shown that Bcr-Abl kinase inhibition with imatinib engages both apoptosis and autophagy; importantly, the latter diminishes the frequency of apoptosis in CML cells.3 Therefore, to improve the response to CML therapy, autophagy must be suppressed during the stress applied by Bcr-Abl kinase inhibition. To do this with precision rather than relying on generalized inhibitors of the autophagic pathway, we need a clear understanding of the signaling complexes engaged by Bcr-Abl to suppress autophagy. Keeping those pathways blocked while activating apoptotic pathways through inhibition of the Bcr-Abl kinase would likely increase the efficacy of imatinib against CML cells and possibly extend it to Bcr-Abl–expressing cells with a highly responsive autophagic pathway (ie, stem cells).4 The article by Sheng et al in this issue of Blood provides a clear description of the signaling cascade that connects Bcr-Abl to negative regulation of autophagy.1 This information may have significance and impact on the clinical management of CML.
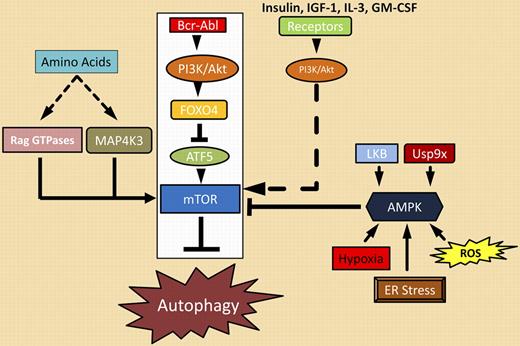
Sheng and colleagues mapped out a clear path from Bcr-Abl to the master autophagy regulator, mTOR, as the major route by which Bcr-Abl represses autophagy. Bcr-Abl activates the PI3K/Akt pathway that phosphorylates many targets including the transcriptional repressor FOXO4. When phosphorylated, FOXO4 cannot repress transcription of ATF5, a gene previously shown to regulate apoptosis in cytokine-dependent cells. In Bcr-Abl–transformed cells, ATF5 takes on a new role as a regulator of autophagy, not apoptosis. Sheng et al demonstrate that ATF5 regulates mTOR expression by binding a previously unidentified ATF5 binding site in the mTOR promoter. Elevated expression of mTOR by ATF5, coupled with mTOR activation by other components of the PI3K/Akt cascade, suppresses autophagy. mTOR activation can be reversed on Bcr-Abl inhibition, resulting in the onset of autophagy, as illustrated in the figure. Unfortunately, imatinib-mediated reversal of this suppression blocks the full impact that Bcr-Abl kinase inhibition has on apoptosis. The observations of Sheng and coworkers provide a clear mechanism for correcting this unwanted effect of imatinib. Based on their model, activation of mTOR combined with Bcr-Abl inhibition should block the induction of autophagy to enhance the apoptotic response. This may translate into direct clinical benefit in terms of depth or kinetics of a molecular response.
Bcr-Abl signaling complexes (boxed in white) and other regulators of autophagy.
Bcr-Abl signaling complexes (boxed in white) and other regulators of autophagy.
However, the central question is how can we exploit this newly mapped Bcr-Abl path to autophagy to improve CML therapy? In addition, do the potential risks in modulating the autophagy pathway outweigh the potential benefits? As noted by many studies, engagement of autophagy can be both friend and foe to tumor cell survival.5 In CML, imatinib clearly engages autophagy, but the degree to which this diverts apoptosis may not significantly influence clinical imatinib responsiveness. On the other hand, the degree of apoptotic escape may be distinct in CML stem cells compared with progenitors, as suggested by earlier studies.4 Therefore, in the CML setting, preserving mTOR activity during imatinib therapy may be key to engaging a greater apoptotic impact, particularly against early CML progenitors or stem cells that are relatively resistant to tyrosine kinase inhibition.
As outlined in the figure, many pathways converge on mTOR. The Bcr-Abl/PI3K/Akt/ATF5/mTOR autophagic suppressor cascade is most relevant in CML, but other pathways may modify it and continue to suppress autophagy during imatinib therapy. For example, Sheng et al demonstrate that a constitutively activated mutant of PI3K blocks induction of autophagy on imatinab treatment. This suggests that activating thePI3K pathway with other growth factors (insulin, IGF-1) or cytokines (IL-3, GM-CSF) might suppress autophagy during imatinib treatment. AMPK (5′-AMP-activated protein kinase), a kinase that is responsive to changes in the cellular ATP/AMP pool, is activated by a number of cellular conditions (reactive oxygen species [ROS], ER stress) and signaling events (LKB kinase, Usp9x deubiquitinase).6 Activated AMPK suppresses mTOR activity by phosphorylating several negative regulators of the complex.2 This suggests that direct inhibition of AMPK, LKB, or Usp9x could block down-regulation of mTOR and prevent autophagy. However, few agents have been described that affect these targets.7,8 The availability of essential amino acids may also suppress mTOR inhibition through activation of additional kinases (MAP4K3) and GTPases (Rag GTPases) as recently illustrated in other tumors.9 The question remains, are these strategies appropriate, beneficial, or safe in the clinical setting? It should be noted that induction of autophagy has been exploited as a therapy in other cancers. CML treatment with Bcr-Abl kinase inhibitors may be an opportunity to examine the benefits and consequences of suppressing autophagy to improve therapy. The detailed map of the Bcr-Abl/autophagy cascade provided by Sheng et al provides a good starting point.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal