Abstract
The HLX gene encoding a diverged homeobox transcription factor has been found to be up-regulated by vascular endothelial growth factor-A (VEGF-A) in endothelial cells. We have now investigated the gene repertoire induced by HLX and its potential biologic function. HLX strongly increased the transcripts for several repulsive cell-guidance proteins including UNC5B, plexin-A1, and semaphorin-3G. In addition, genes for transcriptional repressors such as HES-1 were up-regulated. In line with these findings, adenoviral overexpression of HLX inhibited endothelial cell migration, sprouting, and vessel formation in vitro and in vivo, whereas proliferation was unaffected. This inhibition of sprouting was caused to a significant part by HLX-mediated up-regulation of UNC5B as shown by short hairpin RNA (shRNA)–mediated down-modulation of the respective mRNA. VEGF-A stimulation of endothelial cells induced elevated levels of HLX over longer time periods resulting in especially high up-regulation of UNC5B mRNA as well as an increase in cells displaying UNC5B at their surface. However, induction of HLX was strongly reduced and UNC5B up-regulation completely abrogated when cells were exposed to hypoxic conditions. These data suggest that HLX may function to balance attractive with repulsive vessel guidance by up-regulating UNC5B and to down-modulate sprouting under normoxic conditions.
Introduction
Vascular endothelial growth factor-A (VEGF-A) is the major trigger of vasculogenesis and angiogenesis during embryogenesis and blood-vessel formation in the adult.1,2 It has also been implicated in pathologic angiogenesis in diseases such as cancer, chronic inflammatory disorders, and retinopathy.3 Whereas several peptide products are generated from the VEGF-A gene by differential splicing, the available data suggest that isoform VEGF-A165 is the predominant form responsible for the major angiogenic effects.4
The gene repertoire induced by VEGF-A mainly via VEGF receptor 2 has been investigated by several groups5-7 ; however, the transcription factors up-regulated by VEGF-A and how they mediate its specific and unique biologic functions remain largely uncharacterized. We have recently identified a group of genes selectively or at least preferentially induced by VEGF-A in endothelial cells, compared with a more general growth factor such as epidermal growth factor (EGF) or inflammatory mediators such as interleukin-1 (IL-1).8 Most prominent up-regulation in the described manner was found for the transcription factors NR4A2 (nuclear receptor subfamily 4 group A member 2), EGR3 (early growth response 3), HLX (H2.0-like homeobox protein), and MEF2C (myocyte-specific enhancer factor 2) suggesting their involvement in specific VEGF-A–triggered responses.5
In this study, we have focused on the investigation of HLX, an evolutionary highly conserved homeobox transcription factor originally detected in the visceral musculature of Drosophila.9 Furthermore, HLX was shown to be expressed in bone marrow–derived CD34+ cells10 and lymphocytes,11-13 as well as in endothelial cells.14 Expression in type 1 helper T lymphocytes seems to play an essential role in establishing heritable expression of the γ-IFN gene by epigenetic mechanisms.11,12 In mouse development, the HLX−/− genotype is lethal around day 15 of embryonic development on a mixed genetic background, the embryos displaying severe hypoplasia of the gut and liver.15,16 In this context, it has been proposed that, during visceral organogenesis, HLX regulates a mesenchymal-epithelial interaction that is required for early aspects of enteric nervous system development.16
There is accumulating evidence that several mechanisms are shared between the development of the nervous and vascular systems.17 During angiogenesis, endothelial tip cells (which lead the growing sprout) sense the environment by dynamically extending filopodia toward attractive signals and retracting them from repulsive ones. In this regard, endothelial sprouts use similar guidance cues as growing nerve fibers do for directional growth and network formation.17 Guidance molecules with a similar role in endothelial tip cells and axonal growth cones include the semaphorins and plexin receptors as well as the secreted netrins and uncoordinated-5 (UNC5) receptors. However, signals and transcription factors that regulate the expression of these guidance cues have not yet been established.
We have now investigated the genes controlled by the transcription factor HLX in endothelial cells. Our data demonstrate that HLX is a specific regulator of cell-guidance molecules, such as UNC5B, plexin-A1 (PLXNA1), and semaphorin-3G (SEMA3G), that presumably display repulsive functions in vessel guidance and/or may prevent inappropriate sprouting. Furthermore, HLX also induces transcriptional repressors including HES-1, SNAI2, and BCOR. After overexpression of HLX, we observed an inhibition of migration of endothelial cells, decreased sprouting, and reduced vessel formation. This inhibition of sprouting was in part caused by UNC5B up-regulated by HLX. Furthermore, we show that VEGF-A can increase UNC5B expression in an HLX-dependent way; however, induction of HLX and consecutively UNC5B was strongly reduced or prevented under hypoxic conditions. We propose that HLX serves to counterbalance the effects of attractive guidance cues by up-regulating the expression of repulsive guidance molecules such as UNC5B.
Methods
Cell culture and materials
Human umbilical vein endothelial cells (HUVECs) were isolated as described previously18 and cultured in EGM-2 medium (Clonetics). HUVECs of passages 3 to 5 were used for experiments.
To apply hypoxic conditions, the cell-culture plates were transferred to a modular incubation chamber (Billups-Rothenberg Inc). This was inflated with 10% CO2 compensated with 90% N2 for 10 minutes, closed tightly, and kept at 37°C for the length of the experiment. Cultures to be analyzed within the first 2 hours after VEGF addition were preincubated under hypoxic conditions for 4 hours.
HEK293 cells (CRL-1573; ATCC) were cultured in minimal essential medium α (MEMα) medium with 10% newborn calf serum (NCS; both from Invitrogen). 293T cells (CRL-11268; ATCC) were cultured in Dulbecco modified Eagle medium (DMEM) with 10% fetal calf serum (FCS; both from Invitrogen). VEGF-A165 and basic fibroblast growth factor (bFGF) were obtained from PromoKine or R&D Systems (Merck).
Construction of recombinant adenoviruses and transduction of cells
A cDNA clone of human HLX (IRALp962M1922Q; http://www.imagenes-bio.de/) was obtained from RZPD. The coding region together with a Flag-tag sequence at the 3′ end was cloned into the pACCMVplpASR+ expression plasmid19 (pAC.HLX). The plasmids pAC.HLX and pJM17 (Microbix Biosystems) were cotransfected into HEK293 cells by the calcium phosphate method (Stratagene). Primary adenoviruses generated were subcloned and purified after amplification in HEK293 cells using CsCl ultracentrifugation.20 The HLX sequence in the viral preparations was confirmed by sequencing. Viral titer was determined using the Adeno-X Rapid Titer Kit (Clontech). An empty control adenovirus20 was also grown and purified as described for HLX adenoviruses. The UNC5B-encoding adenovirus was a generous gift of Drs. Noriaki Kitamura and Hirofumi Arakawa (National Cancer Center, Tokyo, Japan).
Production of recombinant lentiviruses and transduction of cells
Plasmids (Mission shRNA pLKO.1-puro) containing the corresponding short hairpin RNA (shRNA) sequences targeted to HLX and UNC5B and a control shRNA plasmid were obtained from Sigma-Aldrich (supplemental Table 3, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The shRNA plasmids together with 2 packaging vectors (pMD2.G and psPAX.1) were cotransfected into 293T cells. Supernatants were harvested after 24 and 48 hours and used for infection mixed with medium in a ratio of 1:1 or 1:2.
RNA preparation
HUVECs were infected in subconfluent state with control (Ad.con) and HLX encoding adenovirus (Ad.HLX) using multiplicity of infections (MOI) of 20 to 40. Then cells were incubated with RNAlater (Ambion) for 1 minute, lysed with TRIzol (Invitrogen), and RNA was extracted.
Real-time RT-PCR analysis
Total RNA (2 μg) was used to synthesize cDNA with Superscript II Reverse Transcriptase and oligodT primer (both from Invitrogen). mRNA levels were measured using real-time reverse transcription–polymerase chain reaction (RT-PCR) detecting SYBR Green I in a LightCycler (Roche Diagnostics). Values were normalized to β2-microglobulin mRNA as internal standard. Oligonucleotide primers were designed using the Primer3 software (http://frodo.wi.mit.edu/primer3/) and are listed in supplemental Table 2.
Affymetrix microarray analysis
cRNA was prepared from total RNA, hybridized to the Human Genome U133 Plus 2.0 Array (Affymetrix), and arrays were scanned according to the manufacturer's protocols (Affymetrix support site, www.affymetrix.com/support/index.affx) as described.21 Robust multiarray average (RMA) signal extraction and normalization were performed as described (http://www.bioconductor.org/).22
Western blot analysis
Cell pellets were lysed in Laemmli buffer. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Immobilon-P membranes (Millipore). The membrane was blocked with 5% skim milk/phosphate-buffered saline (PBS) and 0.1% Tween 20 (PBST) and incubated overnight with the primary antibody at 4°C. The membrane was washed and incubated with peroxidase-conjugated secondary antibody for 1 hour. The membrane was incubated with ECL Plus reagent (GE Healthcare) and exposed to x-ray film. Bands were quantified in scanned film images by measuring tonality using Adobe Photoshop software.
Antibodies used were: mouse-anti–human HLX (Abnova), mouse-anti–human GAPDH (glyceraldehyde 3-phosphate dehydrogenase; Chemicon), and secondary horseradish peroxidase–conjugated enhanced chemiluminescence (ECL) sheep-anti–mouse antibodies (GE Healthcare).
Flow cytometry
After treatment with accutase (PAA), cells were harvested, fixed with 4% paraformaldehyde, permeabilized with 0.05% Triton X-100 (Sigma-Aldrich), and blocked with 5% bovine serum albumin (BSA)–PBS. Cells were stained with 5 μg/mL humanized UNC5B antibody 4 (Genentech) for 1 hour at 4°C followed by the secondary antibody Alexa Fluor 647 goat–anti-human immunoglobulin G (IgG; Invitrogen). Binding was assessed using a FACSCalibur (BD Biosciences).
Immunocytochemistry
HUVECs were grown in fibronectin-coated chamber slides. Cells were fixed with 4% paraformaldehyde and blocked with 1% BSA/PBS. To reveal HLX, cells were also permeabilized with 0.1% Triton X-100/PBS. Slides were stained with: affinity-purified rabbit–anti-human HLX antibodies13 raised against a GST-HLX fusion protein (1:500 dilution); anti-human UNC5B antibody 4 (20 μg/mL; Genentech); secondary Alexa Fluor 568 goat–anti-rabbit IgG at a dilution of 1:5000 or Alexa Fluor 647 goat–anti-human IgG at 1:5000 (Molecular Probes; Invitrogen). Slides were mounted with DAKO fluorescence medium (DAKO). Images were taken at room temperature using an inverted Nikon Diaphot TMD microscope with a 50-fold oil immersion objective and a cooled charge-coupled device camera (Kappa DX30) with the manufacturer's software (Kappa ImageBase).
Proliferation assay
HUVECs were infected with adenoviruses using a MOI of 8. The cells were trypsinized on the following day and seeded into 96-well plates (5000 cells/well). Proliferation was determined by measuring the total protein content using the sulforhodamine B (SRB) colorimetric assay.23 Experiments were performed with 5 wells per data point.
Transwell migration assay
One day after infection, 5 × 104 HUVECs were seeded into the upper well of a gelatinized transwell with a pore size of 8 μm (Corning). After the addition of 50 ng/mL VEGF-A165 to the lower chamber, the transwell was incubated for 4 hours at 37°C and the migration of cells toward the lower chamber was determined. Cells were fixed with methanol and stained with 1 μg/mL 4′,6-Diamidin-2-phenylindol (DAPI; Sigma-Aldrich). Cells in the lower chamber were counted in 5 randomly chosen microscopic fields photographed using a Nikon Diaphot TMD microscope and a CCD camera (Kappa GmbH).
Generation of endothelial cell spheroids
HUVECs were transduced at a MOI of 10 to 20 or infected with 0.5 to 1 mL of lentivirus. Cells were then used for the spheroid-based in vitro or in vivo assays. Cells were used 24 hours after infection with the adenoviruses or 48 hours after infection with the lentiviruses to generate spheroids. Transduction efficiency of all viruses was regularly controlled.
Spheroids of a defined cell number were generated from nontransduced or transduced cells as described in Korff et al.24 Briefly, HUVECs were suspended in medium containing 0.25% (wt/vol) methylcellulose and grown in hanging drops overnight. Single spheroid with a defined cell number of approximately 400 (for the in vitro assay) or 100 (for the in vivo assay) cells/spheroid were obtained.
Spheroid-based in vitro angiogenesis assay
HUVEC spheroids were embedded into rat collagen gels as described25 with the following modifications: 50 spheroids of 400 cells per spheroid were mixed with 80% Methocel/20% FCS; 0.1 mL of basal endothelial cell growth medium (ECGM; PromoCell) without or containing VEGF or bFGF (25 ng/mL each) was layered onto the top of the formed gel. After 24 hours, sprouts were quantified by measuring the total sprout length grown out of each spheroid on pictures taken on the Nikon microscope.
In vivo implantation of spheroids
Spheroids were harvested and carefully suspended in 500 μL of Matrigel (growth factor reduced; BD Biosciences) and fibrinogen (2 mg/mL; Calbiochem, Merck) containing VEGF plus bFGF (500 ng/mL each). Gel formation was initiated by the addition of thrombin (0.4 U; Calbiochem).
Spheroid suspensions containing approximately 1000 spheroids of 100 cells each were injected subcutaneously on each side lateral to the abdominal midline region into 4- to 6-week-old SCID mice. Two plugs were implanted per mouse, each experimental group consisted of 8 mice. After 14 days, mice were killed, the harvested plugs were fixed overnight (0.5 g/L calcium acetate, 5 g/L zinc acetate, 5 g/L zinc chloride in 0.1M Tris, pH 7.4) and analyzed by immunohistochemistry. Animal procedures were carried out in accordance to guidelines of the local committee for animal experimentation (DKFZ Heidelberg and RP Karlsruhe).
Immunohistochemistry
Zinc-fixed Matrigel plugs were embedded in paraffin. Plugs were sectioned at 8 μm. Primary antibodies used were: mouse–anti-human CD34 (QBEND10; Novocastra); sheep–anti-human CD31 (DAKO). Antigen retrieval for anti-human–CD34 stainings was performed by digestion with Proteinase K (Sigma-Aldrich) at 37°C for 15 minutes. The secondary antibodies donkey–anti-sheep Alexa 488 (Invitrogen), and biotinylated goat–anti-mouse (Zymed; Invitrogen) were detected by streptavidin-peroxidase conjugate (DAKO) and the DAB substrate chromogen system (DAKO).
Quantification of the human grafted vasculature and statistical analysis
Sections were prepared across the entire implant size and 3 sections were selected from the beginning, the center, and the end. Microvessel density (MVD) analysis was performed using immunohistochemistry for human CD31 and CD34. Slides were mounted with DAKO fluorescent mounting medium (DAKO). Images of the complete plug area were taken using an Olympus IX80 microscope with a 20-fold objective and the inbuilt F-view camera. Pictures were assembled by multiple alignment and fluorescent signals of the complete plug matrix were calculated as vessel number per mm2 (Cell-F imaging software; Olympus).
Results
HLX up-regulates genes for cell-guidance proteins and transcriptional repressors
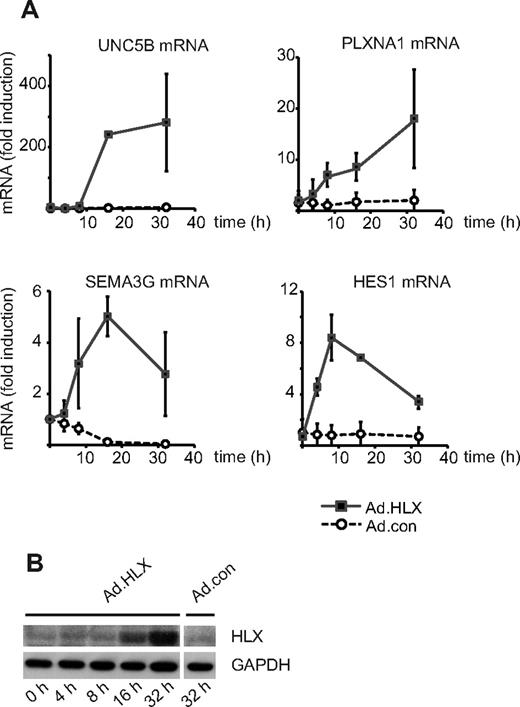
The gene encoding the homeobox transcription factor HLX has previously been identified by us to be induced by VEGF in endothelial cells, suggesting the involvement of HLX in VEGF-mediated transcriptional responses.5 We therefore investigated the transcriptomic program induced by HLX. An adenoviral expression construct encoding a Flag-tagged HLX cDNA was generated and used to transduce HUVECs in parallel with control adenovirus. mRNA was extracted from cells between 4 and 32 hours after infection and subjected to Affymetrix microarray analysis (GEO GSE13054). Fifty-three genes were identified to be induced > 2.5-fold 16 hours after infection (see supplemental Table 1). This group of genes was analyzed according to the categories surface receptors, secreted proteins, cytoplasmic signaling proteins, and transcription factors. Thereby, it became apparent that among the strongest induced genes were those for guidance receptors with proposed repulsive functions in the guidance of endothelial sprouts, namely UNC5B and plexin-A1. Furthermore, transcripts for a secreted guidance molecule, SEMA3G, and for several transcription factors with repressive function were found to be up-regulated. One example is the repressor HES-1, which has been described as a direct target gene of the Notch-signaling pathway. Table 1 displays the most strongly induced genes, which were confirmed by real-time RT-PCR. The kinetics of the up-regulation of mRNAs for UNC5B, PLXNA1, SEMA3G, and HES-1 as measured by real-time RT-PCR shows that the relative up-regulation was most pronounced for UNC5B (Figure 1A). The accumulation of recombinant HLX protein after transduction with Ad.HLX is depicted in Figure 1B.
Selected genes up-regulated by HLX in endothelial cells
| UniGene ID . | Entrez gene . | Gene symbol . | Gene title . | Ad.HLX/Ad.con . | |||
|---|---|---|---|---|---|---|---|
| 4 h . | 8 h . | 16 h . | 32 h . | ||||
| Surface receptors | |||||||
| Hs.585457 | 219699 | UNC5B | unc-5 homolog B (C elegans) | 1.41 | 2.23 | 5.71 | 7.14 |
| Hs.432329 | 5361 | PLXNA1 | Plexin A1 | 1.15 | 2.96 | 4.61 | 8.38 |
| Hs.132781 | 9466 | IL27RA | Interleukin 27 receptor, alpha | 0.91 | 1.86 | 4.18 | 5.53 |
| Secreted proteins | |||||||
| Hs.9315 | 56944 | OLFML3 | Olfactomedin-like 3 | 1.09 | 1.72 | 4.62 | 23.68 |
| Hs.59729 | 56920 | SEMA3G | Sema domain, immunoglobulin domain, short basic domain, secreted (semaphorin) 3G | 1.27 | 2.28 | 2.88 | 4.89 |
| Cytoplasmic (signaling) proteins | |||||||
| Hs.417050 | 8900 | CCNA1 | Cyclin A1 | 0.78 | 2.15 | 3.79 | 2.96 |
| Hs.2128 | 1847 | DUSP5 | Dual-specificity phosphatase 5 | 1.07 | 2.38 | 3.76 | 3.54 |
| Hs.381167 | 1992 | SERPINB1 | Serpin peptidase inhibitor, clade B (ovalbumin), member 1 | 0.93 | 1.33 | 3.15 | 4.25 |
| Transcription factors | |||||||
| Hs.360174 | 6591 | SNAI2 | Snail homolog 2 (Drosophila) | 1.58 | 3.87 | 4.51 | 6.91 |
| Hs.525704 | 3725 | JUN | Jun oncogene | 1.48 | 1.51 | 4.06 | 3.14 |
| Hs.250666 | 3280 | HES1 | Hairy and enhancer of split 1 (Drosophila) | 2.13 | 3.63 | 3.49 | 4.37 |
| Hs.659681 | 54880 | BCOR | BCL6 corepressor | 2.01 | 2.54 | 3.45 | 5.37 |
| UniGene ID . | Entrez gene . | Gene symbol . | Gene title . | Ad.HLX/Ad.con . | |||
|---|---|---|---|---|---|---|---|
| 4 h . | 8 h . | 16 h . | 32 h . | ||||
| Surface receptors | |||||||
| Hs.585457 | 219699 | UNC5B | unc-5 homolog B (C elegans) | 1.41 | 2.23 | 5.71 | 7.14 |
| Hs.432329 | 5361 | PLXNA1 | Plexin A1 | 1.15 | 2.96 | 4.61 | 8.38 |
| Hs.132781 | 9466 | IL27RA | Interleukin 27 receptor, alpha | 0.91 | 1.86 | 4.18 | 5.53 |
| Secreted proteins | |||||||
| Hs.9315 | 56944 | OLFML3 | Olfactomedin-like 3 | 1.09 | 1.72 | 4.62 | 23.68 |
| Hs.59729 | 56920 | SEMA3G | Sema domain, immunoglobulin domain, short basic domain, secreted (semaphorin) 3G | 1.27 | 2.28 | 2.88 | 4.89 |
| Cytoplasmic (signaling) proteins | |||||||
| Hs.417050 | 8900 | CCNA1 | Cyclin A1 | 0.78 | 2.15 | 3.79 | 2.96 |
| Hs.2128 | 1847 | DUSP5 | Dual-specificity phosphatase 5 | 1.07 | 2.38 | 3.76 | 3.54 |
| Hs.381167 | 1992 | SERPINB1 | Serpin peptidase inhibitor, clade B (ovalbumin), member 1 | 0.93 | 1.33 | 3.15 | 4.25 |
| Transcription factors | |||||||
| Hs.360174 | 6591 | SNAI2 | Snail homolog 2 (Drosophila) | 1.58 | 3.87 | 4.51 | 6.91 |
| Hs.525704 | 3725 | JUN | Jun oncogene | 1.48 | 1.51 | 4.06 | 3.14 |
| Hs.250666 | 3280 | HES1 | Hairy and enhancer of split 1 (Drosophila) | 2.13 | 3.63 | 3.49 | 4.37 |
| Hs.659681 | 54880 | BCOR | BCL6 corepressor | 2.01 | 2.54 | 3.45 | 5.37 |
Total RNA was isolated from HUVECs after infection with HLX-encoding (Ad.HLX) and control adenoviruses (Ad.con) for 4, 8, 16, and 32 hours. RNA was subjected to microarray analysis using the Affymetrix Human Genome U133 Plus 2.0 Array. Changes in gene expression induced in Ad.HLX relative to Ad.con-infected cultures are displayed as fold-induction at the given time points. The full set of Affymetrix data is available in the GEO database under GSE13054.
HLX up-regulates genes for repellent cell-guidance molecules and a transcriptional repressor of the Notch signaling pathway. (A) Real-time RT-PCR analysis of UNC5B, PLXNA1, SEMA3G, and HES1 mRNA: HUVECs were transduced for 4, 8, 16, and 32 hours with recombinant adenoviruses encoding HLX (Ad.HLX) or control viruses (Ad.con) using an MOI of 20. Total RNA was isolated and real-time RT-PCR performed as described in “Real-time RT-PCR analysis.” Values were normalized to β2-microglobulin mRNA as internal standard. Mean values ± SD calculated from triplicate wells are depicted as fold induction of the mRNA levels obtained after infection with Ad.HLX (■) or Ad.con (○) compared with noninfected cells. Results of 1 representative experiment of 3 independently performed are shown. (B) Western blot analysis of HLX: Total cell lysates were prepared from HUVECs nontransduced (0 hours) or tranduced with Ad.HLX (4, 8, 16, and 32 hours) or with Ad.con for 32 hours using an MOI of 20. Cells were harvested and proteins were separated by SDS- PAGE. Membranes were probed with anti-HLX and anti-GAPDH antibodies.
HLX up-regulates genes for repellent cell-guidance molecules and a transcriptional repressor of the Notch signaling pathway. (A) Real-time RT-PCR analysis of UNC5B, PLXNA1, SEMA3G, and HES1 mRNA: HUVECs were transduced for 4, 8, 16, and 32 hours with recombinant adenoviruses encoding HLX (Ad.HLX) or control viruses (Ad.con) using an MOI of 20. Total RNA was isolated and real-time RT-PCR performed as described in “Real-time RT-PCR analysis.” Values were normalized to β2-microglobulin mRNA as internal standard. Mean values ± SD calculated from triplicate wells are depicted as fold induction of the mRNA levels obtained after infection with Ad.HLX (■) or Ad.con (○) compared with noninfected cells. Results of 1 representative experiment of 3 independently performed are shown. (B) Western blot analysis of HLX: Total cell lysates were prepared from HUVECs nontransduced (0 hours) or tranduced with Ad.HLX (4, 8, 16, and 32 hours) or with Ad.con for 32 hours using an MOI of 20. Cells were harvested and proteins were separated by SDS- PAGE. Membranes were probed with anti-HLX and anti-GAPDH antibodies.
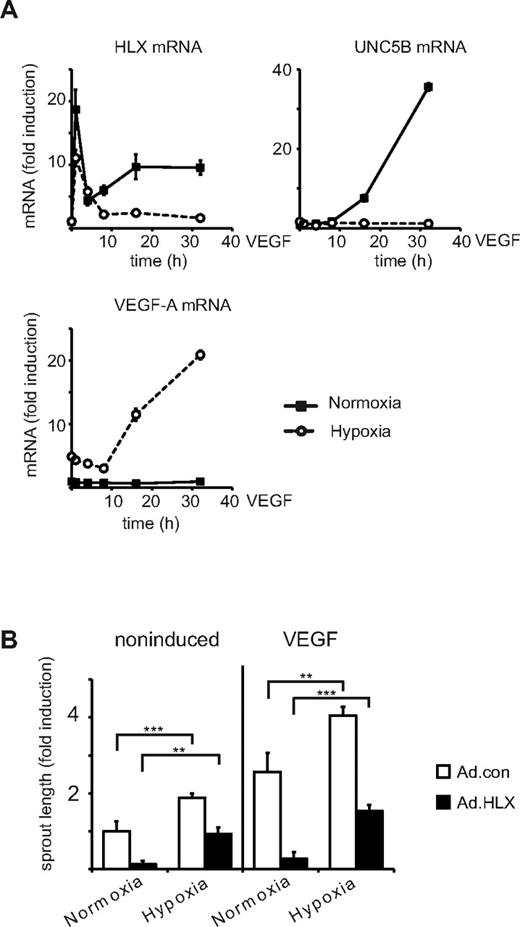
VEGF most prominently up-regulates UNC5B in an HLX-dependent way
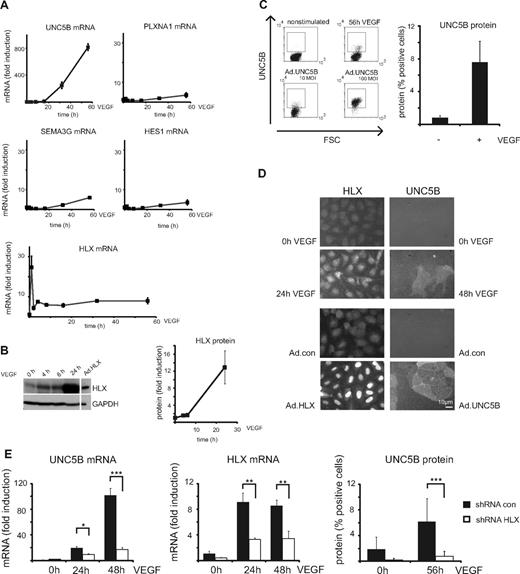
To evaluate whether VEGF up-regulates genes for repellent guidance molecules, which could be mediated by HLX as a secondary transcriptional response to VEGF, we examined mRNA levels for UNC5B, PLXNA1, SEMA3G, and HES-1 by real-time RT-PCR. From these, preferentially UNC5B mRNA was found to be increasingly up-regulated after VEGF stimulation starting between 2 and 4 hours, being 10-fold increased at 16 hours and reaching levels several hundred-fold of the starting values between 24 and 56 hours (Figure 2A, supplemental Figure 1A). This up-regulation of UNC5B mRNA was reflected in the appearance of a fraction of cells displaying UNC5B at their surface as shown by flow cytometry (Figure 2C) and cytoimmunochemistry (Figure 2D).
VEGF up-regulates HLX and UNC5B in endothelial cells. (A) Real-time RT-PCR analysis of UNC5B, PLXNA1, SEMA3G, HES1, and HLX mRNA: HUVECs were cultured to density, starved overnight in EBM-2 medium without supplements and then induced with 100 ng of VEGF/mL for 1, 2, 4, 8, 16, 32, and 56 hours. Total RNA was isolated and mRNA levels were determined by real-time RT-PCR. All values were normalized to β2-microglobulin mRNA as internal standard. Results displayed represent the mean of fold induction ± SEM of the respective mRNA levels calculated from triplicate wells. One representative experiment of 3 performed is shown. (B) Western blot analysis of HLX: Total cell lysates were prepared from HUVECs induced with 100 ng/mL VEGF for 4, 6, and 24 hours and HUVECs transduced with adenoviruses encoding HLX (Ad.HLX) for 72 hours. Cells were harvested and proteins were separated by SDS-PAGE. Membranes were probed with anti-HLX and anti-GAPDH antibodies. The left panel displays one exemplary blot, the right panel the quantification obtained from 3 independent experiments. (C) Flow cytometric analysis of UNC5B surface expression after VEGF induction: HUVECs starved as described in panel A were induced with VEGF-A for 56 hours or kept without stimulation. Cells were detached from the plates, stained with UNC5B and Alexa Fluor 647–labeled goat–anti-human IgG antibodies, and subjected to flow cytometry. The left panel displays representative dot blots of untreated, 56-hour VEGF-induced and control samples transduced for 56 hours with an UNC5B adenovirus. The right panel shows the quantification of 3 independent experiments performed in triplicates. Mean percentage of positive cells ± SEM calculated from at least 3 independent experiments is displayed. (D) Immunocytochemistry analysis of HLX and UNC5B after VEGF induction: HUVECs were induced for 24 and 48 hours; cells were fixed and stained with anti-HLX and anti-UNC5B antibodies. The top panels display the VEGF induction, the lower panels a control experiment using transduction with HLX and UNC5B-expressing adenoviruses. (E) Up-regulation of UNC5B by VEGF-A is HLX-dependent: HUVECs transduced with lentiviruses containing shRNA.con and shRNA.HLX for 48 hours were starved overnight and then induced with VEGF for 24, 48, or 56 hours as indicated. Cells were harvested and UNC5B mRNA and protein were analyzed by real-time RT-PCR and flow cytometry, respectively. The left and middle panels display fold induction of UNC5B mRNA ± SD or HLX mRNA ± SD (as a control) in comparison to noninduced control shRNA cells as calculated from 1 experiment representative of 4 performed in triplicates. The right panel displays the percentage of UNC5B-positive cells ± SD as measured by flow cytometry in a representative experiment performed in triplicates.
VEGF up-regulates HLX and UNC5B in endothelial cells. (A) Real-time RT-PCR analysis of UNC5B, PLXNA1, SEMA3G, HES1, and HLX mRNA: HUVECs were cultured to density, starved overnight in EBM-2 medium without supplements and then induced with 100 ng of VEGF/mL for 1, 2, 4, 8, 16, 32, and 56 hours. Total RNA was isolated and mRNA levels were determined by real-time RT-PCR. All values were normalized to β2-microglobulin mRNA as internal standard. Results displayed represent the mean of fold induction ± SEM of the respective mRNA levels calculated from triplicate wells. One representative experiment of 3 performed is shown. (B) Western blot analysis of HLX: Total cell lysates were prepared from HUVECs induced with 100 ng/mL VEGF for 4, 6, and 24 hours and HUVECs transduced with adenoviruses encoding HLX (Ad.HLX) for 72 hours. Cells were harvested and proteins were separated by SDS-PAGE. Membranes were probed with anti-HLX and anti-GAPDH antibodies. The left panel displays one exemplary blot, the right panel the quantification obtained from 3 independent experiments. (C) Flow cytometric analysis of UNC5B surface expression after VEGF induction: HUVECs starved as described in panel A were induced with VEGF-A for 56 hours or kept without stimulation. Cells were detached from the plates, stained with UNC5B and Alexa Fluor 647–labeled goat–anti-human IgG antibodies, and subjected to flow cytometry. The left panel displays representative dot blots of untreated, 56-hour VEGF-induced and control samples transduced for 56 hours with an UNC5B adenovirus. The right panel shows the quantification of 3 independent experiments performed in triplicates. Mean percentage of positive cells ± SEM calculated from at least 3 independent experiments is displayed. (D) Immunocytochemistry analysis of HLX and UNC5B after VEGF induction: HUVECs were induced for 24 and 48 hours; cells were fixed and stained with anti-HLX and anti-UNC5B antibodies. The top panels display the VEGF induction, the lower panels a control experiment using transduction with HLX and UNC5B-expressing adenoviruses. (E) Up-regulation of UNC5B by VEGF-A is HLX-dependent: HUVECs transduced with lentiviruses containing shRNA.con and shRNA.HLX for 48 hours were starved overnight and then induced with VEGF for 24, 48, or 56 hours as indicated. Cells were harvested and UNC5B mRNA and protein were analyzed by real-time RT-PCR and flow cytometry, respectively. The left and middle panels display fold induction of UNC5B mRNA ± SD or HLX mRNA ± SD (as a control) in comparison to noninduced control shRNA cells as calculated from 1 experiment representative of 4 performed in triplicates. The right panel displays the percentage of UNC5B-positive cells ± SD as measured by flow cytometry in a representative experiment performed in triplicates.
In the same samples, HLX mRNA already displayed a rapid peak value after 1 hour of stimulation and thereafter stayed 5- to 10-fold elevated up to 56 hours. HLX protein was increased starting at 4 hours and reaching 10-fold higher levels at later time points as shown by Western blot analysis (Figure 2B). The VEGF-induced HLX accumulated in the nuclei (Figure 2D). It therefore seemed possible that VEGF-induced HLX is involved in the up-regulation of UNC5B. We further investigated how general the up-regulation of HLX by VEGF would be in endothelial cells of different origin. This indicated that VEGF indeed induces HLX in a broad range of different endothelial cell types obtained from various sources including large vessel and microvascular endothelial cells. The induction rates were comparable with those observed with different HUVEC isolates ranging from 5- to 30-fold with a tendency of somewhat lower inducibility in microvascular cells (supplemental Figure 1B).
Next we tested whether HLX would directly mediate the observed up-regulation of UNC5B by VEGF. Indeed, down-modulation of HLX using a respective shRNA expressed from a lentiviral vector reduced the VEGF-induced accumulation of UNC5B mRNA and protein by > 80% (Figure 2E). This showed that UNC5B up-regulation by VEGF is mediated via HLX.
HLX inhibits migration of endothelial cells, but proliferation is unaffected
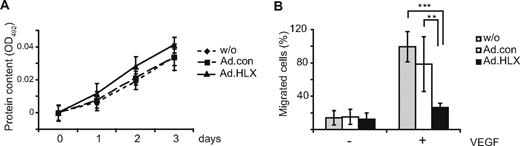
Considering that HLX appeared to preferentially up-regulate UNC5B and other repellent guidance cues and a transcriptional repressor of the Notch pathway, we next investigated to what extent HLX would affect proliferation and/or migration of endothelial cells. When HUVEC were infected with HLX-encoding adenoviruses, no significant influence of HLX overexpression on the growth of cells could be detected in comparison to control adenovirus-infected or noninfected cells (Figure 3A). However, in a transwell system, migration toward VEGF was inhibited by approximately 80% compared with cells infected with control adenovirus or noninfected cells (Figure 3B).
HLX does not affect the proliferation of endothelial cells, whereas migration is strongly reduced. (A) Proliferation assay: HUVECs were infected with HLX-encoding adenoviruses (Ad.HLX ▴) or control viruses (Ad.con ■) using an MOI of 8 or remained noninfected (without ♦). After 24 hours, 5 × 103 cells were seeded per well into 96-well plates; 0, 1, 2, and 3 days after seeding the protein content was measured at OD492 using the SRB assay. The values display the increase in protein content over the indicated time period ± SD calculated from 5 wells each. One experiment of 3 with similar results is shown. (B) Migration assay: HUVECs were transduced with Ad.HLX or Ad.con with a MOI of 8 each or remained noninfected. Three days after infection, cells were seeded into a transwell system and the migration of the cells from the upper chamber toward the lower chamber containing medium with 50 ng of VEGF/mL or without VEGF was scored 4 hours after seeding of the cells. Cells migrated into the lower chamber were visualized by staining with DAPI. Five random microscopic fields per transwell were photographed at a magnification of 10-fold and the stained cells were counted. Values display the percentage of migrated cells in the individual samples in comparison to the VEGF-induced migration in samples containing noninfected cells arbitrarily set to 100%. Mean values were calculated from 2 independent experiments with triplicates each ± SD. Inhibition of migration by Ad.HLX was significant as calculated by t test (**P < .005, ***P < .001).
HLX does not affect the proliferation of endothelial cells, whereas migration is strongly reduced. (A) Proliferation assay: HUVECs were infected with HLX-encoding adenoviruses (Ad.HLX ▴) or control viruses (Ad.con ■) using an MOI of 8 or remained noninfected (without ♦). After 24 hours, 5 × 103 cells were seeded per well into 96-well plates; 0, 1, 2, and 3 days after seeding the protein content was measured at OD492 using the SRB assay. The values display the increase in protein content over the indicated time period ± SD calculated from 5 wells each. One experiment of 3 with similar results is shown. (B) Migration assay: HUVECs were transduced with Ad.HLX or Ad.con with a MOI of 8 each or remained noninfected. Three days after infection, cells were seeded into a transwell system and the migration of the cells from the upper chamber toward the lower chamber containing medium with 50 ng of VEGF/mL or without VEGF was scored 4 hours after seeding of the cells. Cells migrated into the lower chamber were visualized by staining with DAPI. Five random microscopic fields per transwell were photographed at a magnification of 10-fold and the stained cells were counted. Values display the percentage of migrated cells in the individual samples in comparison to the VEGF-induced migration in samples containing noninfected cells arbitrarily set to 100%. Mean values were calculated from 2 independent experiments with triplicates each ± SD. Inhibition of migration by Ad.HLX was significant as calculated by t test (**P < .005, ***P < .001).
We have further assessed the potential triggering of apoptosis by HLX overexpression, but no signs of apoptosis were detectable 4 days after infection (supplemental Figure 2). This suggests that, in endothelial cells, HLX specifically interferes with a migratory mechanism, while it does not affect proliferation and apoptotic signaling pathways.
HLX and UNC5B strongly inhibit sprouting of endothelial cells in vitro
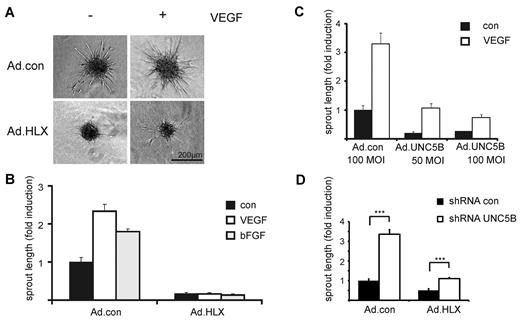
To investigate the effect of HLX on angiogenesis and particularly on sprouting of endothelial cells, we first used an in vitro angiogenesis assay, which evaluates sprout formation of cells embedded as spheroids in a collagen matrix. In this assay, HLX overexpression strongly inhibited sprouting of cells induced by VEGF or bFGF (Figure 4A-B). When decreasing MOIs of virus infection were used, the inhibiting effect diminished in a dose-dependent manner (supplemental Figure 3).
HLX causes strong inhibition of sprouting which is mediated to a significant part by UNC5B. HUVECs were infected with Ad.HLX, Ad.UNC5B, or Ad.con. The following day spheroids of infected and noninfected endothelial cells were generated as described in “Spheroid-based in vitro angiogenesis assay.” The spheroids were embedded into collagen gels and induced with VEGF or bFGF (25 ng/mL) or cultured without cytokine stimulation. After 24 hours, the spheroids were fixed and photographic images were taken for quantification. (A) Representative images of spheroids generated from Ad.HLX- or Ad.con-infected cells (10 MOI) and induced with VEGF (+) or left untreated (−) are displayed. (B) Quantification of inhibition of sprouting by overexpression of HLX: analyses of sprout lengths were performed by measuring the total lengths of sprouts for 10 spheroids each on microscopic images using the ImageJ software (http://www.uhnres.utoronto.ca/facilities/wcif/imagej/). Results are displayed as mean values ± SEM of the fold induction of sprout lengths observed compared with noninduced samples infected with control viruses (arbitrarily set to 1). One representative experiment of 4 performed is shown. (C) Quantification of inhibition of sprouting by overexpression of UNC5B: Analyses were as described in panel B. One representative experiment of 3 is shown. (D) Quantification of increase in sprouting by down-modulation of UNC5B in endothelial cells infected with Ad.con or Ad.HLX: HUVECs were first transduced with lentiviruses for control shRNA or shRNA targeted against UNC5B. After 1 day, cells were also infected with 15 MOI of Ad.con or Ad.HLX. The following day, spheroids were prepared and the next day incorporated into collagen gels in the presence of VEGF-A. Total sprout lengths of 15 spheroids each were measured. The shown values are calculated from 4 independent experiments and display the relative sprout length ± SEM in comparison to the samples transduced with Ad.con and shRNA.con. ***P < .001, t test.
HLX causes strong inhibition of sprouting which is mediated to a significant part by UNC5B. HUVECs were infected with Ad.HLX, Ad.UNC5B, or Ad.con. The following day spheroids of infected and noninfected endothelial cells were generated as described in “Spheroid-based in vitro angiogenesis assay.” The spheroids were embedded into collagen gels and induced with VEGF or bFGF (25 ng/mL) or cultured without cytokine stimulation. After 24 hours, the spheroids were fixed and photographic images were taken for quantification. (A) Representative images of spheroids generated from Ad.HLX- or Ad.con-infected cells (10 MOI) and induced with VEGF (+) or left untreated (−) are displayed. (B) Quantification of inhibition of sprouting by overexpression of HLX: analyses of sprout lengths were performed by measuring the total lengths of sprouts for 10 spheroids each on microscopic images using the ImageJ software (http://www.uhnres.utoronto.ca/facilities/wcif/imagej/). Results are displayed as mean values ± SEM of the fold induction of sprout lengths observed compared with noninduced samples infected with control viruses (arbitrarily set to 1). One representative experiment of 4 performed is shown. (C) Quantification of inhibition of sprouting by overexpression of UNC5B: Analyses were as described in panel B. One representative experiment of 3 is shown. (D) Quantification of increase in sprouting by down-modulation of UNC5B in endothelial cells infected with Ad.con or Ad.HLX: HUVECs were first transduced with lentiviruses for control shRNA or shRNA targeted against UNC5B. After 1 day, cells were also infected with 15 MOI of Ad.con or Ad.HLX. The following day, spheroids were prepared and the next day incorporated into collagen gels in the presence of VEGF-A. Total sprout lengths of 15 spheroids each were measured. The shown values are calculated from 4 independent experiments and display the relative sprout length ± SEM in comparison to the samples transduced with Ad.con and shRNA.con. ***P < .001, t test.
To evaluate whether UNC5B expression could mediate the inhibition of sprouting, we first used an adenovirus for overexpression of UNC5B. Indeed, ectopic UNC5B expression was able to strongly reduce sprouting activity (Figure 4C). This indicated that the inhibitory effect of HLX overexpression is at least in part mediated via UNC5B.
Inhibition of sprouting by HLX is mediated to a significant part by UNC5B
To establish to which extent UNC5B would contribute to the inhibition of sprouting observed after HLX overexpression, we used lentiviral expression of shRNA for the down-modulation of UNC5B mRNA. On average, a 60% down-modulation of the UNC5B mRNA was achieved after VEGF induction at the 56-hour time point (supplemental Figure 4A). The results show that a reduction in the HLX-mediated up-regulation of UNC5B led to significantly decreased inhibition of sprouting (Figure 4D). This shows that the inhibitory effect of HLX overexpression is at least to a significant part caused by UNC5B up-regulation.
It was further intriguing that in the VEGF-induced control samples, down-modulation of UNC5B also caused a strong increase of sprouting activity as evidenced by the cumulative sprout length depicted in Figure 4D. This effect was primarily composed of a higher number of sprouts (supplemental Figure 4B) suggesting that UNC5B up-regulated in VEGF-induced endothelial cells reduces sprout initiation.
Up-regulation of HLX and UNC5B is strongly reduced under hypoxic conditions
Furthermore, we tested the hypothesis that a potential reason for the up-regulation of inhibitory pathways and molecules by VEGF would be the down-modulation of sprouting in a negative feedback loop when normoxic conditions are restored after neovascularization. Therefore, we comparatively evaluated the effects of VEGF on HLX and UNC5B mRNAs under normoxic and hypoxic conditions. The obtained results show that under hypoxia the VEGF-inducible levels of HLX mRNA were strongly reduced, especially at later time points, and the up-regulation of UNC5B mRNA was completely prevented (Figure 5A). In contrast hypoxia induced VEGF mRNA as previously shown.26
Under hypoxic conditions VEGF-mediated up-regulation of HLX and UNC5B mRNA is strongly reduced whereas sprouting activity is increased. (A) Comparison of VEGF-A effects on HLX, UNC5B, and VEGF-A mRNA under hypoxic and normoxic conditions: Starved HUVECs were treated with VEGF-A and were kept under normoxic or hypoxic conditions for 4, 8, 16, and 32 hours. The sample for the 1-hour value was kept under hypoxic conditions already for 4 hours before addition of VEGF. Cells were harvested and the RNA was isolated and subjected to real-time RT-PCR analysis. One representative experiment of 3 performed in triplicates is displayed. The values depict the mean of triplicates ± SEM. (B) Comparison of sprouting activity under hypoxic and normoxic conditions: HUVECs infected with Ad.con or Ad.HLX (20 MOI each) for 1 day were used in the spheroid sprouting assay. Normoxic or hypoxic conditions were used immediately after embedding into the collagen gel concomitant with VEGF addition. Results are displayed as fold induction ± SEM of total sprout length in comparison to spheroids incubated at normoxic conditions without VEGF. **P < .005, ***P < .001, t test.
Under hypoxic conditions VEGF-mediated up-regulation of HLX and UNC5B mRNA is strongly reduced whereas sprouting activity is increased. (A) Comparison of VEGF-A effects on HLX, UNC5B, and VEGF-A mRNA under hypoxic and normoxic conditions: Starved HUVECs were treated with VEGF-A and were kept under normoxic or hypoxic conditions for 4, 8, 16, and 32 hours. The sample for the 1-hour value was kept under hypoxic conditions already for 4 hours before addition of VEGF. Cells were harvested and the RNA was isolated and subjected to real-time RT-PCR analysis. One representative experiment of 3 performed in triplicates is displayed. The values depict the mean of triplicates ± SEM. (B) Comparison of sprouting activity under hypoxic and normoxic conditions: HUVECs infected with Ad.con or Ad.HLX (20 MOI each) for 1 day were used in the spheroid sprouting assay. Normoxic or hypoxic conditions were used immediately after embedding into the collagen gel concomitant with VEGF addition. Results are displayed as fold induction ± SEM of total sprout length in comparison to spheroids incubated at normoxic conditions without VEGF. **P < .005, ***P < .001, t test.
When we performed the spheroid sprouting assay under hypoxic conditions we observed a significant increase of sprouting supporting that hypoxia has an additive effect on VEGF-induced sprouting. Hypoxia- plus VEGF-induced sprouting could be inhibited by overexpression of HLX; however, the relative inhibition appeared less pronounced compared with the inhibition under normoxic conditions (Figure 5B). This suggests that HLX-mediated induction of UNC5B is involved in down-modulation of sprouting under normoxic conditions and hypoxia would facilitate sprouting by reducing UNC5B expression.
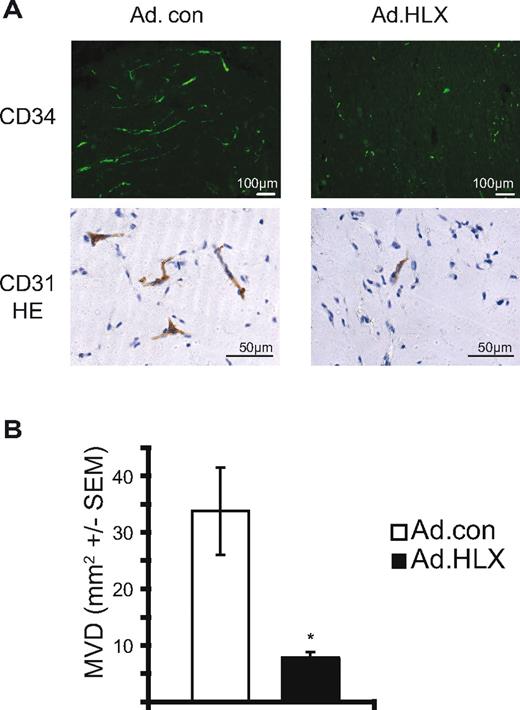
Overexpression of HLX inhibits vessel formation in vivo
To test whether overexpression of HLX would exert an inhibiting effect on angiogenesis in vivo, we further used an in vivo angiogenesis assay with human endothelial cells in mice.27 In this assay, HUVEC spheroids infected with HLX or control adenoviruses were suspended in a mixture of Matrigel and fibrinogen supplemented with growth factors and implanted subcutaneously into SCID mice. Grafted human endothelial cells vascularize the plug and anastomose with the mouse vasculature to give rise to a perfused vascular network (see supplemental Figure 5). Plugs were harvested after 14 days and analyzed by immunohistochemistry.
A significant reduction in the density and length of vessels was observed in vascular networks originating from HLX overexpressing HUVECs compared with control cells (Figure 6). The inhibition was similarly pronounced as the inhibition of sprouting in vitro supporting that HLX plays a comparable role in vivo.
HLX reduces vessel formation in an endothelial spheroid xenografting assay in vivo. HUVEC were infected with HLX-encoding adenoviruses and control viruses with an MOI of 20. The infected cells were used to generate endothelial cell spheroids consisting of approximately 100 cells/spheroid. Spheroids were suspended in a Matrigel/fibrinogen mixture containing 500 ng/mL VEGF and bFGF. After addition of thrombin the spheroid suspension was injected subcutaneously into SCID mice (2 plugs/mouse, 8 mice/experimental group). Plugs were harvested after 14 days, fixed, and analyzed by immunohistochemistry using CD31 or CD34 or antibodies. (A) Representative images of sections of plugs containing HUVECs infected with Ad.HLX or Ad.con and stained with anti-human CD34 antibodies or anti-human CD31 antibodies plus hematoxylin. (B) Evaluation of vessel formation was performed by measuring the vessel areas stained for CD34 on 16 images of 8 sections. The MVD per mm2 ± SEM is depicted for plugs containing vessels with Ad.HLX (■) and Ad.con (□) infected HUVEC. *P < .05, t test.
HLX reduces vessel formation in an endothelial spheroid xenografting assay in vivo. HUVEC were infected with HLX-encoding adenoviruses and control viruses with an MOI of 20. The infected cells were used to generate endothelial cell spheroids consisting of approximately 100 cells/spheroid. Spheroids were suspended in a Matrigel/fibrinogen mixture containing 500 ng/mL VEGF and bFGF. After addition of thrombin the spheroid suspension was injected subcutaneously into SCID mice (2 plugs/mouse, 8 mice/experimental group). Plugs were harvested after 14 days, fixed, and analyzed by immunohistochemistry using CD31 or CD34 or antibodies. (A) Representative images of sections of plugs containing HUVECs infected with Ad.HLX or Ad.con and stained with anti-human CD34 antibodies or anti-human CD31 antibodies plus hematoxylin. (B) Evaluation of vessel formation was performed by measuring the vessel areas stained for CD34 on 16 images of 8 sections. The MVD per mm2 ± SEM is depicted for plugs containing vessels with Ad.HLX (■) and Ad.con (□) infected HUVEC. *P < .05, t test.
Discussion
Several members of the homeobox family have been implicated in the development of the cardiovascular system as well as in vascular remodeling in the adult and have been proposed to be important to many pathologies including atherosclerosis and tumor angiogenesis.28-30
We have recently identified HLX,5,8 a diverged homeobox gene, so far not known to be involved in vascular remodeling, as being preferentially up-regulated in endothelial cells by VEGF-A and to some extent also by another main angiogenic mediator, bFGF.5,31 HLX has previously been observed to be expressed in HUVEC, in placental endothelial cells and in trophoblasts.6,14,32,33 However, no precise function and detailed mechanism of action has yet been defined for HLX in the vascular system. Because our experiments neither identified an induction of HLX expression by inflammatory mediators nor by other more general growth factors such as EGF,5 we proposed that HLX might mediate a specific function of VEGF-A and bFGF related to angiogenesis and/or vascular remodeling.
In the present study, we have undertaken an analysis of downstream target genes of HLX by gene profiling after overexpression in endothelial cells (supplemental Table 1). It was intriguing that HLX can be a prominent inducer of genes implicated in the negative regulation of the sprouting process, including genes for repulsive guidance cues such as UNC5B, PLXNA1, and SEMA3G as well as a transcriptional repressor, HES-1 (Table 1).
UNC5B belongs to the uncoordinated-5 family of transmembrane receptors which consists of 4 members. From these, UNC5A plays a pivotal role in neurons for axon guidance, whereas UNC5B is largely restricted to the vascular system.34 It has been demonstrated that on binding to its soluble ligand netrin-1, angiogenesis is inhibited.17,35 A primarily repulsive role of UNC5B and its ligands in vessel formation is further indicated by the finding that disruption of the UNC5B gene in mice leads to increased extensions of tip cell filopodia, excessive vessel branching, and abnormal navigation.34 UNC5B is expressed in growing embryonic vessels and is then down-regulated in the quiescent adult vasculature, but reexpressed on stimulation of sprouting angiogenesis, for example, in tumor angiogenesis.
PLXNA1 and SEMA3G are members of another complex system regulating axonal and vessel guidance.36,37 SEMA3G has recently been found to be a primarily endothelial cell–expressed isoform.38 In general, members of the class 3 semaphorin family bind to neuropilin-1 or neuropilin-2 in a complex with plexins as coreceptors.37,39 As neuropilins are also coreceptors for various VEGF isoforms, these interactions interfere with VEGF receptor signaling and mediate repulsive signaling via the plexin receptors.
The transcriptional repressor HES-1 is a hallmark of the activated Notch pathway.40 This could indicate a link to tip and stalk cell differentiation as it has been shown that tip cells via Dll4 activate the Notch receptor in neighboring cells. Thereby, stalk cells are inhibited to sprout and are prevented from becoming additional tip cells.41,42 Because HLX induces HES-1, it is possible that this might support the stalk cell phenotype.
Based on further functional data that, in line with the repulsive and repressive functions of the described molecules, HLX overexpression strongly inhibited sprouting of endothelial cells, we started a systematic analysis to define which of the genes would be most significantly involved in HLX- and VEGF-regulated mechanisms. The obtained data showed that UNC5B especially is intimately linked in expression and function to HLX and VEGF-A. First, from the 4 genes analyzed by real-time RT-PCR, which included UNC5B, PLXNA1, SEMA3G and HES-1, overexpression of HLX up-regulated UNC5B mRNA several hundred-fold, which was by far the strongest effect (Figure 1). Second, we observed that in VEGF-A–containing cultures UNC5B mRNA displayed a continuous several hundred-fold up-regulation over several days, whereas PLXNA1, SEMA3G, and HES1 mRNAs increased only modestly around 5-fold (Figure 2A). The VEGF induction of UNC5B mRNA was also reflected in the appearance of UNC5B protein at the surface of a fraction of the cells (Figure 2C). UNC5B up-regulation was preceded by the induction of continuously elevated levels of HLX mRNA resulting in > 10-fold nuclear accumulation of HLX protein at 24 hours (Figure 2B,D). This was in line with the possibility that HLX is involved in up-regulating transcription of the UNC5B gene. Importantly, down-modulation of HLX mRNA by shRNA reduced VEGF-inducible UNC5B mRNA and protein by > 80% (Figure 2E). This directly confirmed that HLX indeed mediates the VEGF-triggered UNC5B induction to a large extent.
Because our data showed that HLX overexpression caused a strong inhibition of migration (Figure 3B) and sprouting of endothelial cells (Figure 4A-B), we first tested the possibility that UNC5B up-regulated by HLX might be decisively involved. Indeed, the obtained data showed that overexpression of UNC5B alone using a recombinant adenovirus caused a strong inhibition of sprouting (Figure 4C). Vice versa, down-modulation of UNC5B in HLX-overexpressing cells significantly reduced inhibition of sprouting restoring activity to the level of control virus–infected cells (Figure 4D). This confirmed that UNC5B expression by itself can cause significant inhibition of sprouting and at least a significant part of the HLX-mediated inhibition. In this context, it is important to mention that down-modulation of UNC5B not only increased sprouting in HLX-suppressed endothelial cells, but also in VEGF-induced control cells reaching 3-fold higher sprouting levels (Figure 4D, supplemental Figure 4B). This may indicate that HLX-mediated inherent expression of UNC5B in VEGF-induced cells reduces sprouting activity. This is in accordance with previous reports that UNC5B is preferentially expressed in growing vessels and capillaries.35
Based on these data and considering a primarily repulsive role, one can principally speculate on 2 nonexclusive potential roles of VEGF-induced HLX and UNC5B for sprouting angiogenesis. It might be possible that UNC5B expression is generally needed to counterbalance the effects of attractive guidance cues. This may be necessary to fine-tune sprouting by preventing excessive sprout initiation under VEGF stimulation and/or to give appropriate direction to the growing sprout by reducing branching. This possibility is in line with the finding that down-modulation of UNC5B in VEGF-induced cells additively increases the number of sprouts (supplemental Figure 4B).
Furthermore, it might be possible that UNC5B is an in-built inhibitory feedback mechanism that serves to adapt sprouting activity to physiologic needs. Such a possibility could be the modulation of UNC5B expression by a hypoxic gradient. It has been previously shown that hypoxia via the transcription factor HIF1α can lead to the up- and down-regulation of several hundred genes.26 We therefore tested the hypothesis that HLX and UNC5B expression in the presence of VEGF could be modulated by hypoxia. Indeed, we find that under hypoxic conditions VEGF induction of HLX mRNA is strongly reduced and accumulation of UNC5B mRNA is prevented. Furthermore, hypoxia increased sprouting additively to VEGF. It is tempting to speculate that this is because of the reduced capacity of the cells to produce UNC5B under hypoxic conditions. These data strongly suggest that one function of the VEGF/HLX/UNC5B induction axis may be to prevent inappropriate sprouting under normoxic conditions and to adapt sprouting activity to a hypoxic gradient.
We have further used an endothelial spheroid xenografting assay to evaluate whether HLX might similarly function in vivo and exert a negative effect on vessel formation. In this assay, HUVEC spheroids are implanted in a Matrigel/fibrinogen matrix below the skin of SCID mice. The human cells can normally give rise to a perfused vascular network fused with murine vessels as indicated by the presence of erythrocytes in the human vessel parts (supplemental Figure 5). Comparable with the in vitro assay, HLX expression strongly reduced the capacity of the cells to form vessels supporting that HLX exerts similar inhibitive function in vivo.
This work defines for the first time a transcription factor apparently controlling a genetic program for the expression of several repulsive molecules. Whereas the data described here show that UNC5B is most closely linked to VEGF induction as well as the inhibitory effects of HLX, it will be further interesting to determine to what extent PLXNA1 and SEMA3G might function in a comparable or rather different way. Considering these newly described properties of HLX, we propose that the factor might be a potent inhibitor of pathologic angiogenesis and could be used as a novel principle to inhibit tumor angiogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Brian Becknell and Michael A. Caligiuri for a generous gift of rabbit anti-HLX antibodies, Drs Noriaki Kitamura and Hirofumi Arakawa for adenoviruses expressing human UNC5B, and Genentech Inc for antibodies to human UNC5B. Furthermore, we are grateful to Dr Johann Wojta and Christoph Kaun for a gift of several macro- and microvascular endothelial cells, the midwives of the Wilhelminen Hospital Vienna for continuous supply with umbilical cords, and Maria Witkowsky for reliable and expert technical assistance in the isolation and culture of endothelial cells. Our thanks belong also to the members of the molecular vascular biology group of the Medical University of Vienna, for technical help and discussions.
This work was supported by grants of the Austrian Science Foundation (P21291-B11) and the European Commission (Health 222995).
C.S. is a recipient of a DOC-fFORTE fellowship of the Austrian Academy of Sciences.
Authorship
Contribution: J.T. did the majority of detailed planning, experimental work, and analysis, in addition to writing parts of the manuscript; B.S., I.H., C.S., K.L., S.G., P.N., R.H.-W., and M.B. performed experiments and analyzed data; H.G.A. contributed to the design of and conclusions from the vessel formation experiments; and E.H. perceived and designed the overall research work and wrote the majority of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for B.S. is Department of Cell Biology, The Scripps Research Institute, La Jolla, CA. The current affiliation for I.H. is Department of Dermatology, University Hospital Essen, Essen, Germany. The current affiliation for P.N. is Department of Hematology/Oncology, Medical University of South Carolina, Charleston, SC.
Correspondence: Dr Erhard Hofer, Department of Vascular Biology and Thrombosis Research, Center for Physiology and Pharmacology, Medical University of Vienna, Lazarettgasse 19, A-1090 Vienna, Austria; e-mail: erhard.hofer@meduniwien.ac.at.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal