Abstract
The interferon-λ (IFNλ) family of cytokines, consisting of interleukin-28A (IFNλ2), IL-28B (IFNλ3), and IL-29 (IFNλ1), have been extensively studied for their antiviral activities. However, little is known about the effect of IFNλ on antigen-presenting cells. In the present study, we show for the first time that IL-29 can increase Toll-like receptor (TLR)–induced IL-12p40 production by human monocyte-derived macrophages. In contrast, IL-29 did not affect monocytes or monocyte-derived dendritic cells (DCs) because of restricted IL-28 receptor α chain expression by macrophages. Furthermore, IL-29–treated macrophages were more responsive to IFNγ, because IL-29 enhanced IFNγ-induced IL-12p40 and tumor necrosis factor (TNF) production by macrophages on R848 stimulation. However, IFNα suppressed IFNγ-induced IL-12p40 and tumor necrosis factor TNF production by human macrophages. The differential effects of IL-29 and IFNα on the responsiveness of macrophages to IFNγ could not be explained by an effect on TLR7 or TLR8 mRNA expression or by altered IL-10 signaling. However, we demonstrated that IL-29 up-regulated, whereas IFNα down-regulated, the surface expression of the IFNγ receptor 1 chain on macrophages, thereby resulting in differential responsiveness of TLR-challenged macrophages to IFNγ. Our findings on the differences between IFNα and IL-29 in modulating TLR-induced cytokine production by macrophages may contribute to understanding the role of IFNs in regulating immunity to pathogens.
Introduction
Over the last years, the interferon-λ (IFNλ) family members have been extensively studied for their antiviral activities. Interleukin-28A (IL-28A, also known as IFNλ2), IL-28B (IFNλ3), and IL-29 (IFNλ1) have been shown to possess potent antiviral activity via mechanisms similar to IFNα despite triggering of a unique IL-28 receptor pair distinct from the IFNα receptor.1-3 At present, IFNα combined with ribavirin is the most efficient therapy used to treat patients chronically infected with the hepatitis C virus (HCV). However, the many side effects and the limited effectiveness shown in a large group of patients mean that alternatives to the standard treatment are needed. Clinical studies are being conducted to examine whether pegylated IL-29 holds promise for future therapeutic use in the treatment of chronic HCV patients.4 Interestingly, polymorphisms close to the IL-28B gene have been reported that are associated with disease progression and response to therapy and have sparked interest in the IFNλ family members.5-8 Numerous studies have examined the antiviral activity of IFNλ,9-13 but little is known about its effect on innate immune cells and their immunoregulatory activity.
Macrophages are crucial innate immune cells that eliminate pathogens and apoptotic cells.14-16 On stimulation of specific pathogen-recognition receptors such as Toll-like receptors (TLR), macrophages produce inflammatory mediators and cytokines such as IL-1β, IL-6, tumor necrosis factor (TNF), and IL-12. IL-12p70, consisting of IL-12p40 and IL-12p35, plays an important role in the development of T helper 1 (Th1)–type responses, which are essential for the clearance of many infections.17 TLR ligands derived from microorganisms are strong inducers of IL-1218,19 ; however, additional signals from cytokines such as IFNγ and IL-4, as well as the interaction between CD40L and CD40, are necessary for the optimal production of IL-12 by monocytes, dendritic cells (DCs), and macrophages.20,21 Both IFNα and IFNγ are able to regulate the production of IL-12. Whereas the production of both IL-12p70 and IL-12p40 is strongly enhanced by IFNγ in antigen-presenting cells,20,22 IFNα has an inhibitory effect on IL-12p40 production by both mice and human monocytes, DCs, and macrophages.22,23 In contrast to the effect on IL-12p40, the production of IL-12p70 is enhanced by exposure to IFNα in monocytes and DCs.24
IFNλ and IFNα interact with different receptors.25-27 The receptor of IFNα is composed of 2 unique receptor chains, IFNαR1 and IFNαR2, whereas the receptor of IFNλ comprises the IL-28 receptor α chain (IL-28RA) and the IL-10 receptor 2 chain (IL-10R2). Whereas IFNαR1, IFNαR2, and IL-10R2 are ubiquitously expressed, IL-28RA appears to be more restricted, and expression of this receptor chain has been reported by plasmacytoid DCs, B cells, epithelial cells, and hepatocytes.1,27-30 The more restricted receptor expression of IL-28RA makes it likely that IL-29 would lead to less adverse effects compared with IFNα if used therapeutically, for example, to treated chronic HCV patients. However, at present, little is known about the effect of IFNλ on innate immune cells and their immunoregulatory activity.
In the present study, we show that although IL-28RA is not expressed by human primary monocytes and monocyte-derived DCs, it is expressed by monocyte-derived macrophages. As a consequence, IL-29 enhances TLR-induced cytokine production of human monocyte-derived macrophages, but does not affect monocytes or monocyte-derived DCs. Unlike the reported similarities in antiviral activity, we show for the first time that IL-29 and IFNα differ in their ability to modulate TLR-induced IL-12p40 production, especially in combination with IFNγ. In addition, IL-29 up-regulates the IFNγR1 chain, whereas IFNα down-regulates this receptor chain, which explains the differential responsiveness of human macrophages to IFNγ. Our findings demonstrate that in addition to its potent antiviral activity, IL-29 plays an important role in modulating cytokine production by macrophages, which may enhance immune responses to pathogens.
Methods
Cell culture and purification
Monocytes were purified from peripheral blood mononuclear cells (PBMCs) obtained from buffy coats (Sanquin) with magnetic CD14 microbeads (Miltenyi Biotec) following the manufacturer's instructions. The purity was always more than 97%. Macrophages were generated from purified monocytes with 10 ng/mL of macrophage-colony stimulating factor (M-CSF; R&D Systems) in 6-well plates (Costar; Corning) at a density of 1.5 × 106 cells/well in 2 mL of RPMI 1640 medium supplemented with 8% research-grade fetal calf serum. On days 2 and 5, half of the medium was refreshed, and on day 6, monocyte-derived macrophages were harvested and used for various purposes. Monocyte-derived DCs were generated from monocytes with 10 ng/mL of IL-4 (eBioscience) and 10 ng/mL of granulocyte–GM-CSF (Leukine; Bayer Healthcare Pharma-ceuticals) in 6-well plates (Costar) at a density of 1.5 × 106 cells/well in 2 mL of RPMI 1640 medium supplemented with 8% research-grade fetal calf serum.
Stimulation of monocyte-derived macrophages, monocytes, and monocyte-derived DCs
To determine which cells respond to IL-29, monocyte-derived macrophages, monocytes, and monocyte-derived DCs were pretreated with IL-29 (100 ng/mL; R&D Systems) for 5 hours and further stimulated for 24 hours with lipopolysaccharide (LPS; 100 ng/mL; InvivoGen) or R848 (1 μg/mL; Alexis Biochemicals) without removing the supernatant. To compare the effects of distinct IFNλ family members and IFNα, monocyte-derived macrophages were pre-exposed to IL-29 (100 ng/mL), IL-28A (100 ng/mL; R&D Systems), IL-28B (100 ng/mL; R&D Systems), or IFNα (INTRON A; 10 ng/mL; Schering-Plough) for 5 hours and further stimulated for 24 hours with LPS (100 ng/mL) or R848 (1 μg/mL). To check the response to IFNγ, monocyte-derived macrophages pretreated with IFNα or IL-29 were further stimulated with IFNγ (10 ng/mL; Miltenyi Biotec) and R848. In some experiments, anti–human IL-10R antibody (anti–IL-10R, clone 3F9; 5 μg/mL; BioLegend) was used to block IL-10 signaling in the cultures. Cytokine production was determined by enzyme-linked immunosorbent assay (ELISA).
Flow cytometric analysis of the expression of the IFN receptors HLA-DR and HLA-ABC
Monocyte-derived macrophages, monocytes, and monocyte-derived DCs were stained with the antibodies IL-28RA–phycoerythrin (IL-28RA-PE; BioLegend) and IL-10R2-biotin (R&D Systems) to evaluate the expression of the IFNλ receptor. Before the addition of antibodies, Fc receptors were blocked to prevent nonspecific staining. Streptavidin-allophycocyanin (BD Pharmingen) was used to visualize IL-10R2. IFNγR1-PE and IFNγR2-PE (BioLegend) were used to determine IFNγ receptor expression on monocyte-derived macrophages pretreated with IL-29 (100 ng/mL), IL-28A (100 ng/mL), IL-28B (100 ng/mL), or IFNα (10 ng/mL). The specificity of the staining was controlled with the appropriate isotype antibodies.
To examine the surface expression of human leukocyte antigen–DR (HLA-DR) and HLA-ABC on the surface of monocyte-derived macrophages, cells were first exposed to IL-29 (100 ng/mL) or IFNα (10 ng/mL) for 5 hours and then further stimulated with IFNγ (10 ng/mL) for another 20 hours. HLA-DR and HLA-ABC expression on macrophages were determined by flow cytometry using antibodies against HLA-DR-peridinin chlorophyll A protein (PerCP)–Cy5.5 (LN3; eBioscience) and HLA-ABC fluorescein isothiocyanate (FITC) (W6/32; BioLegend). The specificity of the staining was controlled with the appropriate isotype antibodies.
Flow cytometric analysis of pSTAT-1 staining
Monocyte-derived macrophages, monocytes, and monocyte-derived DCs were stimulated with IL-29 or IFNα for 20 minutes. Stimulated cells were immediately fixed with Phosflow Lyse/Fix (BD Biosciences) and then permeabilized with Phosflow Perm Buffer III (BD Biosciences). Cells were then incubated with mouse anti-pSTAT1–Alexa Fluor 488 (eBioscience), and the phosphorylation state of STAT-1 was measured by flow cytometry (FASCanto II; BD Biosciences).
Quantification of gene expression by RT-PCR
To determine the effect of IL-29 and IFNα on TLR7 and TLR8 mRNA expression, monocyte-derived macrophages were stimulated with IL-29 (100 ng/mL) or IFNα (10 ng/mL) for 5 hours, lysed using TRI Reagent (Sigma-Aldrich), and stored at −80°C. To determine the IL-12p40, IL-12p35, and IL-12p19 mRNA expression in macrophages, monocyte-derived macrophages were stimulated with IL-29 (100 ng/mL) or IFNα (10 ng/mL) for 5 hours and then further stimulated for 5 hours with R848 (1 μg/mL) plus IFNγ (10 ng/mL). The cells were lysed using TRI Reagent (Sigma-Aldrich) and stored at −80°C.
Total RNA from monocyte-derived macrophages was extracted using the NucleoSpin RNA II kit (MACHEREY-NAGEL) according to the manufacturer's instructions. RNA was quantified using a Nanodrop ND-1000 (Thermo Fisher Scientific). cDNA was prepared using the iScript cDNA synthesis kit (Bio-Rad). All real-time polymerase chain reactions (RT-PCRs) were performed in Bio-Rad optical 96-well plates using a MyIQ5 detection system (Bio-Rad). SYBR-Green present in the MasterMix Plus (Eurogentec) was used for quantification. Primers for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (forward 5′-TGCACCACCAACTGCTTAGC-3′ and reverse 5′-GGCATGGACTGTGGTCATGAG-3′) and TLR7 (forward 5′-AATGTCACAGCCGTCCCTAC-3′ and reverse 5′-GCGCATCAAAAGCATTTACA-3′) were designed to determine the TLR7 mRNA expression. Primer probes for GAPDH (Hs00266705_g1), TLR8 (Hs00152972_m1), IL-12p40 (Hs01011518_m1), IL-12p35 (Hs01073447_m1), and IL-23p19 (Hs00372324_m1) were purchased from Applied Biosystems. The expression of target genes was normalized to GAPDH using the formula: 2−ΔCt, ΔCt = CtTLR − CtGAPDH.
Immunoassay for detection of cytokines in supernatant
The concentrations of cytokines in the supernatant were determined using sandwich ELISA specific for IL-12p40 (C8.6 and C8.3 antibody pairs; BioLegend), and Ready-Set-Go kits for IL-12p70, IL-23, IL-10, and TNF (all from eBioscience). The detection limits for IL-10, IL-12p70, IL-23, and TNF were 15 pg/mL and for IL-12p40 30 pg/mL.
Statistics
Values are expressed as mean values unless indicated otherwise. Data were analyzed with Prism 5.0 software (GraphPad) using the Mann-Whitney t test to compare variables between 2 independent groups. In all analyses, a 2-tailed P value less than .05 (confidence internal 95%) was considered statistically significant.
Results
Monocyte-derived macrophages, but not monocytes or monocyte-derived DCs, respond to IL-29 because of the restricted IL-28RA expression by monocyte-derived macrophages
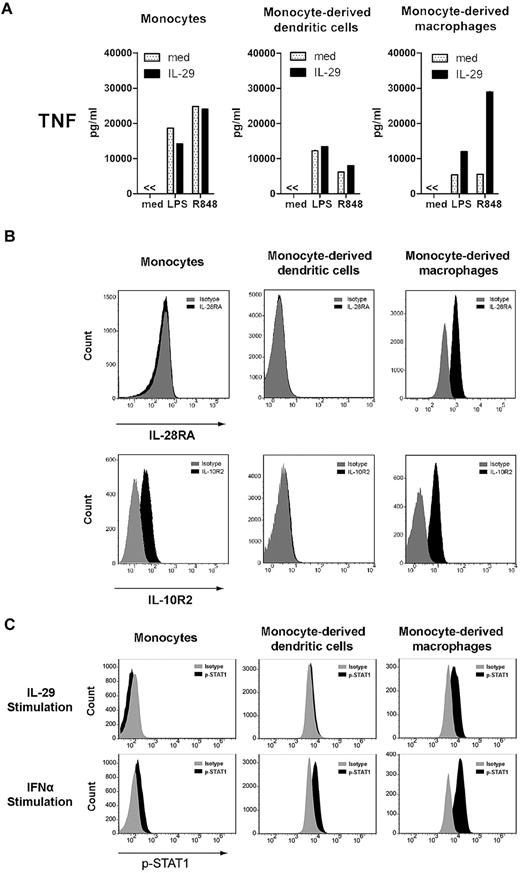
To examine the role of IL-29 in modulating immune responses, we first compared the responsiveness of different innate target cells to IL-29. For this purpose, human circulating monocytes, monocyte-derived DCs, and monocyte-derived macrophages were challenged with IL-29 in combination with LPS or R848. As shown in Figure 1A, IL-29 enhanced the levels of TNF produced by LPS-stimulated, monocyte-derived macrophages by 2-fold and R848-induced responses were enhanced 5-fold. However, neither TLR-stimulated monocytes nor monocyte-derived DCs responded to IL-29, as indicated by their TNF production. Neither monocytes nor monocyte-derived DCs responded to increasing doses of IL-29 (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) or to being exposed to IL-29 for different pretreatment periods (supplemental Figure 1B). Monocytes remained unresponsive to IL-29 when cultured in the presence of M-CSF for 24 hours (data not shown).
Monocyte-derived macrophages, but not monocytes nor monocyte-derived DCs, respond to IL-29 because of the restricted IL-28RA expression by monocyte-derived macrophages. (A) Monocytes, monocyte-derived DCs, and monocyte-derived macrophages were pretreated with IL-29 for 5 hours, and then further stimulated with LPS or R848 for 24 hours. TNF production was determined by ELISA. The values depicted show representative data from 11 independent experiments. (B) Monocytes, monocyte-derived DCs, and monocyte-derived macrophages were stained with antibodies against IL-28RA and IL-10R2 to evaluate the expression of the IFNλ receptor by flow cytometry. The specificity of the staining was controlled with the appropriate isotype antibodies. The histograms depicted show representative data from 10 independent experiments. (C) Monocytes, monocyte-derived DCs, and monocyte-derived macrophages were stimulated with IL-29 or IFNα for 20 minutes, and then cells were fixed and permeabilized. The phosphorylation of STAT-1 was measured by flow cytometry (FACSCanto II; BD Biosciences).
Monocyte-derived macrophages, but not monocytes nor monocyte-derived DCs, respond to IL-29 because of the restricted IL-28RA expression by monocyte-derived macrophages. (A) Monocytes, monocyte-derived DCs, and monocyte-derived macrophages were pretreated with IL-29 for 5 hours, and then further stimulated with LPS or R848 for 24 hours. TNF production was determined by ELISA. The values depicted show representative data from 11 independent experiments. (B) Monocytes, monocyte-derived DCs, and monocyte-derived macrophages were stained with antibodies against IL-28RA and IL-10R2 to evaluate the expression of the IFNλ receptor by flow cytometry. The specificity of the staining was controlled with the appropriate isotype antibodies. The histograms depicted show representative data from 10 independent experiments. (C) Monocytes, monocyte-derived DCs, and monocyte-derived macrophages were stimulated with IL-29 or IFNα for 20 minutes, and then cells were fixed and permeabilized. The phosphorylation of STAT-1 was measured by flow cytometry (FACSCanto II; BD Biosciences).
The receptor of IL-29, consisting of IL-28RA and IL-10R2, is expressed by a limited number of cell types, including plasmacytoid DCs, B cells, epithelial cells, and hepatocytes.1,27-30 To examine whether the differential responsiveness of human monocyte-derived macrophages, monocytes, and DCs to IL-29 can be explained by their receptor expression, flow cytometric analysis for IL-28RA and IL-10R2 expression was performed. Although IL-10R2 is expressed by monocyte-derived macrophages, monocytes, and weakly by DCs, IL-28RA expression was only observed in monocyte-derived macrophages, not in monocytes or monocyte-derived DCs (Figure 1B). In addition, phosphorylation of STAT-1 on exposure to IL-29 was only observed in monocyte-derived macrophages, not in monocytes or monocyte-derived DCs (Figure 1C). These data indicate that the differential responsiveness of human monocyte-derived macrophages, monocytes, and DCs to IL-29 is likely because of restricted IL-28RA expression by monocyte-derived macrophages.
IL-29 enhances TLR-induced IL-12p40 production by human monocyte-derived macrophages
Although much is known about the antiviral activity of IFNλ, its immunoregulatory activity on immune cells is still poorly understood. To examine this, we determined whether IL-29 affects cytokine production by TLR-stimulated monocyte-derived macrophages. We compared the effect of IL-29 on macrophages with IFNα to determine whether these cytokines modulate macrophages responses in a similar manner. This is especially relevant because IL-29 and type I IFN induce antiviral activity via similar mechanisms.1-3 Furthermore, because macrophages are highly responsive to IFNγ, we also included IFNγ in our analysis.
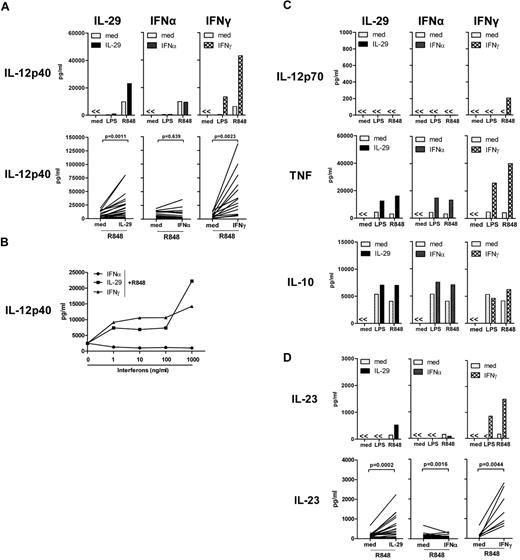
As shown in Figure 2A, low or undetectable IL-12p40 was produced by monocyte-derived macrophages in response to LPS irrespective of the addition of IL-29. However, on R848 stimulation, IL-29 increased the IL-12p40 production by monocyte-derived macrophages by approximately 2-fold, which was observed in the majority of donors (Figure 2A). In agreement with previous reports,20,22,23 no modulation of the levels of R848-induced IL-12p40 was observed by IFNα-treated, monocyte-derived macrophages, whereas IFNγ strongly enhanced IL-12p40 levels by macrophages on TLR ligation (Figure 2A). The enhancement of R848-induced IL-12p40 production by IL-29 was dose dependent, which was also observed for IFNγ (Figure 2B). However, even at relatively high IFNα concentrations, no increase of IL-12p40 production was observed; in fact, there was a mild decrease (Figure 2B). Reduced IL-12p40 production by IFNα was not due to IFNα-induced cytotoxicity (supplemental Figure 2A). This demonstrates that the distinct ability of IL-29 and IFNα to modulate IL-12p40 levels is not the result of suboptimal dosing of IFNα. Although IL-29 and IFNα regulate IL-12p40 production differently, IL-29 has similar effects as IFNα or IFNγ in enhancing TNF and IL-10 production by monocyte-derived macrophages in response to R848 stimulation (Figure 2C). Furthermore, on R848 stimulation, the production of IL-12p70 was only observed in IFNγ-treated monocyte-derived macrophages in 5 of 15 experiments, but not in macrophages treated with IL-29 or IFNα (Figure 2C). However, the production of bioactive IL-23 (a heterodimer consisting of an IL-12p40 and an IL-23p19 chain) following stimulation in the presence of IL-29 reflected the observed effect on IL-12p40 production, which suggests that IL-12p40 production contributes to the levels of bioactive IL-23 (Figure 2D). In summary, IL-29 has similar effects to IFNγ, but not IFNα, in augmenting IL-12p40 production by human monocyte-derived macrophages on TLR ligation.
IL-29 enhances TLR-induced IL-12p40 production by human monocyte-derived macrophages. (A) Monocyte-derived macrophages were pretreated with IL-29 (100 ng/mL), IFNα (10 ng/mL), or IFNγ (10 ng/mL) for 5 hours, and then further stimulated with LPS or R848. The level of IL-12p40 in the supernatants was determined by ELISA. The values depicted show representative data from 27 independent experiments. The increase of IL-12p40 by IL-29 in monocyte-derived macrophages was observed in 19 of 27 healthy individuals. (B) Monocyte-derived macrophages were pretreated with IL-29, IFNα, or IFNγ at the indicated concentrations for 5 hours, and then further stimulated with R848. The level of IL-12p40 in the supernatants was determined by ELISA. The values depicted show representative data from 3 independent experiments. Medium, IL-29, IFNα, or IFNγ alone did not induce IL-12p40 production by R848-stimulated, monocyte-derived macrophages. (C) Monocyte-derived macrophages were stimulated as described for panel A. The concentrations of IL-12p70, TNF, and IL-10 were determined in the supernatants using ELISA. Macrophages from 10 of 15 healthy individuals showed undetectable levels of IL-12p70 in response to IFNγ and R848 stimulation. (D) Monocyte-derived macrophages were pretreated with IL-29 (100 ng/mL), IFNα (10 ng/mL), or IFNγ (10 ng/mL) for 5 hours, and then further stimulated with LPS or R848. The level of IL-23 in the supernatants was determined by ELISA.
IL-29 enhances TLR-induced IL-12p40 production by human monocyte-derived macrophages. (A) Monocyte-derived macrophages were pretreated with IL-29 (100 ng/mL), IFNα (10 ng/mL), or IFNγ (10 ng/mL) for 5 hours, and then further stimulated with LPS or R848. The level of IL-12p40 in the supernatants was determined by ELISA. The values depicted show representative data from 27 independent experiments. The increase of IL-12p40 by IL-29 in monocyte-derived macrophages was observed in 19 of 27 healthy individuals. (B) Monocyte-derived macrophages were pretreated with IL-29, IFNα, or IFNγ at the indicated concentrations for 5 hours, and then further stimulated with R848. The level of IL-12p40 in the supernatants was determined by ELISA. The values depicted show representative data from 3 independent experiments. Medium, IL-29, IFNα, or IFNγ alone did not induce IL-12p40 production by R848-stimulated, monocyte-derived macrophages. (C) Monocyte-derived macrophages were stimulated as described for panel A. The concentrations of IL-12p70, TNF, and IL-10 were determined in the supernatants using ELISA. Macrophages from 10 of 15 healthy individuals showed undetectable levels of IL-12p70 in response to IFNγ and R848 stimulation. (D) Monocyte-derived macrophages were pretreated with IL-29 (100 ng/mL), IFNα (10 ng/mL), or IFNγ (10 ng/mL) for 5 hours, and then further stimulated with LPS or R848. The level of IL-23 in the supernatants was determined by ELISA.
IL-28A and IL-28B also enhance R848-induced IL-12p40 production by human monocyte-derived macrophages
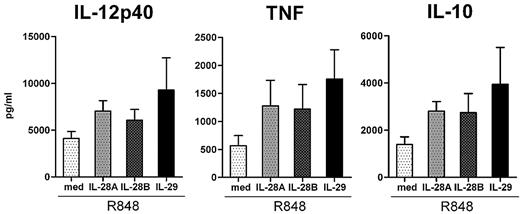
We next examined whether IL-28A and IL-28B also affected TLR-induced cytokine production by human monocyte-derived macrophages. As shown in Figure 3, both IL-28A and IL-28B were able to increase IL-12p40, TNF, and IL-10 production by monocyte-derived macrophages in response to R848 stimulation, although the effects of IL-28A and IL-28B were weaker than that of IL-29. Similarly, both IL-28A and IL-28B enhanced TNF and IL-10 production by monocyte-derived macrophages in response to LPS stimulation (data not shown). These data indicate that the effects of IL-28A and IL-28B are similar to that of IL-29 in monocyte-derived macrophages on R848 stimulation, and all lead to an enhanced production of IL-12p40, TNF, and IL-10.
Similar to IL-29, IL-28A and IL-28B enhance R848-induced IL-12p40, TNF, and IL-10 production by human monocyte-derived macrophages. Monocyte-derived macrophages were pretreated with IL-28A, IL-28B, and IL-29 (all 100 ng/mL) for 5 hours, and then further stimulated with R848. The levels of IL-12p40, TNF, and IL-10 in the supernatants were determined by ELISA. The values depicted show the means ± SE from 7 independent experiments.
Similar to IL-29, IL-28A and IL-28B enhance R848-induced IL-12p40, TNF, and IL-10 production by human monocyte-derived macrophages. Monocyte-derived macrophages were pretreated with IL-28A, IL-28B, and IL-29 (all 100 ng/mL) for 5 hours, and then further stimulated with R848. The levels of IL-12p40, TNF, and IL-10 in the supernatants were determined by ELISA. The values depicted show the means ± SE from 7 independent experiments.
IL-29 enhances IFNγ-induced IL-12p40 production by monocyte-derived macrophages in response to R848 stimulation, whereas IFNγ-induced IL-12p40 production is suppressed by IFNα
Previously, it has been reported that the combination of IL-29 and IFNγ synergistically inhibited HCV replication in Huh7 cell lines by inducing the expression of multiple genes, and exerted stronger antiviral activity than the combination of IL-29 and IFNα or the combination of IFNα and IFNγ.31 Based on these findings, we further examined the effect of IL-29 on IL-12p40 production by TLR-stimulated, monocyte-derived macrophages by combining IL-29 with IFNγ, which is also known to enhance IL-12p40 production.32
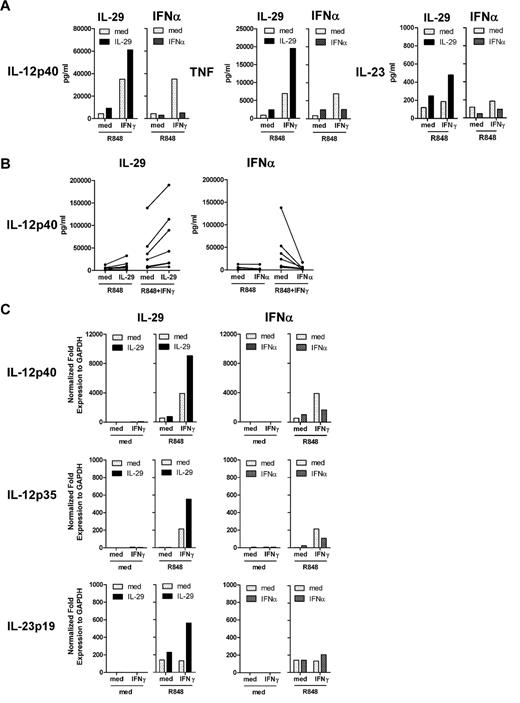
Both IL-29 and IFNγ augmented IL-12p40 production by R848-challenged, monocyte-derived macrophages (Figure 2A and Figure 4B). However, on R848 stimulation, IFNγ induced higher levels of IL-12p40 production in IL-29–pretreated, monocyte-derived macrophages than in macrophages without IL-29 pretreatment (61 and 35 ng/mL, respectively; Figure 4A). This effect was observed in IL-29–pretreated macrophages from the majority of healthy donors (Figure 4B). The additive effect of IL-29 and IFNγ was also observed for TNF production in IL-29–pretreated, monocyte-derived macrophages (Figure 4A). In addition, IL-29 and IFNγ synergistically up-regulated IL-12p40 mRNA expression in R848-challenged, monocyte-derived macrophages (Figure 4C). Although IL-12p70 production was not detectable in the majority of the experiments, we found that IL-29 increased the level of IFNγ-induced IL-12p35 mRNA expression in monocyte-derived macrophages on R848 stimulation (Figure 4C). IL-29–treated macrophages had higher levels of IL-23p19 mRNA expression on IFNγ plus R848 stimulation compared with macrophages that were not treated with IL-29 (Figure 4C). These data indicate that IL-29 can cooperate with IFNγ in inducing high levels of IL-12p40 by monocyte-derived macrophages. Although IFNγ potently induced IL-12p40 production by monocyte-derived macrophages on R848 stimulation, this induction of IL-12p40 by IFNγ was suppressed by IFNα pretreatment (from 35 to 5 ng/mL; Figure 4A), which is in agreement with the finding that IFNα-pretreated macrophages had a lower expression of IL-12p40 mRNA in response to IFNγ plus R848 stimulation (Figure 4C). Failure to induce IL-12p40 production by IFNγ was observed in IFNα-pretreated, monocyte-derived macrophages in all experiments performed (Figure 4B), which could not be explained by enhanced cytotoxicity after exposure to IFNα (supplemental Figure 2B). This inhibitory effect of IFNα was also observed for TNF production (Figure 4A). These data demonstrate that IFNα potently suppresses the responsiveness of monocyte-derived macrophages to subsequent stimulation with IFNγ on TLR ligation.
IL-29 enhances, but IFNα suppresses, IFNγ-induced IL-12p40 production by human monocyte-derived macrophages in response to R848 stimulation. (A-B) Monocyte-derived macrophages were pretreated with IL-29 or IFNα for 5 hours, and then further stimulated with IFNγ in combination with R848. IL-12p40, TNF, and IL-23 production were determined by ELISA. The values depicted show representative data from 7 independent experiments. (C) Monocyte-derived macrophages were pretreated with IL-29 or IFNα for 5 hours, and then further stimulated with IFNγ in combination with R848 for another 5 hours. IL-12p40, IL-12p35, and IL-23p19 mRNA expression in macrophages was quantified by RT-PCR. The values depicted show representative data from 2 independent experiments.
IL-29 enhances, but IFNα suppresses, IFNγ-induced IL-12p40 production by human monocyte-derived macrophages in response to R848 stimulation. (A-B) Monocyte-derived macrophages were pretreated with IL-29 or IFNα for 5 hours, and then further stimulated with IFNγ in combination with R848. IL-12p40, TNF, and IL-23 production were determined by ELISA. The values depicted show representative data from 7 independent experiments. (C) Monocyte-derived macrophages were pretreated with IL-29 or IFNα for 5 hours, and then further stimulated with IFNγ in combination with R848 for another 5 hours. IL-12p40, IL-12p35, and IL-23p19 mRNA expression in macrophages was quantified by RT-PCR. The values depicted show representative data from 2 independent experiments.
Our findings demonstrate that IL-29 pretreatment renders monocyte-derived macrophages more responsive to IFNγ stimulation, as indicated by their IL-12p40 production in response to R848, whereas IFNα suppresses IFNγ-induced IL-12p40 production by human macrophages on TLR ligation.
IL-10 is not involved in the differential regulation of IFNγ-induced IL-12p40 production by IL-29 and IFNα in human monocyte-derived macrophages on R848 stimulation
We previously showed that endogenous IL-10 produced by murine macrophages on TLR ligation suppresses the induction of IL-12 and TNF.33 To examine whether the activity of IL-10 is involved in the distinct regulation of IFNγ-induced IL-12p40 production by IL-29 or IFNα-pretreated human macrophages on TLR ligation, we blocked the IL-10 receptor with antibodies.
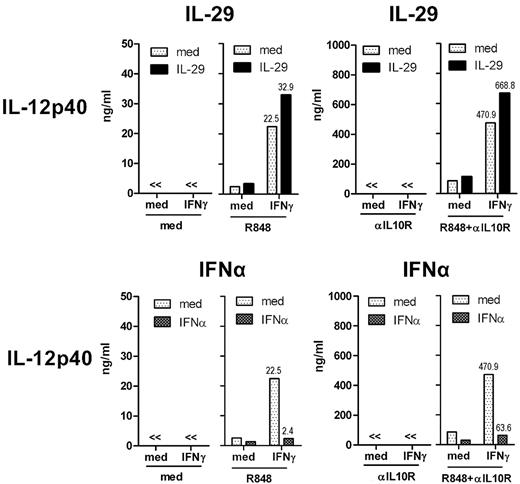
As shown in Figure 5, IFNγ-induced IL-12p40 levels were increased approximately 20-fold by blocking the IL-10 receptor in monocyte-derived macrophages on R848 stimulation (from 23 to 471 ng/mL), indicating that IFNγ-induced IL-12p40 by R848-challenged, monocyte-derived macrophages was strongly suppressed by endogenous IL-10. We showed that IL-29–treated macrophages were more responsive to IFNγ as evidenced by IL-12p40 production on R848 stimulation (Figure 4A and Figure 5 top panel). The additive effect of IL-29 and IFNγ on human macrophages was still observed in the absence of IL-10 signaling, as shown by IL-12p40 production (Figure 5 top panel). IFNα-pretreated, monocyte-derived macrophages had impaired IFNγ-induced IL-12p40 production in response to R848 stimulation (Figure 4 and Figure 5 bottom panel). On IL-10 receptor blockade, IFNα pretreatment did not restore IFNγ-induced IL-12p40 production by monocyte-derived macrophages (Figure 5 bottom panel). These results show that IL-10 is not involved in the differential regulation of IFNγ-induced IL-12p40 production by IL-29 and IFNα in human macrophages on R848 stimulation.
IL-10 is not involved in the differential regulation of IFNγ-induced IL-12p40 production by IL-29 and IFNα in human monocyte-derived macrophages on R848 stimulation. Monocyte-derived macrophages were pretreated with IL-29 or IFNα for 5 hours, and then further stimulated with IFNγ and R848. Anti–human IL-10 receptor antibody (αIL10R, 5 μg/mL) was added to some conditions to block the IL-10 receptor. The level of IL-12p40 in the supernatants was determined by ELISA. The values depicted show representative data from 3 independent experiments.
IL-10 is not involved in the differential regulation of IFNγ-induced IL-12p40 production by IL-29 and IFNα in human monocyte-derived macrophages on R848 stimulation. Monocyte-derived macrophages were pretreated with IL-29 or IFNα for 5 hours, and then further stimulated with IFNγ and R848. Anti–human IL-10 receptor antibody (αIL10R, 5 μg/mL) was added to some conditions to block the IL-10 receptor. The level of IL-12p40 in the supernatants was determined by ELISA. The values depicted show representative data from 3 independent experiments.
Both IL-29 and IFNα up-regulate TLR8 mRNA expression in human monocyte-derived macrophages
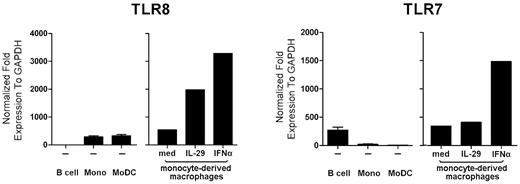
A possible explanation for the distinct regulation of IFNγ-induced IL-12p40 production by IL-29 and IFNα in human macrophages is a different effect of these 2 cytokines on TLR mRNA expression. As shown in Figure 6, on incubation with IL-29 or IFNα for 5 hours, a 3- to 4-fold increase of TLR8 mRNA expression was observed in monocyte-derived macrophages. The regulation of TLR7 by IL-29 or IFNα in macrophages was next investigated, because TLR7 is also the receptor for R848. Whereas IL-29 did not increase TLR7 mRNA expression, IFNα up-regulated TLR7 mRNA expression in monocyte-derived macrophages by 3-fold (Figure 6). Because both IL-29 and IFNα up-regulated TLR8 mRNA expression, TLR expression does not explain our findings on the different IFNγ-induced IL-12p40 production by IL-29 and IFNα in macrophages.
Both IL-29 and IFNα up-regulate TLR8 mRNA expression. Monocyte-derived macrophages were stimulated with medium, IL-29, or IFNα for 5 hours, and then the mRNA expression of TLR7 and TLR8 was measured by RT-PCR. B cells, monocytes, and monocyte-derived DCs were included as controls. The values depicted show representative data from 5 independent experiments.
Both IL-29 and IFNα up-regulate TLR8 mRNA expression. Monocyte-derived macrophages were stimulated with medium, IL-29, or IFNα for 5 hours, and then the mRNA expression of TLR7 and TLR8 was measured by RT-PCR. B cells, monocytes, and monocyte-derived DCs were included as controls. The values depicted show representative data from 5 independent experiments.
IL-29 enhances, but IFNα suppresses, IFNγ-induced IL-12p40 production by human monocyte-derived macrophages via differential regulation of IFNγR1 expression
The regulation of TLR mRNA expression by IL-29 and IFNα could not explain the observation that IL-29 and IFNα differentially affected IFNγ-induced IL-12p40 production by human macrophages. We next investigated whether the receptor for IFNγ (IFNγR) is differentially regulated by IL-29 and IFNα.
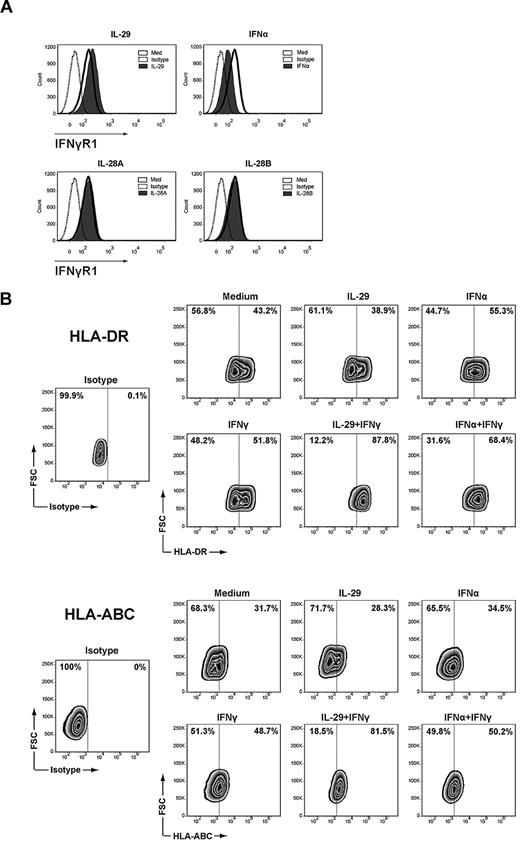
As shown in Figure 7A, IFNγR1 expression on the membrane of monocyte-derived macrophages was up-regulated after incubation with IL-29 for 5 hours, whereas no up-regulation of IFNγR1 was observed in macrophages incubated with IL-28A or IL-28B for the same amount of time. In contrast, down-regulation of IFNγR1 on the membrane of macrophages was observed on IFNα treatment (Figure 7A). However, neither IL-29 nor IFNα affected the expression of IFNγR2 on monocyte-derived macrophages (data not shown). These data strongly suggest that IL-29 and IFNα alter the response of macrophages to IFNγ via distinct regulation of the expression of surface IFNγR1 on monocyte-derived macrophages.
IL-29 and IFNα differentially regulate the surface IFNγR1 expression on monocyte-derived macrophages. (A) Monocyte-derived macrophages were treated with IL-29, IL-28A, IL-28B, or IFNα for 5 hours, and then stained with antibodies against IFNγR1. The specificity of the staining was controlled with the appropriate isotype antibodies. (B) Monocyte-derived macrophages were treated with IL-29 or IFNα for 5 hours, and then further stimulated with IFNγ (10 ng/mL) for another 20 hours. Cells were then harvested and stained with antibodies against HLA-DR and HLA-ABC. The specificity of the staining was controlled with the appropriate isotype antibodies.
IL-29 and IFNα differentially regulate the surface IFNγR1 expression on monocyte-derived macrophages. (A) Monocyte-derived macrophages were treated with IL-29, IL-28A, IL-28B, or IFNα for 5 hours, and then stained with antibodies against IFNγR1. The specificity of the staining was controlled with the appropriate isotype antibodies. (B) Monocyte-derived macrophages were treated with IL-29 or IFNα for 5 hours, and then further stimulated with IFNγ (10 ng/mL) for another 20 hours. Cells were then harvested and stained with antibodies against HLA-DR and HLA-ABC. The specificity of the staining was controlled with the appropriate isotype antibodies.
To further examine whether the additive effect between IL-29 and IFNγ also occurs in the absence of TLR signaling, we investigated the effect of exposure to IL-29, IFNα, and IFNγ on the surface expression of HLA-DR and HLA-ABC on monocyte-derived macrophages. As shown in Figure 7B, pretreatment of macrophages with IL-29 did not affect HLA-DR or HLA-ABC expression. However, pretreatment of macrophages with IL-29 resulted in a strong up-regulation of HLA-DR and HLA-ABC in response to IFNγ. These findings indicate that the synergistic effect between IL-29 and IFNγ also occurs in the absence of TLR triggering.
Discussion
We examined the effect of IL-29 on human antigen-presenting cells and show that only monocyte-derived macrophages, but not monocytes nor monocyte-derived DCs, respond to IL-29, which is explained by the levels of IL-28RA expression. We also demonstrate that IL-29 enhances TLR-induced IL-12p40 production by monocyte-derived macrophages from the majority of healthy individuals. Furthermore, IL-29 enhances IFNγ-induced IL-12p40 and TNF production by monocyte-derived macrophages on TLR ligation, whereas IFNα suppresses IFNγ-induced IL-12p40 and TNF production, indicating that IL-29 and IFNα differentially affect the response of monocyte-derived macrophages to IFNγ. The differential effects of IL-29 and IFNα on the responsiveness of these macrophages to IFNγ is not via modulation of TLR7 or TLR8 mRNA expression and is independent of IL-10 signaling. However, we show that IL-29 up-regulates, whereas IFNα down-regulates, the surface expression of IFNγR1, resulting in differential responsiveness of TLR-challenged, monocyte-derived macrophages to IFNγ.
It is thought that IL-28RA is not expressed in leukocytes, except for B cells and plasmacytoid DCs.28,29,34 Our finding that human monocyte-derived macrophages express relatively high levels of IL-28RA and strongly respond to IL-29 has not been reported before. However, murine macrophages infected with HIV are responsive to IFNλ.13 We now show that monocytes and monocyte-derived DCs do not express IL-28RA, whereas monocyte-derived macrophages express the receptor and are responsive to IL-29. Our finding that monocytes do not express IL-28RA is in agreement with previous studies,3,35 and the expression of IL-28RA cannot be induced by short-time exposure of monocytes to M-CSF (data not shown). Monocyte-derived DCs were reported by one group of investigators to express IL-28RA mRNA, as shown by RT-PCR.35 However, using flow cytometry, we were unable to determine the expression of the IL-28RA protein on monocyte-derived DCs and we were unable to show responsiveness to IL-29. In agreement with results using cultured, monocyte-derived DCs, freshly isolated BDCA-1+ myeloid DCs purified from blood also did not respond to IL-29 on stimulation with poly-I:C (supplemental Figure 3).
We show that IL-29 is able to enhance TLR-induced IL-12p40 production by monocyte-derived macrophages from the majority of healthy individuals. In addition, IL-28A and IL-28B, the other members of the IFNλ family, have a similar effect to IL-29 in increasing IL-12p40 production by TLR-stimulated, monocyte-derived macrophages. It was shown previously that IL-29 alone, in the absence of TLR ligation, induces high levels of the pro-inflammatory cytokines IL-6 and IL-8 by human PBMCs, an effect that was shown to be further enhanced by LPS.36 In that study, monocytes were found to be the major responders to IL-29. However, we and several other groups3,35 have clearly shown that the receptor of IFNλ is not expressed in human primary monocytes, and we were unable to observe the induction of cytokines by IL-29 in the absence of TLR ligation. It has since been reported that plasmacytoid DCs are also responsive to IL-29,29,37 so it is possible that the pro-inflammatory cytokines induced by IL-29 in human PBMCs were derived from plasmacytoid DCs and not from monocytes.
In addition to enhancement of TLR-induced cytokine production by IL-29 in monocyte-derived macrophages, we also observed that IFNλ renders macrophages more responsive to exposure to IFNγ. This is important, because IFNγ is essential for host resistance to many intracellular pathogens38 and is produced by natural killer cells and Th1 cells on infection. It has been reported that mouse macrophages, as well as human monocytes and myeloid DCs, are able to produce high levels of IL-29 on TLR ligation.24,39 In addition, plasmacytoid DCs produce IFNλ and IFNα early on viral infection.27,40 The induction of IL-29 as an early event following challenge with bacterially or virally derived pathogens may result in priming of macrophages, and subsequent boosting of the activation of macrophages induced by IFNγ secreted from other cell types such as natural killer cells and Th1 cells. The expression profile of IL-28RA allows that only certain cell types such as macrophages become activated, which may limit the damage caused by the early activation of macrophages. In addition, we show that the effect of IL-29 is weaker than that of IFNγ, as indicated by IL-12p40 and TNF production, which was also demonstrated before by examining STAT1 phosphorylation in Huh7 cell lines.31
IL-29 and IFNα show a distinct effect on cytokine production by human monocyte-derived macrophages. It is generally accepted that IFNα exerts an inhibitory effect on IL-12p40 production by human antigen-presenting cells.22,23 Indeed, we also observe this inhibitory effect on IL-12p40 production by macrophages, although this effect is weak in most healthy individuals. As an important component of the bioactive cytokines, IL-12p40 is produced in large excess over IL-12p70 and IL-23 by antigen-presenting cells on TLR ligation.41 The biologic function of IL-12p40 is still not fully understood. The down-regulation of IL-12p40 by IFNα may affect the production of the bioactive IL-12 family cytokines by TLR-activated macrophages, although IL-12p70 was not detectable in the supernatant of IFNα-treated macrophages in response to TLR ligation.
We have shown that IL-29 and IFNα differentially modulate the function of human macrophages, including their responses to IFNγ. The up-regulation of TLR8 mRNA expression by IL-29 could enhance the activation of macrophages by IFNγ. However, the weak response to IFNγ by IFNα-treated, monocyte-derived macrophages is not via IL-10 and is not due to the down-regulation of TLR7 or TLR8, because IFNα enhances TLR7 and TLR8 mRNA expression. However, our study demonstrates that IFNα down-regulates IFNγR1 expression on macrophages, whereas IL-29 up-regulates it. This may provide an explanation for the differences between IL-29 and IFNα-pretreated, monocyte-derived macrophages in responding to IFNγ. It has been reported that, in contrast to high doses of type I IFN, in certain experimental systems, low-dose type I IFN can prime cells, leading to enhanced cytoplasmic accumulation of STAT1 and making these cells more responsive to IFNγ.42 Thus, it is tempting to speculate that IL-29 acts in a manner similar to low-dose type I IFN. However, at present, no such studies have been conducted for IL-29. To our knowledge, up-regulation of IFNγR1 by IL-29 in human macrophages has not been previously reported. Because IFNγ is essential for host resistance to many intracellular pathogens,38 it will be important to examine in future studies whether IL-29 improves the clearance of bacterial infections such as Listeria monocytogenes and Mycobacterium tuberculosis. The ability of IFNα to down-regulate IFNγR1 expression that we observed in human macrophages, which explains their reduced response to IFNγ, is in agreement with a recent study on L monocytogenes infection in mice.43 In that study, it was observed that IFNα/β produced during L monocytogenes infection down-regulated IFNγR expression by macrophages and DCs and therefore reduced responsiveness to IFNγ.41-45 Several other bacteria also seem to benefit from IFNα production during their infection46-49 ; some of these, such as M tuberculosis, are known to suppress cellular responses to IFNγ.50
Because of its potent antiviral activity, clinical trials are ongoing to examine the efficacy and safety of peg-IL-29 in the treatment of patients with chronic HCV infection. A modest decline of serum HCV RNA levels was recently reported in the majority of patients.4 The potential importance of IFNλ in the treatment of patients with chronic HCV infection is further suggested by the observation that polymorphism near the IL-28B gene predict the efficacy of standard-of-care antiviral therapy for chronic HCV.5-8 We showed that IL-29 enhances IL-12p40 by human macrophages and that IL-29 pretreatment primes the activation of macrophages induced by IFNγ. With respect to the hepatotropic nature of HCV, it is important to examine in future studies whether Kupffer cells in the liver also respond to IFNλ and play a role in controlling the HCV RNA levels via enhanced Th1 activity (in addition to antiviral activity).
In summary, we found that human monocyte-derived macrophages, but not monocytes or monocyte-derived DCs, respond to IL-29. IL-29 and IFNα differentially modulate the function of macrophages, including their responses to IFNγ. The opposing immunomodulatory effects of IFNλ and IFNα on human macrophages described in this study further enhance our understanding of the complex cross-talk between different types of IFNs, as well as the effects of IFNλ in the treatment of infections with diverse pathogens.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Mark Claassen for critically reading the manuscript and Duygu Turgut for her excellent technical assistance.
Authorship
Contributions: B.L. designed and performed research, analyzed data, and wrote the paper; H.L.A.J. designed research and wrote the paper; and A.B. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: A. Boonstra, PhD, Department of Gastroenterology and Hepatology, Erasmus MC, University Medical Center Rotterdam, ‘s-Gravendijkwal 230, Room L-455, 3015 CE Rotterdam, The Netherlands; e-mail: p.a.boonstra@erasmusmc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal