Abstract
Osteonecrosis is a severe glucocorticoid-induced complication of acute lymphoblastic leukemia treatment. We prospectively screened children (n = 364) with magnetic resonance imaging of hips and knees, regardless of symptoms; the cumulative incidence of any (grade 1-4) versus symptomatic (grade 2-4) osteonecrosis was 71.8% versus 17.6%, respectively. We investigated whether age, race, sex, acute lymphoblastic leukemia treatment arm, body mass, serum lipids, albumin and cortisol levels, dexamethasone pharmacokinetics, and genome-wide germline genetic polymorphisms were associated with symptomatic osteonecrosis. Age more than 10 years (odds ratio, = 4.85; 95% confidence interval, 2.5-9.2; P = .00001) and more intensive treatment (odds ratio = 2.5; 95% confidence interval, 1.2-4.9; P = .011) were risk factors and included as covariates in all analyses. Lower albumin (P = .05) and elevated cholesterol (P = .02) associated with symptomatic osteonecrosis, and severe (grade 3 or 4) osteonecrosis was linked to poor dexamethasone clearance (P = .0005). Adjusting for clinical features, polymorphisms of ACP1 (eg, rs12714403, P = 1.9 × 10−6, odds ratio = 5.6; 95% confidence interval, 2.7-11.3), which regulates lipid levels and osteoblast differentiation, were associated with risk of osteonecrosis as well as with lower albumin and higher cholesterol. Overall, older age, lower albumin, higher lipid levels, and dexamethasone exposure were associated with osteonecrosis and may be linked by inherited genomic variation.
MedscapeEDUCATION Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 2556.
Disclosures
William E. Evans serves on the boards of Aldagen, Chemores (Scientific Advisory Board, European Research Consortium), ParagonDx (Scientific Advisory Board, Inactive), and the National Cancer Institute (NCI) Board of Scientific Counselors. He received NCI grants R37 CA36401, P30 CA21765, CA 90628, and CA116201 from the National Institute of General Medical Sciences Pharmacogenomics Research Network. He also holds a patent with St Jude Children's Research Hospital and receives royalty from TPMT genotyping tests. Mary V. Relling receives a portion of the income St Jude Children's Research Hospital receives from licensing patent rights related to TPMT polymorphisms and GGH polymorphisms. She also receives funding for investigator-initiated research on the pharmacology of asparaginase from Sigma-Tau Pharmaceuticals. The remaining authors; the Associate Editor Crystal L. Mackall; and the CME questions author Charles P. Vega, University of California, Irvine, CA, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Specify the risk factor most associated with osteonecrosis among children treated for ALL
Evaluate risk factors for symptomatic (grades 2-4) osteonecrosis among children treated for ALL
Evaluate risk factors for severe (grades 3-4) osteonecrosis among children treated for ALL
Analyze genetic risk factors for osteonecrosis among children treated for ALL
Release date: February 24, 2011; Expiration date: February 24, 2012
Introduction
Cure rates for pediatric acute lymphoblastic leukemia (ALL) have improved, such that almost 90% of patients are cured with current therapy.1,2 These high cure rates have been partly achieved by intensification of chemotherapy,3,4 which can also result in toxicity and decreased quality of life. Glucocorticoids (dexamethasone and prednisone) play a pivotal role in improving the cure rates in ALL but can cause osteonecrosis, a potentially debilitating toxicity.3,5-11 In severe cases, osteonecrosis results in joint collapse, requiring total joint replacement.12
There are several mechanisms by which glucocorticoids may induce osteonecrosis. These agents cause hyperlipidemia, hypercoagulation, and hypofibrinolysis.13,14 Pathogenesis may involve intravascular thrombotic occlusion and extravascular lipid deposition by fat emboli. Glucocorticoids can increase intraosseous lipocyte size, leading to an increase in bone marrow pressure and intraosseous circulatory disturbances, reduced intramedullary blood flow, marrow ischemia, and apoptosis followed by necrosis.15,16 Glucocorticoids also cause direct toxicity to osteocytes.17
Clinical risk factors for osteonecrosis have included adolescent age between 10 and 20 years,5,8,11,18 female sex,11 white race,11 and higher body mass index.6 Some ALL treatment regimens have a much higher frequency of osteonecrosis than others, suggesting that some nonglucocorticoid drugs (eg, asparaginase and methotrexate) may modify the risk of osteonecrosis.7 Hypercoagulability15,19 and hyperlipidemia20 have been associated with osteonecrosis in clinical settings outside of ALL treatment. Multiple candidate gene studies have indicated several polymorphisms in genes putatively related to the development of osteonecross, such as SERPINE 1,21 VDR,5 and CYP3A4,22 but there are conflicting results,21,23 no genome-wide association studies (GWAS) have been performed, and most genomic studies have failed to account extensively for nongenetic risk factors.
To diagnose osteonecrosis, prior studies have depended on diagnostic imaging in symptomatic patients. Because of nonspecific symptoms, osteonecrosis might not have been detected in some patients with ALL. For the first time, in this study, we prospectively screened patients for osteonecrosis with magnetic resonance imaging (MRI) of hips and knees during the first 6 to 8 months of continuation therapy, regardless of symptoms. This allowed us to unambiguously assign osteonecrosis status to each patient. We performed a comprehensive analysis to determine the clinical, biochemical, pharmacokinetic, and pharmacogenetic risk factors for osteonecrosis.
Methods
Patients
Children with newly diagnosed ALL (n = 498) were enrolled on a frontline protocol, St Jude Total XV (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).2 This study was approved by the St Jude Institutional Review Board, and informed consent was obtained from patients who were 18 years old and, in the case of younger patients, from their parents or guardians, in accordance with the Declaration of Helsinki. Patients were assigned to low-, standard-, or high-risk treatment arms based on their risk of ALL relapse.2 Younger children were more likely to be assigned to the low-risk (LR) treatment arm, whereas older children were more likely to be assigned to the standard- or high-risk (SR/HR) treatment arm.
Detection of osteonecrosis
Irrespective of symptoms, the Total XV protocol called for patients to be screened for osteonecrosis via prospective MRI of the hips and knees after the completion of reinduction I (weeks 7-9), and reinduction II (weeks 17-19 of continuation therapy), and at the completion of therapy.12 Not all MRI studies were obtained as planned because of scheduling conflicts, but 365 patients were evaluable by MRI (supplemental Figure 1). Osteonecrosis at the time of each MRI was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 3.0, and osteonecrosis was categorized as absent (grade 0), asymptomatic (grade 1), or moderate (grade 2), severe (grade 3), or disabling (grade 4). Patients with worsening symptoms were reevaluated by further imaging and, if required, were considered for surgery based on symptoms and imaging findings; however, one patient had asymptomatic osteonecrosis, but because of significant joint involvement by imaging, this patient received surgical core decompression and was excluded from further analysis (supplemental Figure 1).
Treatment regimen and sample collection
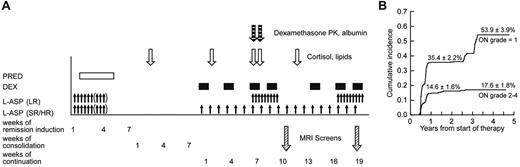
After a common remission induction and consolidation phase, subsequent doses of glucocorticoid therapy differed by treatment arm2 (Figure 1A). Dexamethasone at 8 mg/m2 per day (LR arm) and 12 mg/m2 per day (SR/HR arm) was given orally for 5 days at weeks 1, 4, and 9 of continuation therapy. During both reinductions I and II (weeks 7, 9, 17, and 19), all patients received dexamethasone at 8 mg/m2 per day orally for 7 to 8 days.
We collected heparinized blood samples on day 15 of consolidation (∼ 30 days after the last glucocorticoid dose) for a “baseline” assessment of serum lipid and cortisol levels (Figure 1A). Additional blood samples were collected on day 1 of week 2 (or week 5), week 7, week 8 (after 7 continuous days of dexamethasone), and week 12 of the continuation phase. Serum cortisol was determined by high-performance liquid chromatography (supplemental data).24 High- and low-density lipoproteins, cholesterol, and triglycerides were determined using a colorimetric enzymatic assay on the Cobas Integra system (Roche Diagnostics). For dexamethasone pharmacokinetic analysis, blood was drawn before and after the morning dexamethasone doses on days 1 and 8 of reinduction I (corresponding to weeks 7 and 8 of continuation treatment),25 and pharmacokinetic parameters were estimated for 365 patients, 214 of whom have been previously described.25
Genotyping
Single nucleotide polymorphism (SNP) genotypying was performed using the Affymetrix Gene Chip Human Mapping Array, using 500K or 6.0 arrays (Affymetrix),26,27 and using a custom-designed Illumina Golden Gate assay28 for 1536 SNPs. Genetic ancestry was determined using STRUCTURE,29,30 and race was categorized as white, black, and other based on inferred genetic ancestry.
GWAS for osteonecrosis
Because the nongenetic factors of age and treatment arm were strongly associated with risk of osteonecrosis in a multiple logistic regression model and confirmed in a stepwise selection method, we used a multiple logistic regression model that adjusted for age and treatment arm to test whether SNP genotypes (categorized as 0, 1, or 2 copies of the B allele) were associated with the risk of symptomatic (grade 2-4) osteonecrosis. The optimal α was estimated as described.31 We performed a subset analysis to identify germline polymorphisms associated with more serious grade 3 or 4 osteonecrosis compared with grade 0 or 1 osteonecrosis.
Statistics
Multiple logistic regression was used to determine the association of pharmacokinetic and pharmacodynamic variables with the risk of osteonecrosis.
Results
Development of asymptomatic and symptomatic osteonecrosis
At the first MRI screen, 215 patients were detected to have grade 0 osteonecrosis, 141 patients with asymptomatic (grade 1) osteonecrosis, and 8 patients with symptomatic (grade 2-4) osteonecrosis. Of the 215 with no osteonecrosis (grade 0), 105 maintained grade 0, 62 worsened to grade 1, 27 patients worsened to grade 2 to 4, and 21 patients did not have a second MRI. Of the 141 patients whose initial screen indicated grade 1 osteonecrosis, 14 resolved to grade 0, 82 maintained grade 1, 34 worsened to grade 2 to 4, and 11 patients did not have a second MRI. Overall, patients who initially had grade 1 osteonecrosis at the first MRI screening were more likely to develop symptomatic grade 2 to 4 osteonecrosis (34 of 130; 26%) compared with patients who were initially negative for osteonecrosis (27 of 194; 14%, P = .008). At 1 year from the start of therapy, the cumulative incidence for symptomatic osteonecrosis (grade 2-4) was 14.6% ± 1.6% (SE) and for grade 1 osteonecrosis was 35.4% ± 2.2% (Figure 1B). At the end of therapy, the cumulative incidence of any osteonecrosis (grades 1-4) was 71.8% ± 3.6%, for grade 2 to 4 osteonecrosis was 17.6% ± 1.8%, and for grade 1 osteonecrosis was 53.9% ± 3.9%. Thus, most symptomatic cases were diagnosed within 1 year.
Therapy, testing, and osteonecrosis on Total XV study. (A) Schema of sample collection, asparaginase and glucocorticoid dosing, and MRI screening. Blood samples were collected on consolidation day 15 and week 2, week 7, week 8, and week 12 of continuation phase of therapy. Prednisone was administered at 40 mg/m2 per day during remission induction. Dexamethasone was given at 12 mg/m2 per day (SR/HR arm) and 8 mg/m2 per day (LR) during continuation weeks 1 to 6 and 10 to 16; and at 8 mg/m2 per day for both treatment arms at weeks 7, 9, 17, and 19. Asparaginase was administered at 10 000 U/m2 per dose intramuscularly 3 times weekly for 6 to 9 doses during remission induction; those in SR/HR arms received 25 000 U/m2 per week from weeks 1 to 19; those in the LR arm received 10 000 U/m2 3 times weekly for 9 doses at weeks 7-9 and 17-19. MRI screens were scheduled to be performed at weeks 9-10 and 19-20. (B) Cumulative incidence of asymptomatic and symptomatic osteonecrosis. The top curve represents the first incidence of asymptomatic osteonecrosis in those whose worst grade was grade 1; and lower curve, the first incidence of symptomatic osteonecrosis in those who eventually developed grade 2 to 4 osteonecrosis.
Therapy, testing, and osteonecrosis on Total XV study. (A) Schema of sample collection, asparaginase and glucocorticoid dosing, and MRI screening. Blood samples were collected on consolidation day 15 and week 2, week 7, week 8, and week 12 of continuation phase of therapy. Prednisone was administered at 40 mg/m2 per day during remission induction. Dexamethasone was given at 12 mg/m2 per day (SR/HR arm) and 8 mg/m2 per day (LR) during continuation weeks 1 to 6 and 10 to 16; and at 8 mg/m2 per day for both treatment arms at weeks 7, 9, 17, and 19. Asparaginase was administered at 10 000 U/m2 per dose intramuscularly 3 times weekly for 6 to 9 doses during remission induction; those in SR/HR arms received 25 000 U/m2 per week from weeks 1 to 19; those in the LR arm received 10 000 U/m2 3 times weekly for 9 doses at weeks 7-9 and 17-19. MRI screens were scheduled to be performed at weeks 9-10 and 19-20. (B) Cumulative incidence of asymptomatic and symptomatic osteonecrosis. The top curve represents the first incidence of asymptomatic osteonecrosis in those whose worst grade was grade 1; and lower curve, the first incidence of symptomatic osteonecrosis in those who eventually developed grade 2 to 4 osteonecrosis.
Risk factors for asymptomatic (grade 1) osteonecrosis
Using logistic regression, of age, sex, race, and treatment arm, only patients in the SR/HR treatment arm were at significantly higher risk to develop asymptomatic (grade 1) osteonecrosis compared with grade 0 osteonecrosis patients (P = .005; supplemental Table 1). However, there was a trend for more grade 1 osteonecrosis in older patients (P = .13)
Risk factors for symptomatic (grade 2-4) osteonecrosis
Of age, sex, race, and treatment arm, only older age and SR/HR treatment arm were significantly associated with symptomatic (grade 2-4) osteonecrosis (supplemental Table 2). Forty-one of the 92 patients (44.6%) older than 10 years of age compared with 28 of the 272 (10%) younger patients developed symptomatic osteonecrosis (odds ratio = 4.85; 95% confidence interval [CI], 2.5-9.2, P = .00001; Figure 2A). Fifty-two of the 175 (29.7%) patients on the SR/HR treatment arm compared with 17 of the 189 (9%) patients on the LR treatment arm developed this complication (odds ratio = 2.5; 95% CI, 1.2-4.9, P = .011; Figure 2B). Symptomatic osteonecrosis risk did not differ by sex or race (Figure 2C-D).
Age and treatment arm were associated with symptomatic osteonecrosis. Percentage of patients who developed grade 2 to 4 osteonecrosis by (A) age, (B) treatment arms, (C) race, and (D) sex.
Age and treatment arm were associated with symptomatic osteonecrosis. Percentage of patients who developed grade 2 to 4 osteonecrosis by (A) age, (B) treatment arms, (C) race, and (D) sex.
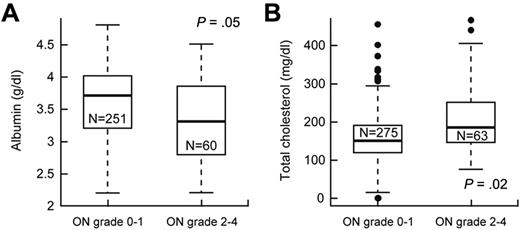
We investigated whether variables, such as serum cortisol, lipids, or albumin, dexamethasone pharmacokinetics, and body mass index, were risk factors for grade 2 to 4 versus grade 0 or 1 osteonecrosis (supplemental Table 3). After adjusting for age and treatment arm, lower serum albumin (P = .05, odds ratio = 0.58; 95% CI, 0.34-1.0) and higher serum cholesterol (P = .020, odds ratio = 1.11; 95% CI, 1.02-1.2) at week 8 (Figure 3) were associated with grade 2 to 4 osteonecrosis.
Lower serum albumin and higher cholesterol were associated with symptomatic osteonecrosis (ON). After adjustment for age and treatment arm, (A) serum albumin levels (g/dL) were lower and (B) cholesterol levels (mg/dL) were higher at week 8 in patients who did versus did not develop grade 2 to 4 osteonecrosis.
Lower serum albumin and higher cholesterol were associated with symptomatic osteonecrosis (ON). After adjustment for age and treatment arm, (A) serum albumin levels (g/dL) were lower and (B) cholesterol levels (mg/dL) were higher at week 8 in patients who did versus did not develop grade 2 to 4 osteonecrosis.
Subset analysis of risk factors for severe (grade 3 or 4) osteonecrosis
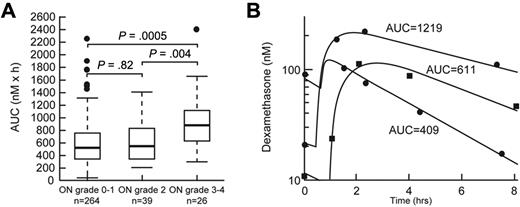
Age older than 10 years (P < .0001) and SR/HR treatment arm (P = .0003) were risk factors for severe (grade 3 or 4) osteonecrosis in univariate analysis (supplemental Table 4). After adjustment for age and treatment arm, higher dexamethasone area under the curve (AUC) at week 8 (P = .0005, supplemental Table 5; Figure 4) and lower serum albumin at week 8 (P = .03, supplemental Table 5) were associated with severe (grade 3 or 4; n = 26) osteonecrosis.
Dexamethasone AUC was associated with severe osteonecrosis. (A) Dexamethasone AUC (nM × h) at week 8 in patients with grade 0 or 1, grade 2, and grade 3 or 4 osteonecrosis (ON). Statistical significance was determined after adjustment for age and treatment arm. (B) Representative plasma concentration versus time curves in patients with low, medium, and higher dexamethasone exposure.
Dexamethasone AUC was associated with severe osteonecrosis. (A) Dexamethasone AUC (nM × h) at week 8 in patients with grade 0 or 1, grade 2, and grade 3 or 4 osteonecrosis (ON). Statistical significance was determined after adjustment for age and treatment arm. (B) Representative plasma concentration versus time curves in patients with low, medium, and higher dexamethasone exposure.
Genomic variation associated with symptomatic osteonecrosis
After adjustment for age and treatment arm, and the interaction between treatment arm and SNP genotype, there were 423 SNPs associated with symptomatic osteonecrosis (P < .001). Of these, 196 SNPs were annotated to genes and 227 SNPs were intergenic (the top 20 SNPs from the GWAS are shown in Table 1). The top 4 SNPs are in the SH3YL1-ACP1 gene locus. Of these, polymorphism in SH3YL1 (rs4241316, P = 1.2 × 10−6 odds ratio = 5.8; 95% CI, 2.9-11.9) is the top ranking SNP, which is in strong linkage disequilibrium (supplemental Figure 2) with 2 SNPs in acid phosphatase-1 (ACP1) (rs12714403, P = 1.9 × 10−6, odds ratio = 5.6; 95% CI, 2.7-11.3; and rs10167992, P = 1.9 × 10−6, odds ratio = 5.6; 95% CI, 2.7-11.3) and another SNP in SH3YL1 (rs10193882, P = 3.6 × 10−6, odds ratio = 5.3; 95% CI, 2.6-10.7; supplemental Figure 2). For example, 22 of 51 (43.1%) patients with at least one C allele developed grade 2 to 4 osteonecrosis compared with only 15.2% with the TT genotype (n = 309) at SH3YL1 SNP rs4241316 (supplemental Figure 3), with similar results for other SNPs in this LD block with ACP1/SH3YL1.
Top 20 SNPs associated with symptomatic (grade 2-4) osteonecrosis
| SNP ID . | Chromosome . | Location* . | Gene . | MAF . | Risk allele . | P . | OR† (95% CI) . | Major allele/minor allele . |
|---|---|---|---|---|---|---|---|---|
| rs4241316 | 2 | 242197 | SH3YL1 | 0.074 | C | 1.2 × 10−6 | 5.8 (2.9-11.9) | T/C |
| rs12714403 | 2 | 263070 | ACP1 | 0.075 | A | 1.9 × 10−6 | 5.6 (2.7-11.3) | G/A |
| rs10167992 | 2 | 253270 | ACP1 | 0.075 | T | 1.9 × 10−6 | 5.6 (2.7-11.3) | C/T |
| rs10193882 | 2 | 236301 | SH3YL1 | 0.078 | T | 3.6 × 10−6 | 5.3 (2.6-10.7) | A/T |
| rs9417254 | 10 | 20055938 | NA | 0.390 | C | 4.6 × 10−6 | 3.8 (2.1-6.6) | T/C |
| rs564065 | 3 | 126296212 | SLC12A8 | 0.460 | G | 1.1 × 10−5 | 3.2 (5.4-1.9) | C/G |
| rs7625035 | 3 | 135210556 | SLCO2A1 | 0.215 | G | 1.6 × 10−5 | 3.0 (1.8-5.0) | A/G |
| rs999828 | 5 | 67311044 | NA | 0.197 | T | 1.9 × 10−5 | 3.5 (2.0-6.3) | C/T |
| rs1446466 | 2 | 164744133 | NA | 0.175 | T | 2 × 10−5 | 3.1 (5.3-1.8) | A/T |
| rs10738147 | 9 | 1002154 | NA | 0.381 | T | 2.2 × 10−5 | 3.0 (5.0-1.8) | C/T |
| rs615231 | 11 | 55977651 | OR5M9 | 0.170 | C | 2.3 × 10−5 | 2.9 (4.6-1.8) | G/C |
| rs7594255 | 2 | 164673066 | NA | 0.164 | C | 2.3 × 10−5 | 3.3 (5.6-1.9) | T/C |
| rs12473215 | 2 | 164698503 | NA | 0.166 | A | 2.7 × 10−5 | 3.1 (5.4-1.8) | T/A |
| rs449725 | 2 | 192993 | NA | 0.103 | C | 2.8 × 10−5 | 4.0 (2.1-7.6) | G/C |
| rs17436622 | 2 | 164743855 | NA | 0.171 | G | 3.1 × 10−5 | 3.0 (5.0-1.8) | A/G |
| rs10078620 | 5 | 67281259 | NA | 0.194 | T | 3.2 × 10−5 | 3.3 (1.9-5.9) | G/T |
| rs9567986 | 13 | 47516171 | NUDT15 | 0.330 | T | 3.3 × 10−5 | 2.7 (1.7-4.3) | C/T |
| rs7398018 | 12 | 130605295 | NA | 0.411 | T | 3.5 × 10−5 | 2.7 (1.7-4.3) | C/T |
| rs4845763 | 1 | 150654762 | CRNN | 0.135 | C | 3.6 × 10−5 | 3.0 (1.8-5.0) | T/C |
| rs2159473 | 7 | 146395930 | CNTNAP2 | 0.362 | A | 3.8 × 10−5 | 3.2 (5.6-1.8) | A/G |
| SNP ID . | Chromosome . | Location* . | Gene . | MAF . | Risk allele . | P . | OR† (95% CI) . | Major allele/minor allele . |
|---|---|---|---|---|---|---|---|---|
| rs4241316 | 2 | 242197 | SH3YL1 | 0.074 | C | 1.2 × 10−6 | 5.8 (2.9-11.9) | T/C |
| rs12714403 | 2 | 263070 | ACP1 | 0.075 | A | 1.9 × 10−6 | 5.6 (2.7-11.3) | G/A |
| rs10167992 | 2 | 253270 | ACP1 | 0.075 | T | 1.9 × 10−6 | 5.6 (2.7-11.3) | C/T |
| rs10193882 | 2 | 236301 | SH3YL1 | 0.078 | T | 3.6 × 10−6 | 5.3 (2.6-10.7) | A/T |
| rs9417254 | 10 | 20055938 | NA | 0.390 | C | 4.6 × 10−6 | 3.8 (2.1-6.6) | T/C |
| rs564065 | 3 | 126296212 | SLC12A8 | 0.460 | G | 1.1 × 10−5 | 3.2 (5.4-1.9) | C/G |
| rs7625035 | 3 | 135210556 | SLCO2A1 | 0.215 | G | 1.6 × 10−5 | 3.0 (1.8-5.0) | A/G |
| rs999828 | 5 | 67311044 | NA | 0.197 | T | 1.9 × 10−5 | 3.5 (2.0-6.3) | C/T |
| rs1446466 | 2 | 164744133 | NA | 0.175 | T | 2 × 10−5 | 3.1 (5.3-1.8) | A/T |
| rs10738147 | 9 | 1002154 | NA | 0.381 | T | 2.2 × 10−5 | 3.0 (5.0-1.8) | C/T |
| rs615231 | 11 | 55977651 | OR5M9 | 0.170 | C | 2.3 × 10−5 | 2.9 (4.6-1.8) | G/C |
| rs7594255 | 2 | 164673066 | NA | 0.164 | C | 2.3 × 10−5 | 3.3 (5.6-1.9) | T/C |
| rs12473215 | 2 | 164698503 | NA | 0.166 | A | 2.7 × 10−5 | 3.1 (5.4-1.8) | T/A |
| rs449725 | 2 | 192993 | NA | 0.103 | C | 2.8 × 10−5 | 4.0 (2.1-7.6) | G/C |
| rs17436622 | 2 | 164743855 | NA | 0.171 | G | 3.1 × 10−5 | 3.0 (5.0-1.8) | A/G |
| rs10078620 | 5 | 67281259 | NA | 0.194 | T | 3.2 × 10−5 | 3.3 (1.9-5.9) | G/T |
| rs9567986 | 13 | 47516171 | NUDT15 | 0.330 | T | 3.3 × 10−5 | 2.7 (1.7-4.3) | C/T |
| rs7398018 | 12 | 130605295 | NA | 0.411 | T | 3.5 × 10−5 | 2.7 (1.7-4.3) | C/T |
| rs4845763 | 1 | 150654762 | CRNN | 0.135 | C | 3.6 × 10−5 | 3.0 (1.8-5.0) | T/C |
| rs2159473 | 7 | 146395930 | CNTNAP2 | 0.362 | A | 3.8 × 10−5 | 3.2 (5.6-1.8) | A/G |
SNP ID indicates SNP identifier according to the dbSNP database; MAF, minor allele frequency; CI, confidence interval; OR, odds ratio; and NA, SNP is not annotated to any gene.
Physical location of SNP based on March 2006 human genome assembly (hg18).
OR is for each additional risk allele. For example, for rs4241316, the OR for developing grade 2 to 4 osteonecrosis is 5.8-fold higher for those with CC versus CT versus TT genotype.
Identification of pleiotropic SNPs
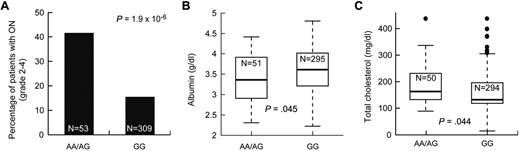
We tested whether the 423 SNPs associated with symptomatic osteonecrosis were also associated with intermediate phenotypes also related to the risk of osteonecrosis, such as low albumin and elevated cholesterol. There were 18 and 9 osteonecrosis-associated SNPs that were associated with low albumin and high cholesterol (P ≤ .05), respectively (supplemental Table 6), including the 5 SNPs in ACP1 and SH3YL1. For example, patients with at least one A allele at ACP1 SNP rs12714403 had a higher risk of symptomatic osteonecrosis, lower albumin, and higher cholesterol levels (P = 1.9 × 10−6, P = .045, and P = .044, respectively, Figure 5). Thus, SNPs in the ACP1-SH3YL1 gene locus were significantly associated with symptomatic osteonecrosis and were also related to lower albumin and higher cholesterol.
SNPs in ACP1 were associated with symptomatic osteonecrosis, lower albumin, and higher cholesterol levels. After adjustment for age and treatment arm, association of SNP genotypes in ACP1 (rs12714403) to (A) grade 2 to 4 osteonecrosis (ON), (B) albumin levels, and (C) cholesterol levels at week 8.
SNPs in ACP1 were associated with symptomatic osteonecrosis, lower albumin, and higher cholesterol levels. After adjustment for age and treatment arm, association of SNP genotypes in ACP1 (rs12714403) to (A) grade 2 to 4 osteonecrosis (ON), (B) albumin levels, and (C) cholesterol levels at week 8.
Identification of SNPs also associated with severe (grade 3 or 4) osteonecrosis
There were 211 SNPs that distinguished patients who developed severe grade 3 or 4 versus grade 0 or 1 osteonecrosis (at a threshold of P < .001). Of these, 86 were annotated to genes and 125 were intergenic. There were 347 SNPs that also distinguished grade 2 to 4 versus grade 0 or 1 osteonecrosis at P < .01 (supplemental Table 7). Overlapping SNPs included those in SH3YL1 and ACP1 genes, as well as in HNRNPC.
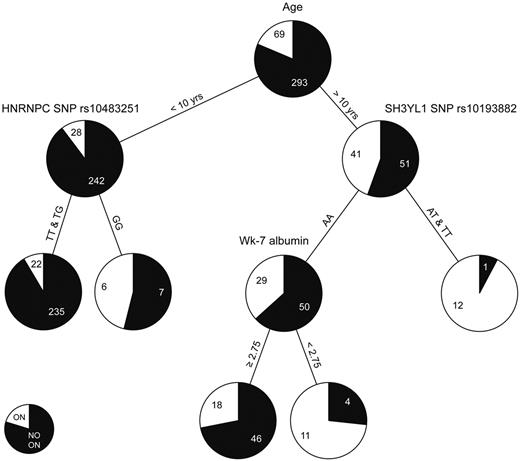
CART analysis
The classification and regression tree (CART) analysis allows sequential division of the cohort by risk factors in order of importance. We used this alternative approach to identify risk factors because age group was so highly associated with the development of symptomatic osteonecrosis. The variables interrogated in the CART analysis are described in supplemental Table 8. For children younger than 10 years of age, those with the GG genotype for a SNP in HNRNPC (rs10483251) had a higher risk for symptomatic osteonecrosis than those with at least one T allele (Figure 6). In patients older than 10 years of age, those with at least one T allele at SNP in SH3YL1 gene (rs10139882) had a higher risk of symptomatic osteonecrosis than those with AA genotype; within older patients with the AA genotype, lower serum albumin levels were risk factors. For the 2 SNPs that were incorporated into the CART analysis (rs10483251 and rs10139882), a similar trend for association with symptomatic osteonecrosis was observed when analyzed for the entire cohort, regardless of age (supplemental Figure 4A-B). Moreover, SNP rs10139882 in SH3YL1 is one of the top 4 SNPs identified in the GWAS (Table 1). In addition, regardless of age, patients with lower serum albumin at week 7 had a higher risk of symptomatic osteonecrosis (supplemental Figure 4C).
CART analysis. Age, sex, race, treatment arm, body mass index, dexamethasone AUC, cholesterol, albumin, and 20 SNPs described in supplemental Table 8 were allowed to compete in the CART analysis. Black and white colors in the pie chart represent the percentage of patients with grade 0 to 1 and grade 2 to 4 osteonecrosis (ON), respectively. Each number indicates the number of patients at the branch point.
CART analysis. Age, sex, race, treatment arm, body mass index, dexamethasone AUC, cholesterol, albumin, and 20 SNPs described in supplemental Table 8 were allowed to compete in the CART analysis. Black and white colors in the pie chart represent the percentage of patients with grade 0 to 1 and grade 2 to 4 osteonecrosis (ON), respectively. Each number indicates the number of patients at the branch point.
Discussion
Osteonecrosis can be a debilitating complication from treatment with glucocorticoids. We investigated genetic and nongenetic risk factors that could predispose to osteonecrosis risk. Because the diagnostic imaging needed to establish the diagnosis of osteonecrosis can be confounded by the patient's threshold for reporting pain and the clinician's threshold for ordering imaging tests, prior studies of osteonecrosis in ALL may have assigned patients with osteonecrosis to the “control” group. In the current study, we avoided this limitation by prospectively screening all patients and assigning patients accurately to various osteonecrosis groups. Using this approach, more than 50% of patients had asymptomatic grade 1 osteonecrosis (radiographic findings only, Figure 1B). However, only 17.6% ± 1.8% of patients had symptomatic grade 2 to 4 osteonecrosis, an incidence higher than earlier studies.10,11 Based on the initial screening MRI of hips and knees, those patients who were initially diagnosed with grade 1 osteonecrosis were significantly more likely to eventually develop symptomatic grade 2 to 4 osteonecrosis (26%) than patients whose initial MRI screen was negative for osteonecrosis (14%) (P = .008).
Similar to earlier studies,5,11,18 age was the most significant risk factor for symptomatic osteonecrosis (odds ratio = 4.85; 95% CI, 2.5-9.2; P = .00001) in multiple regression (supplemental Table 2) as well as CART analysis (Figure 6). Of those patients older than 10 years of age, 44.6% developed symptomatic osteonecrosis compared with 10% in younger patients (Figure 2A; supplemental Table 2). Interestingly, in the United Kingdom ALL-12 study, adolescents younger than 20 years had a higher risk of osteonecrosis compared with patients older than 20 years of age.8 Together, these findings indicate that patients between 10 and 20 years are more prone to develop glucocorticoid-induced symptomatic osteonecrosis than any other age group, suggesting that hormonal and physiologic changes resulting from puberty and the maturing phase of bone in adolescents may make them more susceptible.32,33 Patients in the SR/HR treatment arm were at higher risk to develop symptomatic osteonecrosis (odds ratio = 2.5; 95% CI, 1.2-4.9; P = .011) compared with LR patients (Figure 2B; supplemental Table 2), even after accounting for older age on the SR/HR treatment arm. Patients in the SR/HR treatment arms received more intensive therapy compared with those in the LR treatment arm2 : dexamethasone at 12 mg/m2 per day and 20 consecutive weeks of asparaginase compared with dexamethasone at 8 mg/m2 per day and only 6 weeks of asparaginase. Sex and race were risk factors in the Children's Cancer Group-1882 study11 but not in our cohort.
Because hypoalbuminemia, a marker of asparaginase treatment, is associated with greater plasma exposure to dexamethasone,25 we hypothesize that asparaginase treatment may potentiate glucocorticoid-induced osteonecrosis24 and be reflected by lower serum albumin. Indeed, we report here, for the first time, that lower serum albumin was also associated with symptomatic osteonecrosis risk (Figures 3A, 6; supplemental Table 3). In non-ALL settings, hyperlipidemia has been associated with risk of osteonecrosis,20 but serum lipids have not previously been studied as osteonecrosis risk factors in ALL. We found that serum lipids increased as expected34,35 in response to dexamethasone and asparaginase treatment, and we did find that higher cholesterol was associated with symptomatic osteonecrosis (Figure 3B; supplemental Table 3). Whether hypercholesterolemia contributed to its etiology or served as a biomarker of steroid and asparaginase exposure is not clear. Variability in the extent of cortisol suppression has been associated with risk of glucocorticoid-induced growth suppression36 ; however, we did not observe an association with osteonecrosis risk.
Because dexamethasone clearance is inversely related to age,25 any relationship between dexamethasone plasma exposure and osteonecrosis will be confounded by the association between age (and treatment arm) and risk of grade 2 to 4 osteonecrosis. Nonetheless, even after adjusting for age and treatment arm, there was a higher dexamethasone AUC (P = .0005; Figure 4) in those with severe (grade 3 or 4) osteonecrosis. Unfortunately, older children also tend to have ALL that is more resistant to dexamethasone compared with younger patients.37 Thus, there is hesitation to recommend lower doses of dexamethasone to offset the lower clearance observed in older patients with ALL.
From our GWAS, we identified a locus on chromosome 2 encoding for ACP1 and SH3YL1 (Table 1) that is associated with symptomatic osteonecrosis risk. Although the lowest P values for these 4 SNPs (P = 1.2 × 10−6, 1.9 × 10−6, 1.9 × 10−6, and 3.6 × 10−6) were higher than the standard threshold of P = 10−7 for GWAS, the pleiotropic association of these SNPs with 2 other phenotypes (ie, higher cholesterol and lower albumin levels, Figure 5; supplemental Table 6), which themselves are “independent” risk factors (Figures 3, 6) for symptomatic osteonecrosis, strengthens the evidence for these SNPs. Our findings are consistent with observations that ACP1 is associated with serum cholesterol and triglyceride levels38 and that it regulates osteoblast differentiation via Src kinase.39 These findings suggest that ACP1 might act via multiple mechanisms to affect bone homeostasis and dexamethasone-induced osteonecrosis. The pleiotropic effects of disease-association SNPs with other related phenotypes has also been observed for several other diseases (eg, PRKCA with asthma and body mass index, and CRP with inflammation, blood pressure, body mass index, insulin, leptin, triglycerides, and norepinephrine).40,41 Further studies are needed to test whether these results replicate in other contexts. We found that a polymorphism in SERPINE1 (rs6092), which was significant in a prior cohort,21 was not associated with osteonecrosis in the current cohort (P = .9). This discordance could be caused by differences in therapy or in assignment of control versus osteonecrosis status in prior studies compared with the current study.
We performed the most comprehensive analysis of nongenetic and genetic risk factors for osteonecrosis to date. Similar to earlier studies, we show that age older than 10 years is the strongest covariate for osteonecrosis. Associations with treatment arm, higher cholesterol, lower albumin, and higher dexamethasone exposure are consistent with the hypothesis that agents such as asparaginase might potentiate the osteonecrotic effect of dexamethasone. The fact that variation in the ACP1-SH3YL1 gene locus was associated with osteonecrosis risk, lower albumin, and higher cholesterol levels suggests that altered lipid homeostasis may have been particularly relevant to the mechanism of osteonecrosis in these patients with ALL, and suggest strategies for altering therapy to minimize this complication in the future.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the clinical staff, research nurses, patients, and their parents for their participation; Dr Wenjian Yang and Diana Chan for computing assistance; Drs Jun Yang, Laura Ramsey, and Lisa Trevino for insightful comments on the manuscript; and Nancy Kornegay for data preparation.
This work was supported by the National Cancer Institute (grants CA 142665, CA 36401, and CA 21765), the National Institutes of Health/National Institute of General Medical Sciences Pharmacogenomics Research Network (U01 GM92666), and ALSAC.
National Institutes of Health
Authorship
Contribution: M.V.R. conceived and designed the project, interpreted the data, and drafted the manuscript; J.D.K. analyzed and interpreted the data and drafted the manuscript; S.C.K. designed the screening study and analyzed the MRIs; J.C.P. determined dexamethasone pharmacokinetics; X.C. assayed dexamethasone and cortisol levels in plasma; G.N. oversaw genotyping; D.P. and C.C. performed the statistical analyses; W.E.E., S.C.H., and C.-H.P. were principal investigators for the clinical protocols; and all authors contributed to the writing of the manuscript.
Conflict-of-interest disclosure: W.E.E. serves on the boards of Aldagen, Chemores (Scientific Advisory Board, European Research Consortium), ParagonDx (Scientific Advisory Board, Inactive), and the National Cancer Institute (NCI) Board of Scientific Counselors. He received NCI grants R37 CA36401, P30 CA21765, CA 90628, and CA116201 from the National Institute of General Medical Sciences Pharmacogenomics Research Network. He also holds a patent with St Jude Children's Research Hospital and receives royalty from TPMT genotyping tests. M.V.R. receives a portion of the income St Jude Children's Research Hospital receives from licensing patent rights related to TPMT polymorphisms and GGH polymorphisms. She also receives funding for investigator-initiated research on the pharmacology of asparaginase from Sigma-Tau Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Mary V. Relling, St Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105-2794; e-mail: mary.relling@stjude.org.