Abstract
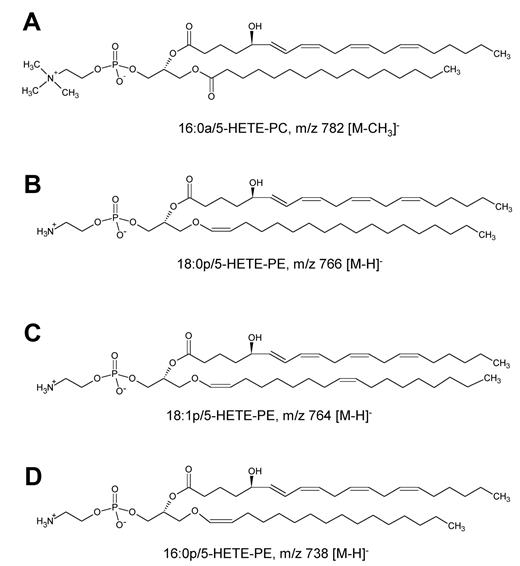
5-Lipoxygenase (5-LOX) plays key roles in infection and allergic responses. Herein, four 5-LOX–derived lipids comprising 5-hydroxyeicosatetraenoic acid (HETE) attached to phospholipids (PLs), either phosphatidylethanolamine (PE) or phosphatidylcholine (18:0p/5-HETE-PE, 18:1p/5-HETE-PE, 16:0p/5-HETE-PE, and 16:0a/5-HETE-PC), were identified in primary human neutrophils. They formed within 2 minutes in response to serum-opsonized Staphylococcus epidermidis or f-methionine-leucine-phenylalanine, with priming by lipopolysaccharide, granulocyte macrophage colony-stimulating factor, or cytochalasin D. Levels generated were similar to free 5-HETE (0.37 ± 0.14 ng vs 0.55 ± 0.18 ng/106 cells, esterified vs free 5-HETE, respectively). They remained cell associated, localizing to nuclear and extranuclear membrane, and were formed by fast esterification of newly synthesized free 5-HETE. Generation also required Ca2+, phospholipase C, cytosolic and secretory phospholipase A2, 5-LOX activating protein, and mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1. 5-HETE-PLs were detected in murine S epidermidis peritonitis, paralleling neutrophil influx, and in effluent from Gram-positive human bacterial peritonitis. Formation of neutrophil extracellular traps was significantly enhanced by 5-LOX inhibition but attenuated by HETE-PE, whereas 5-HETE-PE enhanced superoxide and interleukin-8 generation. Thus, new molecular species of oxidized PL formed by human neutrophils during bacterial infection are identified and characterized.
Introduction
Lipoxygenases (LOXs) are a family of lipid peroxidizing enzymes that oxidize free arachidonate, forming eicosanoids. Neutrophils express the 5-LOX isoform, which generates important regulators of the immune response, including 5-hydroxyeicosatetraenoic acid (HETE), leukotriene B4, resolvins, lipoxins, and the cysteinyl leukotrienes.1 5-LOX shows a distinct induction profile and tissue distribution to other mammalian LOX isoforms, and its free acid products participate in inflammation through enhancing leukocyte activation and mediating bronchoconstriction or vasoconstriction.2 Inhibitor studies and genetic mutants have placed 5-LOX at the center of the immune response regulating adhesion, chemotaxis, and bacterial killing in several disease models.3,4 However, the known free acid products do not fully account for its bioactivity in vivo. For example, adding leukotrienes does not restore adhesion or chemotaxis of LOX-inhibited neutrophils in vitro or lung injury in vivo.2,5 In addition, free acid 5-LOX metabolites, including 5-HETE and leukotrienes, do not compensate for inhibition of LOX during activation of murine peritoneal macrophages for tumoricidal function.6 Although lipoxins and resolvins may also participate in these models, they are unlikely to account for all the biologic activity of 5-LOX, in particular because they are inhibitors of chemotaxis rather than activators.7 Collectively, these observations infer the participation of further uncharacterized molecular species in mediating the biologic actions of 5-LOX and indicate that the identification of novel lipids derived from this pathway is an important and clinically relevant goal. Recently, families of phospholipid-esterified HETEs (HETE-PLs) were shown to be acutely generated by LOXs in monocytes/macrophages and platelets.8-10 Thus, we reasoned that similar lipids might be generated by neutrophils under agonist-activated conditions. More than 20 years ago, studies on the esterification of [14C]arachidonate into neutrophil membranes and its endogenous transformation into free 5-HETE showed that a proportion of 5-LOX products became esterified into phospholipids (PLs), including phosphatidylethanolamine (PE) and phosphatidylcholine (PC).11,12 However, at that time, even with the use of what were then state-of-the-art techniques, it was not possible to determine the specific molecular species generated. Since then, development of new generation liquid chromatography/mass spectrometry tandem mass spectrometry (LC/MS/MS) has allowed such questions to be answered, because lipids present in complex biologic samples at relatively low concentrations can now be efficiently separated and analyzed to a greater degree of sensitivity than before as intact molecular species. In separate studies on the fate of exogenous HETEs in neutrophils, 15-HETE was selectively incorporated into phosphatidylinositol (PI) but 5-HETE only slightly (2%-3%) into PC.13 After its incorporation, ionophore-stimulated release of 15-HETE led to its transformation to bioactive secondary products.13 Other studies on 5-HETE incorporation into membranes in adrenal glomerulosa cells showed that 5-HETE present in neutral lipids could modulate aldosterone release.14 This supports the idea that HETEs esterified to larger functional groups can signal in cellular systems.
Herein, we use a targeted lipidomic approach to search for new lipids generated by 5-LOX in agonist-activated neutrophils. A new family comprising 4 PL-esterified eicosanoids is structurally characterized, and mechanisms of their formation and biologic actions in response to bacterial products demonstrated in vitro.
Methods
Materials
Eicosanoid and PLs standards were from Cayman Chemicals and Avanti Polar Lipids. High-performance liquid chromatography–grade solvents were from Fisher Scientific. Inhibitors of intracellular signaling pathways (MK-886; BEL, cytosolic phospholipase A2α [cPLA2α] Inhibitor; U-73122; wortmannin; GF 109203X; PD98059; p38 mitogen-activated protein kinase [MAPK] Inhibitor; and OOEPC) were from Calbiochem. All other reagents were from Sigma-Aldrich unless otherwise stated.
Isolation and activation of human neutrophils
Whole blood was obtained from healthy volunteers free from nonsteroidal anti-inflammatory drugs for ≥ 14 days. Ethical permission for all donations was obtained from local research ethics committee (South East Wales Ethics Committee and the National Research Ethics Service), and all human participants gave written informed consent. Human neutrophils were isolated as previously described, resuspended in a small volume of Ca2+-free Krebs buffer, counted, and kept on ice.15 Neutrophils (3 × 106 cells) were incubated for 10 minutes at 37°C in the presence of 2.5mM CaCl2, 1.25mM MgCl2 with/without signaling inhibitors, before the addition of activating reagent. When priming agents were used, cells were preincubated for 30 minutes at 37°C before activation. In some experiments, 5-HETE-d8 was added to cells (1nmole/106 cells) for 15 minutes, 37°. Unless otherwise stated, cells were activated for 15 minutes at 37°C. For opsonization, live Staphylococcus epidermidis was incubated with or without 10% serum at a density of 108 colony-forming unit (cfu)/mL at 37°C for 20 minutes with agitation. Neutrophils (3 × 106 cells) were prewarmed for 10 minutes and then incubated with f-methionine-leucine-phenylalanine (fMLP; 1μM; 15 minutes) or live S epidermidis (30 × 106 cfu) for 15 minutes or 1 hour, before the addition of internal standards and lipid extraction as described in “Lipid extraction” below.
Lipid extraction
Lipids were reduced with 1mM SnCl2 for 10 minutes at room temperature. For quantitation, internal standards (15-hydroxyeicosatetraenoic acid-d8, di-14:0-phosphotidylethanolamine, and di-14:0-phosphotidylcholine; 0.5 ng each) were added. Lipids were then extracted by adding a solvent mixture (1M acetic acid, propan-2-ol, hexane; 2:20:30, vol/vol/vol) to the sample at a ratio of 2.5 mL of solvent mixture per milliliter of sample, vortexing, and then adding 2.5 mL of hexane. After vortexing and centrifugation, lipids were recovered in the upper hexane layer. The lipids were then re-extracted by the addition of 2.5 mL of hexane. The combined hexane layers were dried, dissolved in methanol, and stored at −80°C before analysis by LC/MS/MS.
Reverse-phase LC/MS/MS of PLs
Lipid extracts were separated with the use of high-performance liquid chromatography as described for precursor scanning (see supplemental data, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), monitoring specific parent-to-daughter transitions in multiple reaction-monitoring (MRM) mode. For quantitation of 5-HETE–containing lipids, the daughter ion m/z 115.2 was used. Product ion spectra were obtained at the apex of the MRM transitions, with the MS operating in ion trap mode. Scans were acquired over 0.65 second, with a linear ion trap fill time of 200 milliseconds and Q0 trapping. For 5-HETE-d8–containing lipids, the daughter ion of m/z 116 was used.
Determining the cellular localization of esterified 5-HETEs
Lipopolysaccharide (LPS)/fMLP-activated neutrophils were pelleted by centrifugation at 400g for 5 minutes. Supernatant was removed, and cells were resuspended in Krebs buffer. Samples were extracted and analyzed with LC/MS/MS. Alternatively, activated neutrophils were disrupted by N2 cavitation. Briefly, esculetin (50μM) and protease inhibitors (mini complete; Roche) were added to LPS/fMLP-activated cells that were transferred to a prechilled N2 pressure chamber. This was pressurized to 3.1 N/m2 for 20 minutes, then the cavitate was collected into EGTA (ethyleneglycoltetraacetic acid; 0.25mM final). The sample was centrifuged at 1000g for 10 minutes, the nuclear pellet was resuspended in Krebs buffer, and the supernatant was ultracentifuged at 180 000g for 20 minutes. The resulting pellet was resuspended in Krebs buffer (membrane pellet containing small vesicles derived from the plasma membrane and other organelles), and the final supernatant comprises cytosol and extracellular media. To confirm the separation of intact nuclei from other cellular constituents, DNA content was determined with the use of the Quant-iT PicoGreen dsDNA kit (Invitrogen).
Peritonitis patient samples
This study was conducted according to the principles expressed in the Declaration of Helsinki and under local ethical guidelines (Bro Taf Health Authority, Wales). The study was approved by the South East Wales Local Ethics Committee under reference number 04WSE04/27. All patients provided written informed consent for the collection of samples and subsequent analysis in accordance with the Declaration of Helsinki. Patients undergoing peritoneal dialysis for renal failure with confirmed bacterial peritonitis or controls (peritoneal drain effluent from stable patients without a prior history of infection) were recruited from the Peritoneal Dialysis Unit, University Hospital of Wales, Cardiff and Vale University Local Health Board. Overnight peritoneal drain effluent was collected from 6 patients presenting with bacterial peritonitis characterized clinically by abdominal pain and a cloudy peritoneal drain effluent (containing > 100 neutrophils/mm3). Standard microbiologic analysis (National Public Health Service for Wales Laboratories, Cardiff and Vale University Local Health Board) identified the causative organisms. All patients were receiving standard antibiotic therapy according to the International Society for Peritoneal Dialysis guidelines for the treatment of peritonitis, and samples were obtained on day 1 (except Klebsiella, day 3).16,17 For comparison, peritoneal lavage fluid was also obtained from 4 stable, infection-free “control” patients undergoing peritoneal dialysis. Lipids were extracted as described earlier.
Murine S epidermidis peritonitis
Animal experiments were undertaken in accordance with the United Kingdom Animals (Scientific Procedures) Act of 1986 performed under Home Office Project License (PPL 30/2269- Regulation of leukocyte trafficking and Fibrosis). Mice were kept in constant temperature cages and given free access to water and standard chow. An inoculum of live S epidermidis (ATCC 12228) was prepared from bacteria harvested during log-phase growth under sterile conditions. Seven- to 8-week-old C57BL/6 mice (25-30 g) were obtained from Charles River and inoculated by intraperitoneal injection of 5 × 108 cfu S epidermidis in 0.5 mL of phosphate-buffered saline, or vehicle alone. At specific time intervals (3, 6, 12, and 18 hours) mice were killed, and the peritoneal cavity was lavaged with sterile phosphate-buffered saline (n = 4 per group). Lipids were extracted as described earlier.
Formation of neutrophil extracellular traps
Neutrophils were allowed to adhere in 12-well plates. Neutrophil extracellular trap (NET) formation was activated with the use of 25 ng/mL phorbol myristate acetate (PMA) for 30 minutes, with/without 10-minute preincubation with 18:0a/15-HETE-PE (10μM), semipure 18:0a/5-HETE-PE (10μM),18 (see supplemental data for details), 18:0a/20:4-PE (10μM), or vehicle control, or 1 μg/mL PMA when preincubated with MK886 (1μM) or zileuton (10μM).19 Cells were pelleted, and supernatant DNA concentrations were quantified with the use of a DNA standard curve, by fluorometry using Sytox Green (5μM; Invitrogen). To visualize NET formation, neutrophils were adhered on glass coverslips pretreated with bovine serum albumin, and NET formation was activated with 25 ng/mL PMA (30 minutes). Cells were fixed and permeabilized, then stained with mouse–anti-Histone (H1), 1:100 (Cambridge Biosciences) or rabbit anti-myeloperoxidase, 1:250 (Calbiochem), then with goat anti–mouse immunoglobulin G–Alexa 488, 1:250, and goat anti–rabbit immunoglobulin G–Alexa 568, 1:250. Coverslips were mounted with the use of Prolong Gold (Invitrogen), and NETs were imaged with an inverted Axiovert S100-TV (Zeiss) microscope with a Hamamatsu ORCA-ER camera, with a ×10 eyepiece and a ×63 objective lens. Image analysis software (ImageJ Version 10.2; National Institutes of Health; http://rsb.info.nih.gov/ij) was used for postacquisition processing.
Superoxide generation assay
Neutrophils (2 × 106 cells/mL) were incubated at 37°C with stirring in 2 mL of Krebs buffer with 2.5mM CaCl2, 1.25mM MgCl2, 50μM cytochrome c, and with or without 18:0a/15- HETE-PE (10μM) for 10 minutes before the addition of fMLP (1μM) or PMA (1μg/mL).20 Absorbance (550 nm) was monitored, and reduced cytochrome c was quantified with ϵ550 = 21.1mM.cm−1.
Statistical analysis
For human neutrophil studies, data are representative of ≥ 3 separate donors, with samples run in triplicate for each experiment. Data are expressed as mean ± SEM. The statistical significance of the difference between 2 sets of data was assessed with the use of an unpaired, 2-tailed Student t test. When the difference between > 2 sets of data was analyzed, 1-way analysis of variance was used, followed by Bonferroni multiple comparisons test, as indicated on legends. P < .05 was considered significant.
Results
Identification of 5-HETE-PLs and structural characterization
Precursor LC/MS/MS identified a series of 4 ions that fragment to generate a daughter ion characteristic of HETE in lipid extracts from ionophore-activated human monocytes. This is described in full in supplemental Figures 1 and 2.
Structural identification of 5-HETE-PLs
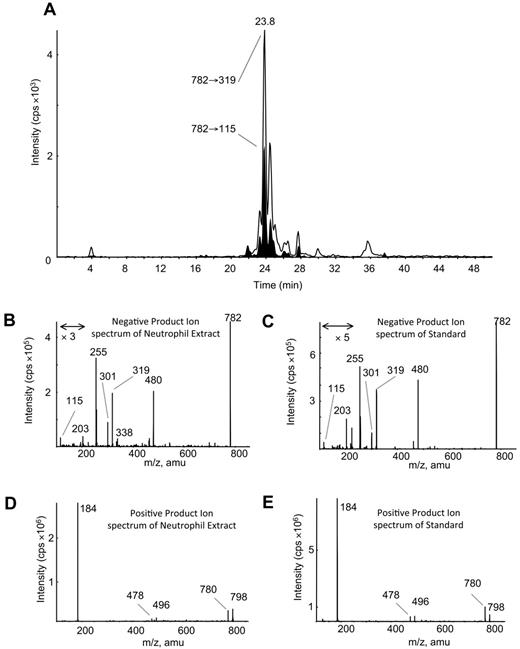
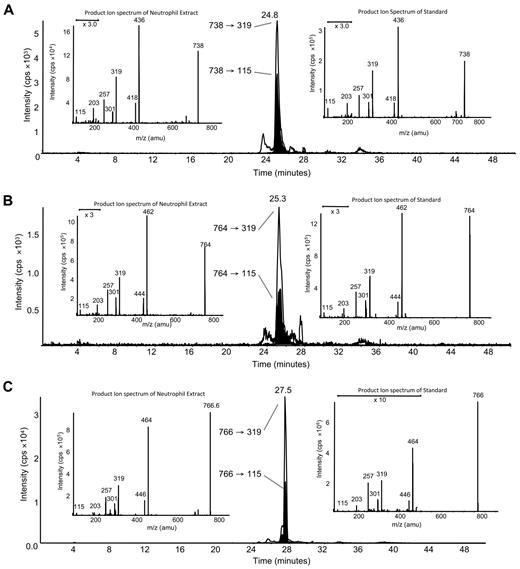
LC/MS/MS analysis with online acquisition of product ion spectra was conducted for the 4 ions, monitored by the parent-to-daughter transition arising from collision-induced dissociation to give daughter ions for HETE at either m/z 319 or 115, the latter being diagnostic for the 5-HETE positional isomer. Lipids were separated with the use of LC/MS/MS in MRM mode, and product ion spectra were acquired at the apex of elution for each lipid by switching the MS to ion trap mode during the LC run. For structural identification, PE and PC standards were oxidized with the use of potato 5-LOX. The LC/MS/MS profile of m/z 782→319 from human neutrophils shows a predominant ion that co-elutes with the m/z 782→115 transition (shown in black on trace), indicating that this lipid generates m/z 319 and m/z 115 daughter ions on collision-induced dissociation (Figure 1A). The negative MS/MS spectrum shows several HETE ions, including m/z 319, 301, and 115 (Figure 1B). There is also a prominent ion at m/z 255, indicating an acyl-linked 16:0 at sn1. On the basis of this, the structure was predicted to be 16:0a/5-HETE-PC (Figure 2). Oxidation of 16:0/20:4-PC with the use of potato 5-LOX, followed by SnCl2 reduction, generated a species with identical LC retention time and MS/MS spectrum (Figure 1C). To further confirm structure, positive LC/MS/MS was undertaken on both neutrophil lipid extract and the standard. In negative mode, PC appears as [M-15]−, thus in positive mode, [M+H]+ is 16 amu greater, appearing at m/z 798 with a prominent daughter ion at m/z 184 (PC head-group), confirming the identity of the neutrophil lipid as 16:0a/12-HETE-PC (Figure 1D-E). Product ion spectra for the other 3 ions (m/z 766, 764, and 738) also show 5-HETE fragments at m/z 319, 301, 257, and 115 but no sn1 ions, indicating that they are plasmalogen or ether lipids (Figure 3). Oxidation of brain PE, which contains a high proportion of plasmalogen but little ether PE, with the use of potato 5-LOX generated ions with identical retention time (RT) and MS/MS spectra (Figure 3). This identifies the lipids as 18:0p/5-HETE-PE, 18:1p/5-HETE-PE, and 16:0p/5-HETE-PE, respectively (Figures 2–3).
Structural identification of m/z 782 as 16:0a/5-HETE-PC with the use of MS/MS. (A) Negative LC/MS/MS of m/z 782→319 and 782→115 from ionophore-activated neutrophil lipid extract. (B) Negative MS/MS spectrum of m/z 782 at 23.8 minutes from neutrophil lipid. (C) Negative MS/MS spectrum of m/z 782 generated by 5-LOX oxidation of 16:0a/20:4-PC, eluting at 23.8 minutes. (D) Positive MS/MS of m/z 782, eluting at 23.8 minutes from neutrophil lipids. (E) Positive MS/MS of m/z 782 generated by 5-LOX oxidation of 16:0a/20:4-PC, eluting at 23.8 minutes.
Structural identification of m/z 782 as 16:0a/5-HETE-PC with the use of MS/MS. (A) Negative LC/MS/MS of m/z 782→319 and 782→115 from ionophore-activated neutrophil lipid extract. (B) Negative MS/MS spectrum of m/z 782 at 23.8 minutes from neutrophil lipid. (C) Negative MS/MS spectrum of m/z 782 generated by 5-LOX oxidation of 16:0a/20:4-PC, eluting at 23.8 minutes. (D) Positive MS/MS of m/z 782, eluting at 23.8 minutes from neutrophil lipids. (E) Positive MS/MS of m/z 782 generated by 5-LOX oxidation of 16:0a/20:4-PC, eluting at 23.8 minutes.
Structural identification of m/z 738, 764, and 766 as plasmalogen PEs with the use of MS/MS. (A) Negative LC/MS/MS of m/z 738→319 and 738→115 from ionophore-activated neutrophil lipid extract. (Left insert) Negative MS/MS spectrum at 24.8 minutes from neutrophil lipid. (Right insert) Negative MS/MS spectrum of m/z 738 generated by 5-LOX oxidation of brain PE, eluting at 24.8 minutes. (B) Negative LC/MS/MS of m/z 764→319 and 764→115 from ionophore-activated neutrophil lipid extract. (Left inset) Negative MS/MS spectrum at 25.3 minutes from neutrophil lipid. (Right inset) Negative MS/MS spectrum of m/z 764 generated by 5-LOX oxidation of brain PE, eluting at 25.3 minutes. (C) Negative LC/MS/MS of m/z 766→319 and 766→115 from ionophore-activated neutrophil lipid extract. (Left inset) Negative MS/MS spectrum at 27.5 minutes from neutrophil lipid. (Right inset) Negative MS/MS spectrum of m/z 766 generated by 5-LOX oxidation of brain PE, eluting at 27.5 minutes.
Structural identification of m/z 738, 764, and 766 as plasmalogen PEs with the use of MS/MS. (A) Negative LC/MS/MS of m/z 738→319 and 738→115 from ionophore-activated neutrophil lipid extract. (Left insert) Negative MS/MS spectrum at 24.8 minutes from neutrophil lipid. (Right insert) Negative MS/MS spectrum of m/z 738 generated by 5-LOX oxidation of brain PE, eluting at 24.8 minutes. (B) Negative LC/MS/MS of m/z 764→319 and 764→115 from ionophore-activated neutrophil lipid extract. (Left inset) Negative MS/MS spectrum at 25.3 minutes from neutrophil lipid. (Right inset) Negative MS/MS spectrum of m/z 764 generated by 5-LOX oxidation of brain PE, eluting at 25.3 minutes. (C) Negative LC/MS/MS of m/z 766→319 and 766→115 from ionophore-activated neutrophil lipid extract. (Left inset) Negative MS/MS spectrum at 27.5 minutes from neutrophil lipid. (Right inset) Negative MS/MS spectrum of m/z 766 generated by 5-LOX oxidation of brain PE, eluting at 27.5 minutes.
5-HETE-PLs are generated acutely in response to agonist activation
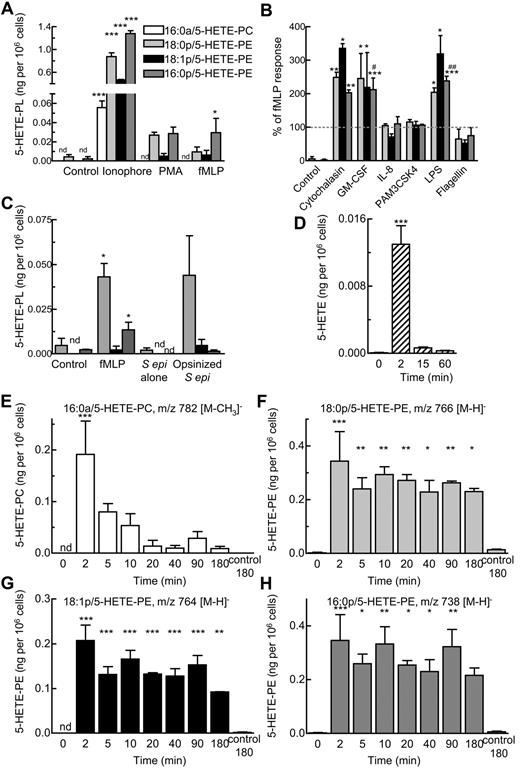
Ionophore activation of neutrophils for 15 minutes stimulated generation of all four 5-HETE-PLs (Figure 4A). PMA or fMLP also caused formation of 5-HETE-PEs, although 5-HETE-PC was generally not found (Figure 4A). Other agents, including platelet activating factor, adenosine triphosphate (ATP), thrombin, trypsin (activate protease activated receptors), interleukin-8 (IL-8), Pam3CSK4, or flagellin did not activate (not shown). Priming with the use of cytochalasin B, granulocyte macrophage colony-stimulating factor, or LPS increased fMLP-dependent formation of 5-HETE-PE by 2- to 3-fold (Figure 4B) and raised levels of 5-HETE-PC above the limit of detection (to ∼ 0.01 ng/106 cells). Pretreatment of neutrophils with IL-8, PAM3CSK4, or flagellin was without effect (Figure 4B). 5-HETE-PEs also formed on incubation of serum-opsonized S epidermidis with neutrophils (Figure 4C). In contrast incubation with nonopsonized bacteria, or sterile cell-free supernatant from S epidermidis did not activate (Figure 4C). After 15 minutes of activation with LPS/fMLP, levels of 5-HETE-PLs were 0.05 ± 0.05 ng 16:0a/5-HETE-PC, 0.13 ± 0.06 ng 18:0p/5-HETE-PE, 0.06 ± 0.02 ng 18:1p/5-HETE-PE, and 0.12 ± 0.03 ng 16:0p/5-HETE-PE per 106 neutrophils (giving a total of 0.37 ± 0.14 ng esterified 5-HETE), compared with 0.55 ± 0.18 ng free 5-HETE per 106 neutrophils (n = 3 separate donors). This equates to ∼ 0.41% and 0.01% of total PE or PC, respectively. For all subsequent experiments, LPS/fMLP was used as agonist, except when pharmacologic inhibitors were used (fMLP alone). Time courses showed that 5-HETE-PC and also free 5-HETE peaked at ∼ 2 minutes, followed by a rapid decline (Figure 4D-E). In contrast, 5-HETE-PEs formed within 2 minutes but remained stable for ≥ 3 hours (Figure 4F-H).
Generation of 5-HETE-PLs by human neutrophils in response to agonists. (A) Neutrophils were activated by ionophore (10μM), PMA (1 μg/mL), or fMLP (1μM) for 15 minutes. (B) Priming of 5-HETE-PE formation by stimulation with fMLP (1μM, 15 minutes) with 30 minutes of preincubation with cytochalasin B (10 μg/mL), granulocyte macrophage colony-stimulating factor (GM-CSF; 1nM), IL-8 (10nM), PAM3CSK4 (1 μg/mL), LPS (1 μg/mL), or flagellin (0.1 μg/mL). (C) Activation by bacteria. Neutrophils (3 × 106 cells) were incubated with fMLP (1μM) or live S epidermidis bacteria (S epi alone; 30 × 106 cfu) or live S epidermidis (Opsin S epi; 30 × 106 cfu) for 15 minutes. n ≥ 3; mean ± SEM data presented from 1 experiment and representative of 3; *P < .05, **P < .01, and ***P < .001 versus Control; #P < .05 and ##P < .01 versus fMLP alone. (D-H) Time course of activation with fMLP (1μM) after 30 minutes of preincubation with LPS (1 μg/mL). Formation of (D) free 5-HETE, (E) 16:0a/5-HETE-PC, (F) 18:0p/5-HETE-PE, (G) 18:1p/5-HETE-PE, (H) 16:0p/5- HETE-PE, and (inset) free 5-HETE. n ≥ 3; *P < .05, **P < .01, and ***P < .001 versus Control (0 minutes).
Generation of 5-HETE-PLs by human neutrophils in response to agonists. (A) Neutrophils were activated by ionophore (10μM), PMA (1 μg/mL), or fMLP (1μM) for 15 minutes. (B) Priming of 5-HETE-PE formation by stimulation with fMLP (1μM, 15 minutes) with 30 minutes of preincubation with cytochalasin B (10 μg/mL), granulocyte macrophage colony-stimulating factor (GM-CSF; 1nM), IL-8 (10nM), PAM3CSK4 (1 μg/mL), LPS (1 μg/mL), or flagellin (0.1 μg/mL). (C) Activation by bacteria. Neutrophils (3 × 106 cells) were incubated with fMLP (1μM) or live S epidermidis bacteria (S epi alone; 30 × 106 cfu) or live S epidermidis (Opsin S epi; 30 × 106 cfu) for 15 minutes. n ≥ 3; mean ± SEM data presented from 1 experiment and representative of 3; *P < .05, **P < .01, and ***P < .001 versus Control; #P < .05 and ##P < .01 versus fMLP alone. (D-H) Time course of activation with fMLP (1μM) after 30 minutes of preincubation with LPS (1 μg/mL). Formation of (D) free 5-HETE, (E) 16:0a/5-HETE-PC, (F) 18:0p/5-HETE-PE, (G) 18:1p/5-HETE-PE, (H) 16:0p/5- HETE-PE, and (inset) free 5-HETE. n ≥ 3; *P < .05, **P < .01, and ***P < .001 versus Control (0 minutes).
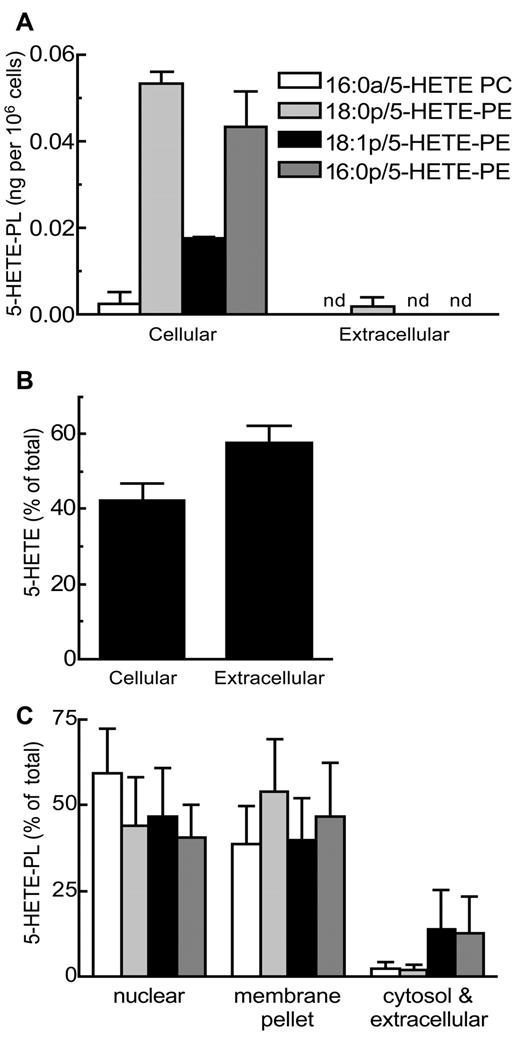
5-HETE-PLs are retained by neutrophils and are present in nuclear and extranuclear membranes
5-HETE-PLs were almost totally retained by the cells, unlike free 5-HETE, of which ∼ 60% was released (Figure 5A-B). Fractionation of membranes after lysis of activated neutrophils by nitrogen cavitation showed that 5-HETE-PLs were localized primarily in both nuclear and extranuclear membrane fractions, rather than cytosol (Figure 5C).
5-HETE-PLs are associated with membrane fractions and not released after activation of neutrophils. Cells were activated with LPS and fMLP. Neutrophils were centrifuged to separate cells from extracellular supernatant before lipid extraction and analysis. (A) 5-HETE-PLs were retained in the cell pellet. (B) Free 5-HETE is both retained in the cell and released into the extracellular media. n ≥ 3; mean ± SEM data presented from 1 experiment and representative of 3. (C) After lysis by N2 cavitation, neutrophils were fractionated by centrifugation before lipid extraction and analysis with LC/MS/MS; mean ± SEM data are pooled from 3 independent experiments.
5-HETE-PLs are associated with membrane fractions and not released after activation of neutrophils. Cells were activated with LPS and fMLP. Neutrophils were centrifuged to separate cells from extracellular supernatant before lipid extraction and analysis. (A) 5-HETE-PLs were retained in the cell pellet. (B) Free 5-HETE is both retained in the cell and released into the extracellular media. n ≥ 3; mean ± SEM data presented from 1 experiment and representative of 3. (C) After lysis by N2 cavitation, neutrophils were fractionated by centrifugation before lipid extraction and analysis with LC/MS/MS; mean ± SEM data are pooled from 3 independent experiments.
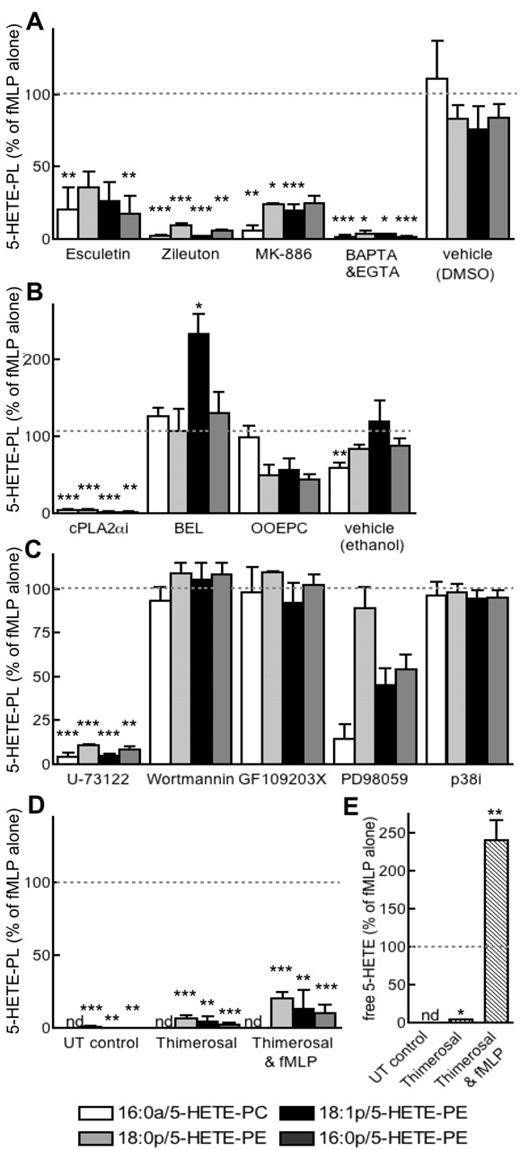
Elucidating the signaling pathways and enzymes required for 5-HETE-PL generation
Several inhibitors of LOX (esculetin, zileuton) or 5-LOX–activating protein (FLAP; MK-886) attenuated 5-HETE-PL generation, confirming the requirement for 5-LOX (Figure 6A). 5-HETE-PL generation was also blocked by Ca2+ chelation (EGTA and BAPTA-AM) or inhibition of cPLA2 (cPLA2αi) or phospholipase C (PLC; U-73112) (Figure 6A-C). Blockade of secretory PLA2 (OOEPC) or MAPK/extracellular signal-regulated kinase (ERK) kinase 1 (MEK1; PD98059) partially attenuated, whereas inhibition of iPLA2, (BEL), p38 MAPK (p38i), PI3 kinase (wortmannin), or protein kinase C (PKC; GF 109203X) had no effect (Figure 6A-C). The inhibitor of fatty acid esterification, thimerosal, reduced 5-HETE-PL generation, and it increased the release of free 5-HETE (Figure 4D-E). Collectively, these data implicate 5-LOX, FLAP, Ca2+, cPLA2, and fatty acid esterification in the formation of 5-HETE-PLs, while suggesting a role for secretory PLA2 and MEK1. Except for thimerosal, pharmacologic inhibitors had the same effect on free 5-HETE as for 5-HETE-PL generation, indicating that common signaling pathways lead to their formation (not shown). In separate experiments, neutrophils were examined for their ability to acutely esterify exogenous 5-HETE, by incubation with 5-HETE-d8 before lipid extraction and analysis with the use of both MRM modes (for specific parent-to-daughter transitions corresponding to expected lipids) or precursor scanning (for m/z 327, for 5-HETE-d8). However, this eicosanoid was not detected either attached to the expected HETE-PLs or to PI or any other PL class (not shown).
Generation of 5-HETE-PLs requires 5-LOX, FLAP, Ca2+, cPLA2, and PLC and occurs by re-esterification of free 5-HETE. Neutrophils were activated with fMLP after preincubation with inhibitors as described. Lipids were extracted and analyzed with the use of LC/MS/MS. (A) Inhibition of 5-LOX (zileuton, 10μM; esculetin, 50μM), FLAP (MK-886; 1μM), cPLA2α Inhibitor (cPLAi; 10μM), or calcium chelation (BAPTA-AM, BAPTA, 10μM; and EGTA, 1mM) prevented formation of 5-HETE-PLs. (B) Inhibition of cPLA2 (cPLA2αi; 1.2μM) or secretary PLA2 (OOEPC; 10μM), but not of iPLA2 (BEL; 100nM), prevented 5-HETE-PL formation. (C) Inhibition of PLC (U-73122; 5μM) or MEK1 (PD98059; 50μM), but not p38 MAPK (p38 MAP Kinase Inhibitor, P38i; 100nM) or PKC (GF109203X; 100nM) prevented 5-HETE-PL formation. (D) Inhibition of lysophospholipid acyltransferase enzymes with thimerosal (100μM) inhibited formation of 5-HETE-PLs by fMLP-activated neutrophils. (E) Thimerosal (100μM) enhanced formation of free 5-HETE by fMLP-activated neutrophils. n ≥ 3; mean ± SEM data presented from 1 experiment and representative of 3; data expressed as percentage of formation in response to fMLP alone. *P < .05, **P < .01, and ***P < .001 versus fMLP alone.
Generation of 5-HETE-PLs requires 5-LOX, FLAP, Ca2+, cPLA2, and PLC and occurs by re-esterification of free 5-HETE. Neutrophils were activated with fMLP after preincubation with inhibitors as described. Lipids were extracted and analyzed with the use of LC/MS/MS. (A) Inhibition of 5-LOX (zileuton, 10μM; esculetin, 50μM), FLAP (MK-886; 1μM), cPLA2α Inhibitor (cPLAi; 10μM), or calcium chelation (BAPTA-AM, BAPTA, 10μM; and EGTA, 1mM) prevented formation of 5-HETE-PLs. (B) Inhibition of cPLA2 (cPLA2αi; 1.2μM) or secretary PLA2 (OOEPC; 10μM), but not of iPLA2 (BEL; 100nM), prevented 5-HETE-PL formation. (C) Inhibition of PLC (U-73122; 5μM) or MEK1 (PD98059; 50μM), but not p38 MAPK (p38 MAP Kinase Inhibitor, P38i; 100nM) or PKC (GF109203X; 100nM) prevented 5-HETE-PL formation. (D) Inhibition of lysophospholipid acyltransferase enzymes with thimerosal (100μM) inhibited formation of 5-HETE-PLs by fMLP-activated neutrophils. (E) Thimerosal (100μM) enhanced formation of free 5-HETE by fMLP-activated neutrophils. n ≥ 3; mean ± SEM data presented from 1 experiment and representative of 3; data expressed as percentage of formation in response to fMLP alone. *P < .05, **P < .01, and ***P < .001 versus fMLP alone.
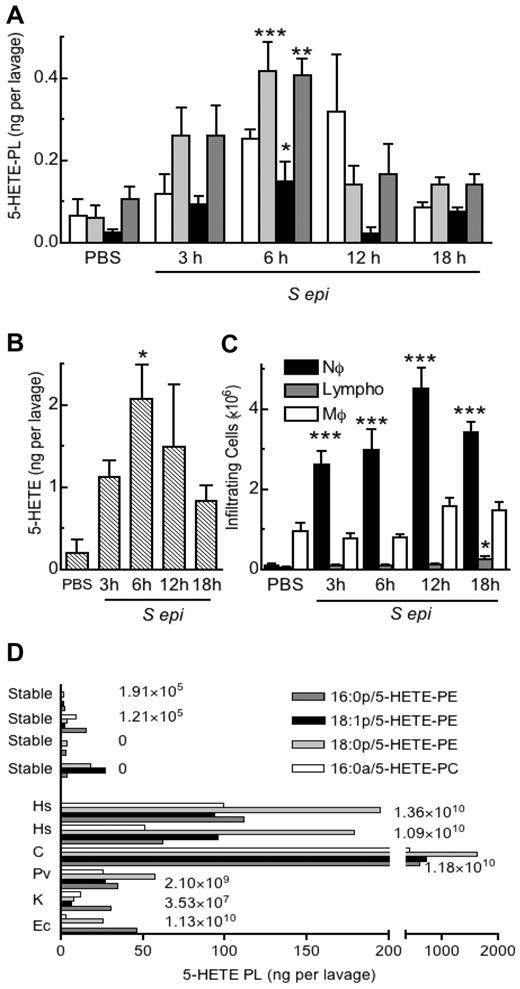
5-HETE-PLs are formed during human and murine bacterial peritonitis
To examine for in vivo generation of 5-HETE-PLs, a well-characterized murine model of bacterial peritonitis induced by S epidermidis was used.21 Administration of live S epidermidis significantly increased levels of free 5-HETE and 5-HETE-PLs by 3 hours, peaking by 6 hours, then returning to near baseline by 18 hours (Figure 7A-B). The profile of product formation was paralleled by neutrophil recruitment, although this peaked closer to 12 hours, suggesting that neutrophil activation of eicosanoid generation occurs early but does not persist (Figure 7C). Other HETE-PE isomers were absent from murine lavage, except for 12-HETE-PE that follows a distinct temporal generation pattern linked to 12/15-LOX expression in resident macrophages (eg, cleared during infection).8 Peritoneal drain effluent was obtained from human patients undergoing continuous ambulatory peritoneal dialysis for renal failure (Table 1). Samples from healthy patients contained little or no 5-HETE-PLs. In contrast, 5-HETE-PLs were significantly elevated in drain effluent from all patients with microbiologically confirmed infection and significantly elevated neutrophil counts, with especially large increases in those with Gram-positive bacteria (Hemolytic streptococcus and Corynebacterium) (Figure 7D). Other HETE-PE isomers, including 12-, 11-, and 8-HETE-PEs were absent, although in the sample with α-Hemolytic streptococcus, 15-HETE-PE was also detected (supplemental Figure 3). The absence of other positional isomers, in particular the nonenzymatically generated 8- and 11-HETE-PEs, supports the idea that 5- and 15-HETE-PEs (or 12-HETE-PE in mouse) are enzymatically generated in bacterial peritonitis, with 5-HETE-PEs most probably originating from infiltrating neutrophils.
5-HETE-PLs are formed during acute bacterial peritonitis in mice and humans. (A-C) Formation of 5-HETE-PLs in murine live bacterial peritonitis. S epidermidis (5 × 108 cfu/mouse) was administered intraperitoneally, and lavage was harvested 3, 6, 12, or 18 hours later (n = 4 mice; mean ± SEM). Lipids were extracted and analyzed with LC/MS/MS (A) Time course of formation of 5-HETE-PLs. (B) Time course of formation of free 5-HETE. (C) Time course of cellular infiltration, neutrophils (Nϕ), macrophages (Mϕ), and lymphocytes (Lympho) n = 4; *P < .05, **P < .01, and **P < .001 versus phosphate-buffered saline (PBS). (D) 5-HETE-PLs were elevated in patients with confirmed bacterial infection but not in lavage from patients who were culture negative or from stable control patients. Lipids were extracted and analyzed with LC/MS/MS The numbers to the right of the bars indicate the number of infiltrating neutrophils per lavage. Hs indicates Hemolytic streptococcus; C, Corynebacterium; Pv, Proteus vulgaris; K, Klebsiella and Ec, Escherichia coli.
5-HETE-PLs are formed during acute bacterial peritonitis in mice and humans. (A-C) Formation of 5-HETE-PLs in murine live bacterial peritonitis. S epidermidis (5 × 108 cfu/mouse) was administered intraperitoneally, and lavage was harvested 3, 6, 12, or 18 hours later (n = 4 mice; mean ± SEM). Lipids were extracted and analyzed with LC/MS/MS (A) Time course of formation of 5-HETE-PLs. (B) Time course of formation of free 5-HETE. (C) Time course of cellular infiltration, neutrophils (Nϕ), macrophages (Mϕ), and lymphocytes (Lympho) n = 4; *P < .05, **P < .01, and **P < .001 versus phosphate-buffered saline (PBS). (D) 5-HETE-PLs were elevated in patients with confirmed bacterial infection but not in lavage from patients who were culture negative or from stable control patients. Lipids were extracted and analyzed with LC/MS/MS The numbers to the right of the bars indicate the number of infiltrating neutrophils per lavage. Hs indicates Hemolytic streptococcus; C, Corynebacterium; Pv, Proteus vulgaris; K, Klebsiella and Ec, Escherichia coli.
Characteristics of patients undergoing peritoneal dialysis
| Patient . | Sex . | Age, y . | Neutrophils, cells/lavage . | Aspirin . | Gram staining . | Microbiologic analysis . |
|---|---|---|---|---|---|---|
| 1 | M | 83 | 1.9 × 105 | No | N/A | N/A (stable) |
| 2 | M | 62 | 1.2 × 105 | No | N/A | N/A (stable) |
| 3 | M | 62 | 0 | Yes | N/A | N/A (stable) |
| 4 | M | 62 | 0 | Yes | N/A | N/A (stable) |
| 5 | F | 66 | 2.1 × 109 | No | Negative | Proteus vulgaris |
| 6 | M | 69 | 3.5 × 107 | Yes | Negative | Klebsiella |
| 7 | M | 55 | 1.1 × 1010 | No | Negative | Escherichia coli |
| 8 | M | 69 | 1.4 × 1010 | Yes | Positive | Hemolytic streptococcus |
| 9 | M | 75 | 1.1 × 1010 | Yes | Positive | Hemolytic streptococcus |
| 10 | M | 61 | 1.2 × 1010 | No | Positive | Corynebacterium |
| Patient . | Sex . | Age, y . | Neutrophils, cells/lavage . | Aspirin . | Gram staining . | Microbiologic analysis . |
|---|---|---|---|---|---|---|
| 1 | M | 83 | 1.9 × 105 | No | N/A | N/A (stable) |
| 2 | M | 62 | 1.2 × 105 | No | N/A | N/A (stable) |
| 3 | M | 62 | 0 | Yes | N/A | N/A (stable) |
| 4 | M | 62 | 0 | Yes | N/A | N/A (stable) |
| 5 | F | 66 | 2.1 × 109 | No | Negative | Proteus vulgaris |
| 6 | M | 69 | 3.5 × 107 | Yes | Negative | Klebsiella |
| 7 | M | 55 | 1.1 × 1010 | No | Negative | Escherichia coli |
| 8 | M | 69 | 1.4 × 1010 | Yes | Positive | Hemolytic streptococcus |
| 9 | M | 75 | 1.1 × 1010 | Yes | Positive | Hemolytic streptococcus |
| 10 | M | 61 | 1.2 × 1010 | No | Positive | Corynebacterium |
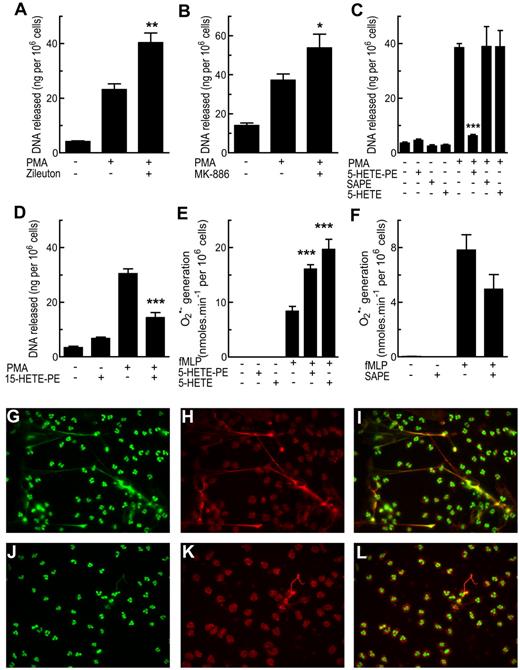
HETE-PLs negatively regulate NET formation
Formation of NETs by PMA-activated neutrophils was significantly increased by zileuton or MK886, indicating a suppressive role for 5-LOX in this process (Figure 8A-B). In contrast, either semipure 18:0a/5-HETE-PE (60% pure, with the remainder 9-HETE-PE) or the monocyte-derived analog, 18:0a/15-HETE-PE, significantly attenuated (P < .01) NET formation, whereas their un-oxidized control (18:0a/20:4-PE, SAPE) and free 5-HETE were without effect (Figure 8C-D,G-L).
HETE-PEs regulate NET formation and superoxide generation by neutrophils. (A-D,G-L) Adherent neutrophils (24-well plate; 106 cells/well) were activated with PMA for 30 minutes, after a 10-minute preincubation with inhibitor or lipid. The supernatant was removed and centrifuged to ensure no cells were present, and NET formation was quantified by fluorescent assay for DNA. (A) Zileuton (10μM) increased NET formation in response to PMA. (B) MK886 (1μM) increased NET formation in response to PMA. (C) Exogenous 5-HETE-PE, but not SAPE (both 10μM), reduced NET formation in response to PMA (D) Exogenous 15-HETE-PE (10μM) reduced NET formation in response to PMA. (E) Superoxide generation was measured by reduction of cytochrome c at 550 nm with activation by 1μM fMLP, after10 minutes of preincubation with 5-HETE-PE or 5-HETE (10μM). (F) Superoxide generation was determined as in panel E with 10 minutes of preincubation with SAPE (1μM). (G-I) Representative fluorescent images of immunostaining of NETs by PMA-activated neutrophils. (J-L) Representative images of neutrophils pretreated with exogenous 15-HETE-PE before activation with PMA. Images show staining for histone H1 (green; G,J), myeloperoxidase (red; H,K), or overlay images (I,L). n = 4; mean ± SEM. Data presented from 1 experiment and representative of ≥ 3; * P < .05, ** P < .01, and *** P < .001 vs activated.
HETE-PEs regulate NET formation and superoxide generation by neutrophils. (A-D,G-L) Adherent neutrophils (24-well plate; 106 cells/well) were activated with PMA for 30 minutes, after a 10-minute preincubation with inhibitor or lipid. The supernatant was removed and centrifuged to ensure no cells were present, and NET formation was quantified by fluorescent assay for DNA. (A) Zileuton (10μM) increased NET formation in response to PMA. (B) MK886 (1μM) increased NET formation in response to PMA. (C) Exogenous 5-HETE-PE, but not SAPE (both 10μM), reduced NET formation in response to PMA (D) Exogenous 15-HETE-PE (10μM) reduced NET formation in response to PMA. (E) Superoxide generation was measured by reduction of cytochrome c at 550 nm with activation by 1μM fMLP, after10 minutes of preincubation with 5-HETE-PE or 5-HETE (10μM). (F) Superoxide generation was determined as in panel E with 10 minutes of preincubation with SAPE (1μM). (G-I) Representative fluorescent images of immunostaining of NETs by PMA-activated neutrophils. (J-L) Representative images of neutrophils pretreated with exogenous 15-HETE-PE before activation with PMA. Images show staining for histone H1 (green; G,J), myeloperoxidase (red; H,K), or overlay images (I,L). n = 4; mean ± SEM. Data presented from 1 experiment and representative of ≥ 3; * P < .05, ** P < .01, and *** P < .001 vs activated.
5-HETE-PE regulates superoxide generation, IL-8 release, but not tumor necrosis factor α generation or degranulation by human neutrophils
Both 5-HETE-PE and free 5-HETE significantly enhanced activity of NADPH, whereas there was no effect of 15-HETE-PE and SAPE was slightly (not significantly) inhibited (Figure 8E-F; supplemental Figure 4G-H). Generation of IL-8 was elevated in response to 5-HETE-PE, abolished by free 5-HETE, and unaffected by 15-HETE-PE or SAPE (supplemental Figure 4B,D) Synthesis of tumor necrosis factor α (TNFα) was not regulated by 15- or 5-HETE-PE, although it was abolished by free 5-HETE (supplemental Figure 4A,C). Finally, there was no effect of 5-HETE-PE, 15-HETE-PE, or SAPE on elastase release (degranulation; supplemental Figure 4E-F).
Discussion
Here, we show with the use of a targeted lipidomic approach that human neutrophils activated by bacterial products or serum-opsonized bacteria acutely generate 4 new 5-LOX–derived lipids, comprising plasmalogen and diacyl PL-esterified 5-HETEs (16:0a/5-HETE-PC, 18:0p/5-HETE-PE, 18:1p/5-HETE-PE, and 16:0p/5-HETE-PE; Figure 2). The rapid rate of formation and cellular localization suggests an autocrine mode of action, consistent with the observation that HETE-PEs regulated formation of NETs and superoxide generation (Figures 4–5,8). HETE-PLs were detected in both murine and human bacterial peritonitis, indicating they form during infections, associated with acute neutrophil influx (Figure 7). Thus, these are a new group of 5-LOX–derived lipids, adding to the known species from this pathway that includes 5-HETE, leukotrienes, resolvins, and lipoxins.
Neutrophil 5-HETE-PL generation was activated in vitro via fMLP receptor by the bacterial peptide fMLP, which is generated by both Gram-positive and negative strains (Figures 1 and 5).22 In vitro priming with either LPS (a Toll-like receptor 4 [TLR4] agonist) or granulocyte macrophage colony-stimulating factor indicates that additional bacterial or host factors further enhance 5-HETE-PL generation; however, the lack of effect of several others indicates selectivity. The inability of TLR2 to activate or prime was confirmed with the use of Pam3CSK4, live S epidermidis, or its cell-free supernatant from S epidermidis, all of which are potent TLR2 agonists (Figure 4C; data not shown).21 In contrast, opsonized S epidermidis in vitro stimulated 5-HETE-PL generation, indicating that serum-derived factors such as antibody and/or complement can activate (Figure 4C). This may represent a global activation mechanism, particularly for strains of bacteria that do not generate direct ligands such as fMLP, probably explaining why S epidermidis could activate in vivo (Figure 7A). The robust generation of 5-HETE-PLs during continuous ambulatory peritoneal dialysis for peritonitis, particularly in Gram-positive infection indicates that these lipids are also formed in human disease (Figure 7D). The variation in levels within the small numbers of patients probably reflects differing levels of neutrophil activation and trafficking between donors, agonist concentrations, severity of infection, organism strain, the stage of disease, and the concomitant antibiotic therapy. There was no correlation with aspirin use (Table 1), although numbers are small. We note that levels of HETE-PLs generated in vitro and in vivo are remarkably similar: 0.41 ± 0.05 ng/106 cells (mouse) or 0.33 ± 0.25 ng/106 cells (human) versus 0.37 ± 0.14 ng/106 cells (in vitro using LPS/fMLP). Thus, generation of 5-HETE-PLs will occur in vivo in response to both Gram-positive and -negative organisms, stimulated by both bacterial and host factors and thus is probably a common response to acute infection.
The fMLP receptor triggers multiple signaling pathways in neutrophils, including Ca2+ mobilization, PKC, PLC, PI3 kinase, p38 MAPK, and ERK1/2. Stimuli that activate p38 MAPK pathway and ERKs cause phosphorylation of 5-LOX, an event implicated in its translocation to the nuclear membrane.23,24 Herein, Ca2+ and PLC were required for formation of 5-HETE-PLs but not p38 MAP kinase (Figure 6). A reduction in free 5-HETE formation was previously observed with the use of PD98059 (which inhibits MEK1 upstream of ERK), and similarly a partial inhibition of 5-HETE-PL generation was found (Figure 6).25 Finally, although artificial activation of PKC with PMA induced formation of 5-HETE-PLs at similar levels to fMLP, lack of inhibition by GF 109203X showed that PKC is not involved when fMLP is the stimulus (Figure 6C). Thus, the involvement of a coordinated signaling cascade, acutely activated during infection, underscores the idea that formation of these lipids is a pathophysiologically relevant event.
The participation of cPLA2, FLAP, and lysophospholipid acyltransferases implicate free arachidonate, its transfer to the 5-LOX active site, and re-esterification after its oxidation in the mechanism of 5-HETE-PL formation (Figure 6). Although 5-LOX, FLAP, and cPLA2 are located at nuclear membrane in activated cells, 5-HETE-PC and -PE were found at both nuclear and extranuclear membranes (Figure 5).26,27 This suggests that either 5-HETE esterification occurs at other sites or that 5-HETE-PLs can be trafficked after their generation. The fast temporal generation of 5-HETE-PLs (within 2 minutes) indicates that formation and esterification of 5-HETE is a tightly regulated event with esterification occurring as fast as generation of the free acid. This is similar to arachidonate reacylation, known to occur on the same timescale as free eicosanoid formation in human neutrophils.28 5-HETE-d8-PE and -PC did not form after incubation with exogenous 5-HETE-d8 (not shown). This suggests a very tight association between PLA2, LOX, and re-esterification pathways within the cell, such that the 5-HETE pool that is destined for esterification is probably distinct from that forming free 5-HETE. This is similar to previous observations for 12-LOX–derived esterified lipids generated by human platelets.9,29 In addition, a previous report showed that, although 15-HETE is avidly esterified into neutrophil PLs, little 5-HETE is incorporated.13 However, we note that these results were obtained with in vitro approaches, and in the future, if it becomes possible to determine the fate of endogenous 5-HETE in vivo, different results may be obtained.
Herein, we found that 5-LOX inhibition enhanced, whereas HETE-PE supplementation inhibited formation of NETs by neutrophils (Figure 8A-D,G-L). These are weblike structures comprising an extracellular scaffold of DNA and harboring neutrophil antimicrobial proteins of granular and nuclear origin that enhance the ability of the cells to trap bacteria in the circulation.30 They are believed to limit both microbial spread and associated damage from neutrophil granular contents.30,31 The processes of NET formation are not well characterized, but profound membrane alterations, including dissolving of nuclear and granular membranes, then rupture of the plasma membrane, occur.30 Thus, 5-HETE-PLs would be ideally placed to influence DNA release through such mechanisms as influencing fluidity and permeability of the membranes directly or altering activity of integral membrane proteins. Because we found that the monocyte-derived analog 15-HETE-PE could also inhibit NET formation (Figure 8D), the mechanism is probably common to structurally related oxidized PLs. Recent studies from our group and others have shown that oxidized lipids, including HETE-PEs and HETE-PCs, inhibit TLR4 signaling most probably through competing with LPS for binding to CD14 and LPS-binding protein.8 Similarly, that effect was common to several structurally related oxidized PLs. The observation that > 1 HETE-PE can also attenuate NET formation may suggest a common mode of action, for example, competing with an endogenous PL for binding to a protein involved in the process. Further work will be required to determine the exact mechanisms involved.
Amounts of HETE-PE added in vitro (3.9 μg/106 cells) that attenuated NET formation were higher than HETE-PL detected in vivo in peritonitis. However, there are several caveats. First, HETE-PLs are poorly taken up by cells (eg, in monocytes; only ∼ 5% over 24 hours; V.B.O., unpublished data, July 2008). Second, the membrane localization of HETE-PLs is likely to lead to a much greater local concentration than estimated by our assays. Enhancement of NET formation by the 5-LOX inhibitors MK886 and zileuton strongly supports the idea that endogenously generated 5-HETE-PLs regulate NET formation (Figure 8A-B).
5-HETE-PE selectively increased IL-8 but not TNFα generation. (supplemental Figure 4A-D). As stated earlier, 15-HETE-PE attenuates LPS induction of TNFα generation by human monocytes.8 However, monocyte experiments were conducted over far longer time periods (24 hours) and with higher serum concentrations (10%) (thus supplementing levels of CD14 and LPS-binding protein). Herein, we chose the shorter incubation times previously described for cytokine generation by neutrophils, because these cells tend to undergo apoptosis later on and thus may not be viable ex vivo by 24 hours.32 This may partially explain why a different effect was observed in neutrophils. Unexpectedly, free 5-HETE totally abolished IL-8 or TNFα generation (supplemental Figure 4C-D), suggesting that free and esterified forms of this lipid act differently to regulate neutrophil functions. Both 5-HETE-PE and 5-HETE (but not 15-HETE-PE or SAPE) significantly enhanced superoxide generation, indicating an involvement of the 5-HETE functional group (Figure 8E-F; supplemental Figure 4G-H). Stimulation of NADPH by 5-HETE is in agreement with a previous report.33 In summary, the studies show that 5-HETE-PE can modulate NET formation, IL-8, and superoxide generation, while having no effect on degranulation or TNFα. Some actions were shared with free 5-HETE (superoxide), whereas others were similar to additional oxidized PE species (NETs).
HETE-PLs appear to have specific effects on the neutrophil, regulating NETs and superoxide but not degranulation. Studies on 5-LOX−/− mice have found that deficiency of the enzyme reduces the ability to mount an effective antimicrobial response.34 This may suggest a protective effect of 5-HETE-PLs; however, other 5-LOX products, including leukotrienes and lipoxins, also modulate leukocyte migration and will influence outcome. Free-acid products of the 5-LOX pathway stimulate killing of bacteria. Both 5-HETE and leukotriene B4 are chemoattractant for neutrophils, whereas leukotriene B4 augments neutrophil phagocytosis in vitro and in vivo.2,3,35 Thus, further work is required to determine whether these new lipids play a promicrobial or antimicrobial role in vivo. Of note, we recently found that related lipids generated by monocytes or platelets also can influence plasma membrane-localized events, including coagulation and TLR4 signaling.8,9 This suggests that PL-esterified eicosanoids generated by immune cells may commonly act in an autocrine manner to regulate membrane biology during acute activation, in particular relating to innate immune responses.
Monocyte/macrophage and platelet LOXs were recently observed to generate complex lipids comprising 15- or 12-HETE attached to PLs.8-10 They form acutely and can regulate cytokine generation and coagulation at the plasma membrane, indicating that they represent new families of bioactive eicosanoid-related complex lipids.8,9 These neutrophil lipids are structurally related; however, there are several key differences. Monocytes/macrophages generate HETE-PEs, but no PCs, and either 12S- or 15S-HETE positional isomers, whereas platelets generate exclusively 12S-HETE-PLs in response to thrombin.8-10 This is consistent with the positional specificity of the LOX isoform expressed, with neutrophils instead generating exclusively 5-HETE–containing isomers. Furthermore, the temporal generation of 5-HETE-PE in murine peritonitis (vs 12-HETE-PE) and its presence in human samples in the absence of nonenzymatic isomers (8-, 11-HETE-PE) further support the idea that these lipids are generated by LOX isoforms (Figure 7).8 The differences in structure, agonist activation pathways, and temporal generation may suggest distinct biologic actions for HETE-PL families generated by different immune cell populations, similar to previous observations for free acid HETE, although common actions such as on NET formation and TLR signaling are already indicated.36,37
PLs are the main structural constituents of mammalian cell membranes where they provide a permeability barrier and participate in a diverse range of cell functions, including motility, secretion, internalization, and signaling. PL peroxidation was for many years assumed to be an uncontrolled pathologic event occurring in vascular disease and inflammation.38,39 Our study shows receptor/agonist stimulation of 4 new lipids comprising 5-HETE attached to specific PL molecular species and their in vivo detection in human and murine infection. The studies indicate that 18:0p/5-HETE-PE, 18:1p/5-HETE-PE, 16:0p/5-HETE-PE, and 16:0a/5-HETE-PC generated by neutrophils are an important addition to the growing family of lipids generated in vivo by LOXs that may regulate host-pathogen responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Martin Davey and Chan-Yu Lin for processing patient samples, and Sally Campbell for helpful suggestions regarding microscopy.
This work was supported by grants from the Wellcome Trust, the Welsh Assembly Government, the British Lung Foundation, Baxter Healthcare, and European Union FP7.
Wellcome Trust
Authorship
Contribution: S.R.C. and V.B.O. designed research; S.R.C., A.P.K.-M., V.J.H., C.J.G., M.J.S., C.P.T., and B.C. performed research; G.W.R. and M.E. contributed new reagents; S.A.J., N.T., P.R.T., and S.K. gave intellectual input; S.R.C. and V.B.O. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Valerie B. O'Donnell, Department of Infection, Immunity, and Biochemistry, School of Medicine, Cardiff University, CF14 4XN, Cardiff, United Kingdom; e-mail: o-donnellvb@cardiff.ac.uk.