Abstract
The aim of this retrospective cohort study was to analyze the impact of surgery on the outcomes and qualities of life (QOL) in patients with intestinal diffuse large B-cell lymphoma (DLBCL). We assessed 345 patients with either localized or disseminated intestinal DLBCL and compared them according to treatment: surgical resection followed by chemotherapy versus chemotherapy alone. In patients with localized disease (Lugano stage I/II), surgery plus chemotherapy yielded a lower relapse rate (15.3%) than did chemotherapy alone (36.8%, P < .001). The 3-year overall survival rate was 91% in the surgery plus chemotherapy group and 62% in the chemotherapy-alone group (P < .001). The predominant pattern in the chemotherapy group was local relapse (27.6%). When rituximab was used with cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP), there was no improvement of the outcomes in patients treated with primary surgical resection. The QOL of patients who underwent surgery and chemotherapy was lower than chemotherapy alone, but its difference was acceptable. Multivariate analysis showed that surgical resection plus chemotherapy was an independent prognostic factor for overall survival. Surgical resection followed by chemotherapy might be an effective treatment strategy with acceptable QOL deterioration for localized intestinal DLBCL. This study was registered at www.clinicaltrials.gov as #NCT01043302.
Introduction
The small and large intestines are the second most common site of primary gastrointestinal (GI) lymphomas, and diffuse large B-cell lymphomas (DLBCL) account for most primary intestinal lymphomas.1-3 Considerable information on primary gastric DLBCL is available, but there are insufficient data on the clinical features and outcomes of primary intestinal DLBCL because it is usually studied as a subgroup of GI lymphomas.1,3-5 Although a few reports focusing on primary intestinal DLBCL have been published,6-9 the sample sizes were small, and the optimal treatment strategies specific for primary intestinal DLBCL have not been established. Various treatment approaches have been tried, such as systemic chemotherapy similar to that used to treat nodal DLBCL and primary surgical resection of intestinal lesions and postoperative chemotherapy.6,10,11 However, the treatment of primary intestinal DLBCL is a controversial issue because none of the published studies was randomized and these studies used mainly Cytoxan, hydroxyrubicin, Oncovin, and prednisone (CHOP) as an adjuvant chemotherapy. The combined treatment of rituximab with CHOP (R-CHOP) is effective for treating nodal DLBCL,12 and R-CHOP might improve the outcome of primary intestinal DLBCL. Considering the probability of surgery-related morbidity and subsequent deterioration of quality of life (QOL), the role of surgery remains to be determined in the era of rituximab. No reports have compared the 2 treatment strategies (surgical resection plus chemotherapy and chemotherapy) in a large study population using rituximab. We performed a multicenter study to analyze the impact of surgical resection on the outcomes and QOL of patients and the role of rituximab in the treatment of intestinal DLBCL.
Methods
Study design and patients
We performed a retrospective cohort study to assess the clinical features and outcomes of primary intestinal DLBCL. The clinical data of 345 patients diagnosed with primary intestinal DLBCL between 1993 and 2009 were gathered from 16 hospitals affiliated with the Consortium for Improving Survival of Lymphoma, a Korean lymphoma study group. Pathologic diagnosis was performed by expert pathologists in lymphoma in each hospital according to the Revised European-American Lymphoma or the World Health Organization classifications. The best method for discriminating primary intestinal DLBCL from systemic DLBCL involving the intestine is not clear. Patients who presented with predominant intestinal lesions were classified as having primary intestinal DLBCL according to the definition proposed in previous reports.13-15
The imaging studies for staging workup were chest and abdomen-pelvis computerized tomography scans. Not all patients underwent colonoscopy and esophagogastroduodenoscopy at baseline as a part of the staging evaluation. Patients were staged according to the Lugano staging system specified for GI lymphomas.16 Stage I is defined as disease confined to the intestine, stage II is defined as disease extending to local (II-1) or distant (II-2) nodes, stage II-E is defined as disease involving adjacent organs or tissues, and stage IV is defined as disseminated extranodal involvement or concomitant supradiaphragmatic lymph node involvement. Although the main analysis was based on a retrospective cohort of primary intestinal DLBCL, we also performed a concurrent cross-sectional survey of the QOL of surviving patients from this retrospective cohort at the time of the study. We surveyed the QOL of 75 patients who had completed their treatment in one of 8 hospitals. At the time of QOL survey, all survey participants had completed their treatment and were in a state of complete response. They provided written informed consent before answering the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire.17 They answered the questionnaire in reference to their current state.
Analysis of clinical features and outcomes
The treatment strategy was decided by the investigators at each participating center, except for surgical conditions that needed urgent surgery, such as perforation or obstruction. We separated patients into 2 groups according to treatment: surgical resection followed by chemotherapy (surgery/chemotherapy group) and chemotherapy alone (chemotherapy group) according to the primary treatment strategy. Surgical resection of a primary tumor mass, such as hemicolectomy or segmental resection and anastomosis, was performed with or without lymph node dissection. The extent of lymph node dissection varied between participating centers and included regional lymph node dissection equivalent to colon adenocarcinoma or resection only of lymph nodes with suspected lymphoma involvement. We compared the clinical features of the 2 groups, including the Eastern Cooperative Oncology Group (ECOG) performance status, serum lactate dehydrogenase concentration, site of involvement, the International Prognostic Index (IPI), Lugano stage, and the presence of B symptoms. Because intestinal involvement itself can cause weight loss, weight loss without fever and night sweats was not considered as a B symptom. Bulky disease was defined as the maximum diameter of a mass larger than 10 cm. To simplify the analysis of response data, the response was defined according to the World Health Organization criteria as follows.18 Complete response (CR) was designated as the disappearance of all lesions and absence of any new tumor lesions. Partial response was defined as a decrease of more than or equal to 50% in each lesion. Progressive disease or relapse was defined as the presence of a newly developed lesion or more than 25% increase in the product of 2 diameters of at least one tumor. Stable disease was defined as the state of neither partial response nor progressive disease.
Cross-sectional assessment of QOL
We compared the QOL of the surgery/chemotherapy and chemotherapy groups. Linear regression analysis was used to analyze the lymphoma-related factors, such as IPI, serum lactate dehydrogenase concentration, B symptoms, involved sites, stage, and patient-related factors, such as age at diagnosis, survival time, and ECOG performance status, to exclude possible confounding factors that can affect QOL. Because we hypothesized that surgery might decrease the patients' QOL, we used a noninferior test with a margin for acceptance of noninferiority as 15% of the difference. Thus, if the lower limit of the 95% confidence interval was within the margin of 15%, the QOL of patients treated with surgery plus chemotherapy was interpreted as not inferior to that of the patients treated with chemotherapy.
Statistical analysis
The χ2 test was used to evaluate the relationships between clinical features and outcomes. Overall survival (OS) was calculated from the date of diagnosis to the date of the final follow-up or death from any cause. Progression-free survival (PFS) was from the date of diagnosis to the date of disease progression, relapse, or death from any cause. Survival was estimated from Kaplan-Meier curves and compared using the log-rank test. The Cox proportional hazard regression model was used in the multivariate analysis to identify prognostic factors. A 2-sided P value less than .05 was considered significant. The institutional review board of each hospital approved this study, and it was registered at www.clinicaltrials.gov as #NCT01043302.
Results
Characteristics of patients
The characteristics of the 345 enrolled patients at diagnosis are summarized in Table 1. The median follow-up duration was 37.7 months (95% confidence interval, 30.19-45.15 months). The median age was 57 years (range, 15-92 years), and the male-to-female ratio was 1.63:1. Most patients had good performance status (≤ ECOG grade 0/1, 83.2%) and localized disease (Lugano stage I/II, 77.1%) usually involving the ileocecal area. Thus, the IPI risk was mainly low or low intermediate (75.0%).
Characteristics of patients (n = 345)
| Characteristic/category . | No. . | % . |
|---|---|---|
| Age, y | ||
| Median (range) | 57 (15-92) | |
| ≤ 60 | 204 | 59.1 |
| > 60 | 141 | 40.9 |
| Sex | ||
| Male | 214 | 62.0 |
| Female | 131 | 38.0 |
| Performance status | ||
| ECOG 0/1 | 287 | 83.2 |
| ECOG ≥ 2 | 58 | 16.8 |
| Serum LDH level | ||
| Normal | 206 | 59.7 |
| Increased | 133 | 38.6 |
| Missing | 6 | 1.7 |
| B symptoms | ||
| Absent | 279 | 80.9 |
| Present | 65 | 18.8 |
| Missing | 1 | 0.3 |
| Extranodal involvements | ||
| < 2 | 267 | 77.4 |
| ≥ 2 | 78 | 22.6 |
| IPI | ||
| Low/low to intermediate | 204/55 | 59.1/15.9 |
| High to intermediate/high | 53/27 | 15.4/7.8 |
| Missing | 6 | 1.7 |
| Lugano stage | ||
| I | 78 | 22.6 |
| II-1 | 106 | 30.7 |
| II-2 | 68 | 19.7 |
| II-E | 14 | 4.1 |
| IV | 79 | 22.9 |
| Bulky disease | ||
| Bulky | 73 | 21.2 |
| Nonbulky | 272 | 78.8 |
| Bone marrow invasion | ||
| Absent | 306 | 88.7 |
| Present | 16 | 4.6 |
| Not evaluated | 23 | 6.7 |
| Location | ||
| Duodenum | 12 | 3.5 |
| Jejunum/ileum | 17/63 | 4.9/18.3 |
| Ileocecum/ascending colon | 175/37 | 50.7/10.7 |
| Transverse/descending colon | 11/3 | 3.2/0.9 |
| Rectosigmoid colon | 14 | 4.0 |
| Multiple sites | 13 | 3.8 |
| Treatment modality | ||
| Surgery/CTx | 188 | 54.5 |
| CTx | 139 | 40.3 |
| Surgery | 12 | 3.5 |
| No treatment | 6 | 1.7 |
| CTx regimen | ||
| CHOP | 134 | 38.8 |
| R-CHOP | 172 | 49.9 |
| Others | 21 | 6.1 |
| Characteristic/category . | No. . | % . |
|---|---|---|
| Age, y | ||
| Median (range) | 57 (15-92) | |
| ≤ 60 | 204 | 59.1 |
| > 60 | 141 | 40.9 |
| Sex | ||
| Male | 214 | 62.0 |
| Female | 131 | 38.0 |
| Performance status | ||
| ECOG 0/1 | 287 | 83.2 |
| ECOG ≥ 2 | 58 | 16.8 |
| Serum LDH level | ||
| Normal | 206 | 59.7 |
| Increased | 133 | 38.6 |
| Missing | 6 | 1.7 |
| B symptoms | ||
| Absent | 279 | 80.9 |
| Present | 65 | 18.8 |
| Missing | 1 | 0.3 |
| Extranodal involvements | ||
| < 2 | 267 | 77.4 |
| ≥ 2 | 78 | 22.6 |
| IPI | ||
| Low/low to intermediate | 204/55 | 59.1/15.9 |
| High to intermediate/high | 53/27 | 15.4/7.8 |
| Missing | 6 | 1.7 |
| Lugano stage | ||
| I | 78 | 22.6 |
| II-1 | 106 | 30.7 |
| II-2 | 68 | 19.7 |
| II-E | 14 | 4.1 |
| IV | 79 | 22.9 |
| Bulky disease | ||
| Bulky | 73 | 21.2 |
| Nonbulky | 272 | 78.8 |
| Bone marrow invasion | ||
| Absent | 306 | 88.7 |
| Present | 16 | 4.6 |
| Not evaluated | 23 | 6.7 |
| Location | ||
| Duodenum | 12 | 3.5 |
| Jejunum/ileum | 17/63 | 4.9/18.3 |
| Ileocecum/ascending colon | 175/37 | 50.7/10.7 |
| Transverse/descending colon | 11/3 | 3.2/0.9 |
| Rectosigmoid colon | 14 | 4.0 |
| Multiple sites | 13 | 3.8 |
| Treatment modality | ||
| Surgery/CTx | 188 | 54.5 |
| CTx | 139 | 40.3 |
| Surgery | 12 | 3.5 |
| No treatment | 6 | 1.7 |
| CTx regimen | ||
| CHOP | 134 | 38.8 |
| R-CHOP | 172 | 49.9 |
| Others | 21 | 6.1 |
LDH indicates lactate dehydrogenase; and CTx, chemotherapy.
More than half of the patients underwent primary surgical resection, and the main chemotherapy included 6 cycles of CHOP or R-CHOP. Surgical resection was performed via open laparotomy, and no patient required permanent or temporary colostomy because most lesions were around the ileocecum/ascending colon. In the surgery/chemotherapy group, the median interval between surgery and chemotherapy was 19.5 days (range, 14-42 days), although it varied between centers. Six patients refused treatment and were lost to follow-up early after diagnosis. Twelve patients underwent only surgery because they refused further chemotherapy because of old age or poor performance status. Among these 12 patients, 10 relapsed or died, and only 2 patients with stage I disease survived to the time of analysis. Because these 18 patients did not fit one of the 2 strategies we chose to assess (surgery/chemotherapy or chemotherapy alone), we excluded them from the treatment outcome analysis. Thus, the treatment outcomes of 327 patients were compared according to treatment strategy: surgery/chemotherapy versus chemotherapy alone.
Treatment outcome of localized disease
The surgery/chemotherapy group had more patients with a lower IPI risk than did the chemotherapy group (P = .006, Table 2). The number of patients with Lugano stage I and II-1 was nonsignificantly greater in the surgery/chemotherapy group, although this was of marginal significance (P = .051, Table 2). The number of patients with bulky disease was significantly higher in the surgery/chemotherapy group (P < .001, Table 2). The number of patients who received radiotherapy after chemotherapy was higher in the chemotherapy group, although only a limited number (n = 12, 13.8%) of patients received radiotherapy. Other characteristics did not differ significantly between the 2 groups (Table 2).
Comparison of patients based on treatment strategy (n = 327)*
| . | Lugano I/II . | Lugano IV . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Surgery/CTx, no. (%; n = 163) . | CTx, no. (%; n = 87) . | P . | Surgery/CTx, no. (%; n = 25) . | CTx, no. (%; n = 52) . | P . | |||||
| Age, y | ||||||||||
| ≤ 60 | 109 | (66.9) | 49 | (56.3) | .130 | 13 | (52.0) | 25 | (48.1) | .810 |
| > 60 | 54 | (33.1) | 38 | (43.7) | 12 | (48.0) | 27 | (51.9) | ||
| Performance status | ||||||||||
| ECOG 0/1 | 146 | (89.6) | 79 | (90.8) | .828 | 18 | (72.0) | 33 | (63.5) | .608 |
| ECOG ≥ 2 | 17 | (10.4) | 8 | (9.2) | 7 | (28.0) | 19 | (36.5) | ||
| Lugano stage | ||||||||||
| I | 49 | (30.1) | 20 | (23.0) | .051 | |||||
| II-1 | 70 | (42.9) | 30 | (34.5) | ||||||
| II-2 | 35 | (21.5) | 33 | (37.9) | ||||||
| II-E | 9 | (5.5) | 4 | (4.6) | ||||||
| IPI | ||||||||||
| Low | 134 | (83.2) | 57 | (65.5) | .006 | 1 | (4.0) | 4 | (7.7) | .239 |
| Low to intermediate | 20 | (12.4) | 21 | (24.1) | 6 | (24.0) | 5 | (9.6) | ||
| High to intermediate | 7 | (4.3) | 9 | (10.3) | 12 | (48.0) | 22 | (42.3) | ||
| High | 0 | (0.0) | 0 | (0.0) | 6 | (24.0) | 21 | (40.4) | ||
| Missing | 2 | |||||||||
| Primary mass size | ||||||||||
| Bulky | 45 | 4 | < .001 | 13 | 8 | .002 | ||||
| Nonbulky | 118 | 83 | 12 | 44 | ||||||
| Reason for surgery | ||||||||||
| Mass resection | 98 | (60.1) | 14 | (56.0) | ||||||
| Obstruction | 51 | (31.3) | 8 | (32.0) | ||||||
| Bleeding | 7 | (4.3) | 1 | (4.0) | ||||||
| Perforation | 7 | (4.3) | 2 | (8.0) | ||||||
| Relapse/progression | 4 | (4.6) | ||||||||
| Perforation after CTx | 4 | (4.6) | 2 | (3.8) | ||||||
| Other complications after CTx | 4 | (4.6) | 1 | (1.9) | ||||||
| Chemotherapy regimen | ||||||||||
| CHOP | 69 | (42.3) | 45 | (51.7) | 0.243 | 5 | (20.0) | 15 | (28.8) | .695 |
| R-CHOP | 87 | (53.4) | 36 | (41.4) | 17 | (68.0) | 33 | (63.5) | ||
| Others | 7 | (4.3) | 6 | (6.9) | 3 | (12.0) | 4 | (7.7) | ||
| Radiotherapy | ||||||||||
| Done | 4 | (2.5) | 12 | (13.8) | .001 | 3 | (12.0) | 7 | (13.5) | > .99 |
| Not done | 159 | (97.5) | 75 | (86.2) | 22 | (88.0) | 45 | (86.5) | ||
| Response | ||||||||||
| CR | 139 | (85.3) | 56 | (64.4) | < .001 | 13 | (52.0) | 24 | (46.2) | .971 |
| PR | 2 | (1.2) | 13 | (14.9) | 5 | (20.0) | 13 | (25.0) | ||
| SD | 2 | (1.2) | 4 | (4.6) | 1 | (4.0) | 2 | (3.8) | ||
| PD | 8 | (4.9) | 9 | (10.3) | 3 | (12.0) | 8 | (15.4) | ||
| NE | 12 | (7.4) | 5 | (5.7) | 3 | (12.0) | 5 | (9.6) | ||
| Relapse/progression | ||||||||||
| Local | 14 | (8.6) | 24 | (27.6) | < .001 | 5 | (20.0) | 6 | (11.5) | .454 |
| Systemic | 11 | (6.7) | 8 | (9.2) | 5 | (20.0) | 16 | (30.8) | ||
| None | 138 | (84.7) | 55 | (63.2) | 15 | (60.0) | 30 | (57.7) | ||
| Survival | ||||||||||
| Alive | 143 | (87.7) | 49 | (56.3) | < .001 | 16 | (64.0) | 23 | (44.2) | .409 |
| Unknown† | 8 | (4.9) | 7 | (8.0) | 1 | (4.0) | 4 | (7.7) | ||
| Dead | 12 | (7.4) | 31 | (35.6) | 8 | (32.0) | 25 | (48.1) | ||
| 3-y PFS | 82% | 52% | < .001 | 52% | 34% | .518 | ||||
| 3-y OS | 91% | 62% | < .001 | 58% | 44% | .303 | ||||
| . | Lugano I/II . | Lugano IV . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Surgery/CTx, no. (%; n = 163) . | CTx, no. (%; n = 87) . | P . | Surgery/CTx, no. (%; n = 25) . | CTx, no. (%; n = 52) . | P . | |||||
| Age, y | ||||||||||
| ≤ 60 | 109 | (66.9) | 49 | (56.3) | .130 | 13 | (52.0) | 25 | (48.1) | .810 |
| > 60 | 54 | (33.1) | 38 | (43.7) | 12 | (48.0) | 27 | (51.9) | ||
| Performance status | ||||||||||
| ECOG 0/1 | 146 | (89.6) | 79 | (90.8) | .828 | 18 | (72.0) | 33 | (63.5) | .608 |
| ECOG ≥ 2 | 17 | (10.4) | 8 | (9.2) | 7 | (28.0) | 19 | (36.5) | ||
| Lugano stage | ||||||||||
| I | 49 | (30.1) | 20 | (23.0) | .051 | |||||
| II-1 | 70 | (42.9) | 30 | (34.5) | ||||||
| II-2 | 35 | (21.5) | 33 | (37.9) | ||||||
| II-E | 9 | (5.5) | 4 | (4.6) | ||||||
| IPI | ||||||||||
| Low | 134 | (83.2) | 57 | (65.5) | .006 | 1 | (4.0) | 4 | (7.7) | .239 |
| Low to intermediate | 20 | (12.4) | 21 | (24.1) | 6 | (24.0) | 5 | (9.6) | ||
| High to intermediate | 7 | (4.3) | 9 | (10.3) | 12 | (48.0) | 22 | (42.3) | ||
| High | 0 | (0.0) | 0 | (0.0) | 6 | (24.0) | 21 | (40.4) | ||
| Missing | 2 | |||||||||
| Primary mass size | ||||||||||
| Bulky | 45 | 4 | < .001 | 13 | 8 | .002 | ||||
| Nonbulky | 118 | 83 | 12 | 44 | ||||||
| Reason for surgery | ||||||||||
| Mass resection | 98 | (60.1) | 14 | (56.0) | ||||||
| Obstruction | 51 | (31.3) | 8 | (32.0) | ||||||
| Bleeding | 7 | (4.3) | 1 | (4.0) | ||||||
| Perforation | 7 | (4.3) | 2 | (8.0) | ||||||
| Relapse/progression | 4 | (4.6) | ||||||||
| Perforation after CTx | 4 | (4.6) | 2 | (3.8) | ||||||
| Other complications after CTx | 4 | (4.6) | 1 | (1.9) | ||||||
| Chemotherapy regimen | ||||||||||
| CHOP | 69 | (42.3) | 45 | (51.7) | 0.243 | 5 | (20.0) | 15 | (28.8) | .695 |
| R-CHOP | 87 | (53.4) | 36 | (41.4) | 17 | (68.0) | 33 | (63.5) | ||
| Others | 7 | (4.3) | 6 | (6.9) | 3 | (12.0) | 4 | (7.7) | ||
| Radiotherapy | ||||||||||
| Done | 4 | (2.5) | 12 | (13.8) | .001 | 3 | (12.0) | 7 | (13.5) | > .99 |
| Not done | 159 | (97.5) | 75 | (86.2) | 22 | (88.0) | 45 | (86.5) | ||
| Response | ||||||||||
| CR | 139 | (85.3) | 56 | (64.4) | < .001 | 13 | (52.0) | 24 | (46.2) | .971 |
| PR | 2 | (1.2) | 13 | (14.9) | 5 | (20.0) | 13 | (25.0) | ||
| SD | 2 | (1.2) | 4 | (4.6) | 1 | (4.0) | 2 | (3.8) | ||
| PD | 8 | (4.9) | 9 | (10.3) | 3 | (12.0) | 8 | (15.4) | ||
| NE | 12 | (7.4) | 5 | (5.7) | 3 | (12.0) | 5 | (9.6) | ||
| Relapse/progression | ||||||||||
| Local | 14 | (8.6) | 24 | (27.6) | < .001 | 5 | (20.0) | 6 | (11.5) | .454 |
| Systemic | 11 | (6.7) | 8 | (9.2) | 5 | (20.0) | 16 | (30.8) | ||
| None | 138 | (84.7) | 55 | (63.2) | 15 | (60.0) | 30 | (57.7) | ||
| Survival | ||||||||||
| Alive | 143 | (87.7) | 49 | (56.3) | < .001 | 16 | (64.0) | 23 | (44.2) | .409 |
| Unknown† | 8 | (4.9) | 7 | (8.0) | 1 | (4.0) | 4 | (7.7) | ||
| Dead | 12 | (7.4) | 31 | (35.6) | 8 | (32.0) | 25 | (48.1) | ||
| 3-y PFS | 82% | 52% | < .001 | 52% | 34% | .518 | ||||
| 3-y OS | 91% | 62% | < .001 | 58% | 44% | .303 | ||||
CTx indicates chemotherapy; PR, partial response; SD, stable disease; PD, progressive disease; and NE, not evaluable.
Among 345 patients, 12 patients had surgery only and 6 patients received no treatment.
The survival status was unknown because of follow-up loss. These patients were censored during the Kaplan-Meier survival analysis.
The most common reason for surgery was primary mass resection for both therapeutic and diagnostic purposes. Presentation as bowel perforation or bleeding was a rare cause for surgery, whereas bowel obstruction was a frequent cause. Among patients treated initially with chemotherapy, 8 underwent surgery because of chemotherapy-related complications, such as perforation (8 of 87, 9.2%), and their median onset time was 3 days after chemotherapy started (range, 2-7 days). Four patients underwent surgery because of relapse or progression during or after chemotherapy (4 of 87, 4.6%).
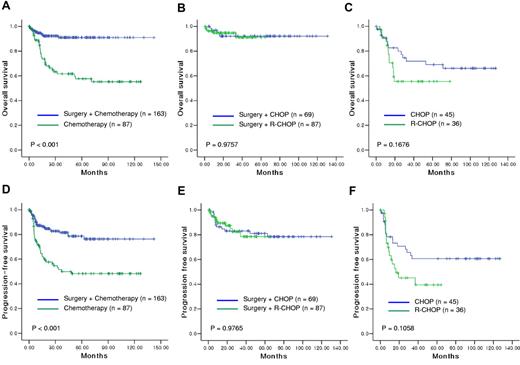
The CR rate was significantly higher in the surgery/chemotherapy group (85.3%) than in the chemotherapy group (64.4%, P < .001). The relapse rate was lower in the surgery/chemotherapy group (25 of 163, 15.3%) than in the chemotherapy group (32 of 87, 36.8%). Local relapse, defined as relapse from the primary site or adjacent sites including regional nodes, was the predominant pattern of relapse in the chemotherapy group (27.6%, Table 2). Thus, both the OS and PFS of the surgery/chemotherapy group were significantly better than those in the chemotherapy-alone group (Figure 1A,D). The 3-year OS was 91% in the surgery/chemotherapy group and 62% in the chemotherapy group (P < .001). In the surgery/chemotherapy group, OS and PFS were not related to Lugano staging (P > .05), but patients with Lugano stage IIE had a worse PFS in the chemotherapy group (P = .014). Because of the differences in IPI scores and Lugano stages between the surgery/chemotherapy group and the chemotherapy group, we performed a subgroup analysis to compare patients with localized disease according to IPI risk category and Lugano stage. In the subgroup analysis, the OS and PFS were significantly longer in the surgery/chemotherapy group than in the chemotherapy group regardless of IPI risk and Lugano stage category (P < .005).
Survival outcomes were compared in patients with localized disease according to the treatment strategy. Surgical resection followed by chemotherapy produced significantly longer OS (A) and PFS (D) than chemotherapy alone. In patients treated with primary surgical resection for localized disease, the postoperative chemotherapy regimen, CHOP or R-CHOP, did not significantly affect OS (B) or PFS (E). In patients treated with chemotherapy alone, OS (C) and PFS (F) did not differ between patients treated with CHOP or R-CHOP.
Survival outcomes were compared in patients with localized disease according to the treatment strategy. Surgical resection followed by chemotherapy produced significantly longer OS (A) and PFS (D) than chemotherapy alone. In patients treated with primary surgical resection for localized disease, the postoperative chemotherapy regimen, CHOP or R-CHOP, did not significantly affect OS (B) or PFS (E). In patients treated with chemotherapy alone, OS (C) and PFS (F) did not differ between patients treated with CHOP or R-CHOP.
Treatment outcome for disseminated disease
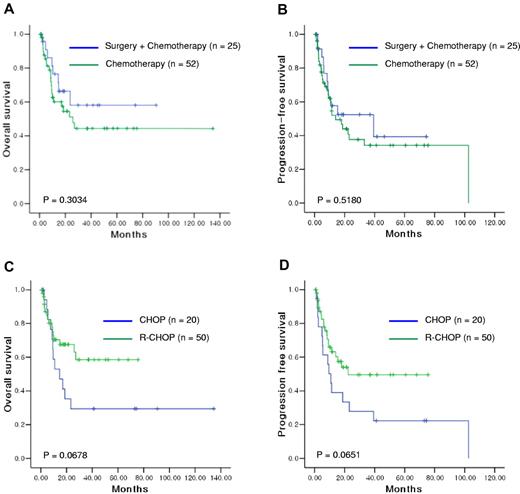
Of the patients with Lugano stage IV, 52 were treated with chemotherapy, and 7 of these patients received follow-up radiotherapy (Table 2). Although disease status was disseminated, 25 patients underwent surgery for diagnostic or therapeutic purposes. The characteristics were balanced between the 2 groups, and the response and relapse rates did not differ between the 2 groups (Table 2). The 3-year OS rate of disseminated disease was significantly less than that of localized disease (47% vs 80%). Comparison of OS and PFS according to the treatment strategy showed no significant differences between the 2 groups (P > .05, Figure 2A-B).
Comparison of survival outcomes in patients with disseminated disease according to the treatment strategy. OS (A) and PFS (B) did not differ between the surgery/chemotherapy and chemotherapy groups. However, comparison according to the use of rituximab showed longer OS (C) and PFS (D) in patients treated with R-CHOP than in those treated with CHOP.
Comparison of survival outcomes in patients with disseminated disease according to the treatment strategy. OS (A) and PFS (B) did not differ between the surgery/chemotherapy and chemotherapy groups. However, comparison according to the use of rituximab showed longer OS (C) and PFS (D) in patients treated with R-CHOP than in those treated with CHOP.
Impact of rituximab on the outcome
In patients with localized disease, 69 patients received CHOP chemotherapy, and 87 patients received R-CHOP after primary surgical resection. The median number of cycles was 6 in both regimens, and the characteristics at diagnosis were balanced between them. However, R-CHOP failed to show a survival benefit over CHOP (P > .05, Figure 1B,E). Thus, regardless of rituximab use, the 3-year OS was more than 90% in patients with localized disease treated with chemotherapy after surgery. In patients with localized disease treated with chemotherapy alone, OS and PFS did not differ significantly between CHOP (n = 45) and R-CHOP (n = 36) (P > .05, Figure 1C,F). Although a small number of patients underwent surgery (n = 25), there was no significant difference in survival between patients with disseminated disease treated with surgery plus CHOP or surgery plus R-CHOP (P > .05). However, when the OS and PFS were compared according to the use of rituximab in patients with disseminated disease, R-CHOP treatment showed an approaching statistical significance for longer OS and PFS than did CHOP treatment (P = .0678, and .0651, respectively; Figure 2C-D). Thus, the 3-year OS was higher in patients treated with R-CHOP (59%) compared with CHOP (29%).
Comparison of QOL
All participants answered the questionnaire in reference to their current QOL in the state of CR; at the time of the QOL survey, their median follow-up duration after CR was 32.3 months (range, 7.1-120.3 months). Thus, 64 survivors from 159 alive patients in the surgery/chemotherapy group participated in the QOL survey, whereas 11 survivors from 72 alive patients treated with chemotherapy alone participated (Table 2). When the mean scores of each parameter were compared between the 2 groups, the lower limit of 95% confidence interval more than 15% was designated as significant as described in “Cross-sectional assessment of QOL.” Among the parameters of functional scales, the physical, role, cognitive, and social functioning did not differ, and only the emotional functioning was worse in the surgery/chemotherapy group. Among the parameters of symptom scales, nausea, vomiting, appetite loss, and financial difficulties did not differ significantly between treatment groups. Although fatigue was inferior in patients receiving surgery based on the definition of inferiority, its difference in mean scores was only 1.67 points (Table 3). However, patients in the surgery/chemotherapy group were significantly inferior to chemotherapy alone in the scale of constipation, diarrhea, insomnia, and dyspnea. As a result, the mean score of global health status was lower in the surgery/chemotherapy group than chemotherapy group (Table 3).
Comparison of quality of life
| Parameter . | Mean score* . | 95% CI for difference (%)† . | Interpretation‡ . | ||
|---|---|---|---|---|---|
| Surgery/CTx (n = 64) . | Chemotherapy (n = 11) . | Upper limit . | Lower limit . | ||
| Functional scales | |||||
| Physical functioning | 73.53 | 74.01 | 11.59 | −12.55 | Not inferior |
| Role functioning | 77.76 | 75.86 | 16.64 | −12.84 | Not inferior |
| Emotional functioning | 78.27 | 87.99 | 3.44 | −22.83 | Inferior |
| Cognitive functioning | 78.64 | 79.19 | 11.44 | −12.56 | Not inferior |
| Social functioning | 79.84 | 73.44 | 23.86 | −11.06 | Not inferior |
| Symptom scales | |||||
| Fatigue | 47.90 | 46.23 | 13.34 | −16.67 | Inferior |
| Nausea and vomiting | 1.50 | 0.01 | 5.05 | −8.79 | Not inferior |
| Pain | 6.52 | 14.50 | 25.14 | −9.19 | Not inferior |
| Dyspnea | 18.01 | 7.35 | 8.31 | −29.63 | Inferior |
| Insomnia | 8.95 | 5.90 | 17.16 | −23.27 | Inferior |
| Appetite loss | 20.40 | 24.42 | 20.31 | −12.47 | Not inferior |
| Constipation | 17.24 | 10.51 | 8.07 | −21.55 | Inferior |
| Diarrhea | 38.73 | 19.87 | 1.82 | −39.55 | Inferior |
| Financial difficulties | 21.08 | 28.38 | 26.64 | −12.24 | Not inferior |
| Global health status | |||||
| Global health | 61.31 | 71.50 | 6.02 | −26.38 | Inferior |
| Parameter . | Mean score* . | 95% CI for difference (%)† . | Interpretation‡ . | ||
|---|---|---|---|---|---|
| Surgery/CTx (n = 64) . | Chemotherapy (n = 11) . | Upper limit . | Lower limit . | ||
| Functional scales | |||||
| Physical functioning | 73.53 | 74.01 | 11.59 | −12.55 | Not inferior |
| Role functioning | 77.76 | 75.86 | 16.64 | −12.84 | Not inferior |
| Emotional functioning | 78.27 | 87.99 | 3.44 | −22.83 | Inferior |
| Cognitive functioning | 78.64 | 79.19 | 11.44 | −12.56 | Not inferior |
| Social functioning | 79.84 | 73.44 | 23.86 | −11.06 | Not inferior |
| Symptom scales | |||||
| Fatigue | 47.90 | 46.23 | 13.34 | −16.67 | Inferior |
| Nausea and vomiting | 1.50 | 0.01 | 5.05 | −8.79 | Not inferior |
| Pain | 6.52 | 14.50 | 25.14 | −9.19 | Not inferior |
| Dyspnea | 18.01 | 7.35 | 8.31 | −29.63 | Inferior |
| Insomnia | 8.95 | 5.90 | 17.16 | −23.27 | Inferior |
| Appetite loss | 20.40 | 24.42 | 20.31 | −12.47 | Not inferior |
| Constipation | 17.24 | 10.51 | 8.07 | −21.55 | Inferior |
| Diarrhea | 38.73 | 19.87 | 1.82 | −39.55 | Inferior |
| Financial difficulties | 21.08 | 28.38 | 26.64 | −12.24 | Not inferior |
| Global health status | |||||
| Global health | 61.31 | 71.50 | 6.02 | −26.38 | Inferior |
The mean scores for each parameter. The higher scores of functional scales and global health status indicate better function and health status. The lower scores of symptom scales indicate that patients have fewer symptoms.
The 95% CI for difference between 2 groups adjusting covariates, such as IPI, serum LDH concentration, B symptoms, involved sites, stage, age at diagnosis, survival time, and ECOG performance status.
If the lower limit of the 95% CI is within the margin of 15%, the parameter of surgery plus chemotherapy can be interpreted as not inferior to that of chemotherapy.
Analysis of prognostic factors
Univariate analysis with binary variables showed that age more than 60 years, performance status more than or equal to ECOG grade 2, increased serum lactate dehydrogenase level, number of extranodal involvements more than or equal to 2, Lugano stage IV, high to intermediate/high IPI risk, and surgery/chemotherapy were prognostic indicators for OS (P < .05). However, multivariate analysis, which included all these factors and used IPI risk as a continuous variable, showed that only treatment strategy based on primary surgical resection followed by chemotherapy was an independent prognostic factor for OS (Table 4). When the multivariate analysis was performed separately for localized and disseminated disease, surgery/chemotherapy was significant only in patients with localized disease (Table 4).
Multivariate analysis of prognostic factors for OS
| Characteristic . | Lugano stage I/II . | Lugano stage IV . | Total . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P . | Hazard ratio . | 95% CI . | P . | Hazard ratio . | 95% CI . | P . | Hazard ratio . | 95% CI . | |
| Age, y | |||||||||
| ≤ 60 vs > 60 | .260 | 1.917 | 0.618-5.940 | .883 | 1.116 | 0.259-4.811 | .165 | 1.823 | 0.781-4.257 |
| Sex | |||||||||
| Male vs female | .871 | 0.938 | 0.433-2.032 | .348 | 0.620 | 0.228-1.683 | .550 | 0.836 | 0.466-1.502 |
| Performance status | |||||||||
| ECOG < 2 vs ≥ 2 | .206 | 2.969 | 0.549-16.048 | .514 | 1.572 | 0.405-6.107 | .178 | 1.983 | 0.732-5.375 |
| Serum LDH level | |||||||||
| Normal vs increased | .412 | 1.633 | 0.507-5.261 | .970 | 1.033 | 0.190-5.610 | .666 | 1.238 | 0.469-3.266 |
| B symptoms | |||||||||
| Absent vs present | .499 | 1.365 | 0.553-3.366 | .397 | 0.573 | 0.158-2.078 | .466 | 1.281 | 0.659-2.492 |
| No. of extranodal involvements | |||||||||
| < 2 vs ≥ 2 | .795 | 0.817 | 0.178-3.761 | .933 | 0.932 | 0.183-4.749 | .719 | 0.827 | 0.294-2.327 |
| Lugano stage | |||||||||
| I/II vs IV | .932 | 1.050 | 0.345-3.200 | ||||||
| IPI | |||||||||
| L vs LI vs HI vs H | .976 | 1.022 | 0.252-4.140 | .169 | 3.271 | 0.605-17.688 | .330 | 1.640 | 0.607-4.431 |
| Bone marrow | |||||||||
| Normal vs invasive | .514 | 2.130 | 0.220-20.615 | .090 | 0.340 | 0.098-1.182 | .392 | 0.639 | 0.229-1.783 |
| Treatment | |||||||||
| Surgery/chemotherapy vs chemotherapy | .001 | 3.963 | 1.772-8.865 | .667 | 0.761 | 0.220-2.636 | .002 | 2.845 | 1.453-5.568 |
| Chemotherapy regimen | |||||||||
| R-CHOP vs CHOP | .340 | 0.692 | 0.325-1.475 | .212 | 1.853 | 0.704-4.881 | .968 | 1.012 | 0.564-1.815 |
| Characteristic . | Lugano stage I/II . | Lugano stage IV . | Total . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P . | Hazard ratio . | 95% CI . | P . | Hazard ratio . | 95% CI . | P . | Hazard ratio . | 95% CI . | |
| Age, y | |||||||||
| ≤ 60 vs > 60 | .260 | 1.917 | 0.618-5.940 | .883 | 1.116 | 0.259-4.811 | .165 | 1.823 | 0.781-4.257 |
| Sex | |||||||||
| Male vs female | .871 | 0.938 | 0.433-2.032 | .348 | 0.620 | 0.228-1.683 | .550 | 0.836 | 0.466-1.502 |
| Performance status | |||||||||
| ECOG < 2 vs ≥ 2 | .206 | 2.969 | 0.549-16.048 | .514 | 1.572 | 0.405-6.107 | .178 | 1.983 | 0.732-5.375 |
| Serum LDH level | |||||||||
| Normal vs increased | .412 | 1.633 | 0.507-5.261 | .970 | 1.033 | 0.190-5.610 | .666 | 1.238 | 0.469-3.266 |
| B symptoms | |||||||||
| Absent vs present | .499 | 1.365 | 0.553-3.366 | .397 | 0.573 | 0.158-2.078 | .466 | 1.281 | 0.659-2.492 |
| No. of extranodal involvements | |||||||||
| < 2 vs ≥ 2 | .795 | 0.817 | 0.178-3.761 | .933 | 0.932 | 0.183-4.749 | .719 | 0.827 | 0.294-2.327 |
| Lugano stage | |||||||||
| I/II vs IV | .932 | 1.050 | 0.345-3.200 | ||||||
| IPI | |||||||||
| L vs LI vs HI vs H | .976 | 1.022 | 0.252-4.140 | .169 | 3.271 | 0.605-17.688 | .330 | 1.640 | 0.607-4.431 |
| Bone marrow | |||||||||
| Normal vs invasive | .514 | 2.130 | 0.220-20.615 | .090 | 0.340 | 0.098-1.182 | .392 | 0.639 | 0.229-1.783 |
| Treatment | |||||||||
| Surgery/chemotherapy vs chemotherapy | .001 | 3.963 | 1.772-8.865 | .667 | 0.761 | 0.220-2.636 | .002 | 2.845 | 1.453-5.568 |
| Chemotherapy regimen | |||||||||
| R-CHOP vs CHOP | .340 | 0.692 | 0.325-1.475 | .212 | 1.853 | 0.704-4.881 | .968 | 1.012 | 0.564-1.815 |
CI indicates confidence interval for hazard ratio; LDH, lactate dehydrogenase; L, low; LI, low to intermediate; HI, high to intermediate; and H, high.
Discussion
Primary intestinal DLBCL presents mainly as a localized disease, and the ileocecal area is the most frequently involved site.11,19 Our patients had mainly localized disease, especially Lugano stages I, II-1, and II-2 (73.0%). Thus, surgical resection of the primary mass followed by postoperative chemotherapy, CHOP, or R-CHOP was performed in more than two-thirds of patients with localized disease (n = 163, Table 2). This combined treatment strategy produced a 3-year OS rate of more than 90% (Figure 1). Although the IPI risk was lower in the surgery/chemotherapy group, the subgroup analysis by IPI risk category also revealed significantly better outcome in the surgery/chemotherapy group than in the chemotherapy group within the same IPI risk category (P < .05). This favorable outcome of surgery/chemotherapy is consistent with that observed in previous prospective studies reporting prolonged survival with a low relapse rate in intestinal B-cell lymphomas.10,11 Surgery plus chemotherapy showed a higher CR rate (85.3%) and a lower relapse rate (15.3%) than the CR (64.4%) and relapse (36.8%) rate for chemotherapy alone (P < .001). Local relapse was more frequent in the chemotherapy alone group. Although this was a retrospective study and data were not available regarding the decision of why patients with localized disease proceeded to surgery plus chemotherapy versus chemotherapy alone, these findings suggest that the better outcome in the surgery/chemotherapy group might be related to the complete resection of the bowel segment. Because it can be difficult to discriminate residual lesions in cases of bowel wall thickening, the underestimation of residual lesions might be another reason for the higher local relapse rate in the chemotherapy group.
In primary gastric DLBCL, the role of surgery has diminished, and treatment strategy has moved toward organ preservation because surgical resection is not superior to chemotherapy plus radiotherapy.20,21 The benefit of radiotherapy might be its curative potential for attacking low-grade lymphoma components because the presence of low-grade lymphoma components can contribute to local relapse.22 Unlike gastric DLBCL, the association of low-grade lymphoma components with the risk of relapse is still controversial in primary intestinal DLBCL; the presence of low-grade components is not consistent among previous studies of intestinal DLBCL.3,19,23 The intestine is less suitable for radiotherapy than is the stomach. Two specific problems with intestinal DLBCL (ie, difficulties in preoperative pathologic diagnosis and the risk of complications requiring surgery) also contribute to the widespread use of surgical resection for intestinal lymphomas. In this study, a substantial number of patients (n = 65, 39.9%) underwent surgery before diagnosis because of obstructions and other complications associated with intestinal lesions.
Surgical resection did not provide any significant benefit to patients with disseminated disease in our study. Thus, more effective chemotherapy may produce a better outcome for disseminated disease, such as nodal DLBCL. Comparison of the outcomes of CHOP and R-CHOP in these patients showed that R-CHOP had a longer 3-year OS (59%) compared with CHOP (29%), although the difference was not statistically significant (P = .0678, Figure 2C). These data suggest that R-CHOP is a better treatment for disseminated disease. However, the addition of rituximab to CHOP failed to show additional survival benefits in terms of localized disease, regardless of surgery (Figure 1B-C,E-F). This suggests that inclusion of rituximab in the chemotherapy regimen might not affect the outcome of localized disease as much as we expected. It also emphasizes the importance of surgical resection to the prognosis for patients with localized intestinal DLBCL, although postoperative chemotherapy is the accepted mainstay in the treatment of this disease.11,23 The value of rituximab cannot be determined before these findings are confirmed in a randomized prospective study.
Even though the role of surgery seemed to be important in the treatment of intestinal DLBCL, surgical resection of the primary mass has been a topic of debate because DLBCL itself is a medical disorder that can be cured by chemotherapy. Thus, the major concern about surgery is the deterioration of QOL. To address this issue, we assessed the QOL of 75 survivors who completed their treatment courses. In the comparison of symptom scales, patients who underwent surgery and chemotherapy were significantly inferior to chemotherapy alone in the scale of fatigue, constipation, diarrhea, insomnia, and dyspnea, whereas other GI tract-associated parameters, such as nausea and vomiting, and appetite loss were not significantly different (Table 3). The global health status of the surgery/chemotherapy group was also inferior to the chemotherapy group. However, the most functional scales did not differ significantly between the 2 groups. Thus, these results suggest that the surgery-associated deterioration of QOL may be acceptable, and the benefits of surgery plus chemotherapy may outweigh this negative effect of surgery on QOL considering the favorable outcome of surgery/chemotherapy.
However, a limitation of the QOL comparison in our study included the number of patients from the chemotherapy group was lower than the surgery/chemotherapy group, which may have biased the results. This imbalance of numbers may be in part associated with that the number of total (163 vs 87) as well as alive patients (159 vs 72) was much higher in the surgery/chemotherapy group than the chemotherapy group. Therefore, a prospective study assessing QOL in the future may help to clarify the effect of surgery on the QOL of patients in this clinical setting.
Multivariate analysis demonstrated that surgical resection followed by chemotherapy was an independent prognostic factor for OS (Table 4). Because most patients with Lugano stage I/II and low IPI risk underwent surgery, the prognostic value was diluted in the multivariate analysis. When the multivariate analysis was done separately for localized and disseminated disease, the prognostic value of surgery plus chemotherapy was significant only for localized disease (Table 4). These findings suggest that patients with localized disease who are medically fit to undergo surgery might have better outcomes.
In conclusion, surgical resection followed by chemotherapy might be recommended as an effective treatment strategy for localized intestinal DLBCL. Surgery-related deterioration of QOL might be an acceptable disadvantage of this strategy. Although the extent of surgery may be associated with the outcome of localized intestinal DLBCL, this issue should be addressed in a future prospective study. Our results also challenge the value of rituximab as a postoperative chemotherapy or primary chemotherapy in treating localized disease. However, this also needs to be defined in a prospective randomized study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Eliseo Guallar (Department of Environmental Health Sciences, Johns Hopkins Bloomberg School of Public Health) and Seonwoo Kim and Sookyoung Woo (Department of Biostatistics, Samsung Biomedical Research Institute) for insightful comments on the statistical analysis and study design.
This work was supported by the Korean Association of Hematology and the Consortium for Improving Survival of Lymphoma.
Authorship
Contribution: S.J.K. designed the research and wrote the manuscript; H.J. Kang, J.S.K., S.Y.O., C.W.C., S.I.L., J.H.W., M.K.K., J.H.K., Y.-C.M., J.-Y.K., J.M.K., I.G.H., H.J. Kim, J.P., and S.O. recruited patients and recorded the clinical data; J.H. and Y.H.K. reviewed and provided the pathology data; and C.S. and W.S.K. designed the experiment and analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cheolwon Suh, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, 388-1 Pungnap-2dong, Songpa-gu, Seoul, 138-736, Korea; e-mail: csuh@amc.seoul.kr; and Won Seog Kim, Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 50 Irwon-dong, Gangnam-gu, Seoul 135-710 Korea; e-mail: wskimsmc@skku.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal