More than 45 years ago, in cells cultured from Burkitt lymphoma (BL), the first human tumor virus—Epstein-Barr virus (EBV)—was discovered. We know now that this human herpes virus is associated with an unusually wide range of different malignancies including Hodgkin and posttransplantation lymphomas as well as different carcinomas. The correlative link between this virus and common malignancies has fueled the field of viral tumorigenesis and revealed a rich biology of EBV's oncogenes but a systematic comparison of EBV-positive lymphomas and this virus' contribution to their transformed phenotype has been lacking.
In this issue of Blood, Vereide and Sugden counterselect EBV and then study different lymphoma cell lines upon EBV's induced loss.1 Their findings support an interesting novel perspective: few viral oncogenes including viral miRNAs likely drive cellular transformation in canonical, endemic BLs but other lymphomas have not evolved that far; their survival and growth still depend on several additional viral functions.
EBV is an unusual pathogen which infects quiescent cells, establishes a latent infection precluding virus de novo synthesis, protects the latently infected cells from apoptosis, and drives them to proliferate. EBV's viral DNA genome does not integrate into the host chromosome but is maintained extrachromosomally as several plasmid copies in the nucleus of the latently infected cell. Plasmid maintenance is an operational term that includes synchronous replication of viral plasmids during S phase as well as their nuclear retention during interphase and mitosis of the host cell.
Plasmid maintenance of EBV DNA relies on a viral protein, EBNA1, which is essential to tether the viral genomes to the machinery of the host cell. EBNA1 also acts as a transcription factor, regulating its own expression and 5 additional latent EBNA genes.2 Beyond the class of 6 EBNAs, 3 latent membrane proteins, numerous micro-RNAs (miRNAs), other noncoding RNA species, and, occasionally, the viral BHRF1 protein (a viral homologue of the large BCL-2 family), score as latent gene products.3-5
Extrachromosomal maintenance of the EBV's plasmid DNA comes at a cost because it can be lost from infected, proliferating cells and is only maintained efficiently if it provides them a selective advantage. Sugden and colleagues have engineered a conditional mutant of EBNA1, which is dominant-negative and mediates the induced loss of EBV genomes in EBV-positive cells at will.1 In this issue, Vereide and Sugden introduced this conditional EBNA1 mutant into members of 3 different classes of EBV-positive lymphomas: canonical BL lymphomas, which express only EBNA1, miRNAs, and other noncoding RNAs; noncanonical Wp-restricted BL lymphomas, which express additional viral latent genes such as BHRF1 and members of the EBNA3 gene family from the viral Wp promoter6 ; and posttransplantation lymphoproliferative disorder lymphomas (PTLDs), which can express all known latent genes of EBV.
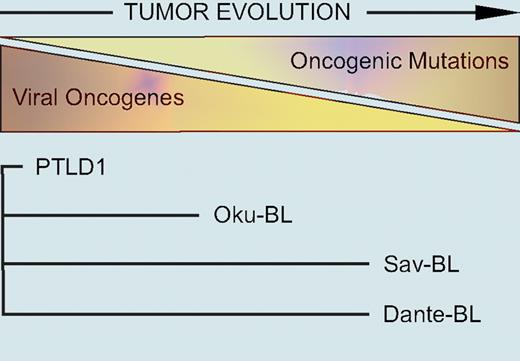
Vereide and Sugden study the resulting phenotypes and substitute cellular candidate genes, such as c-myc and bcl-xL, to compensate for EBV's induced loss. They find that cells derived from PTLDs entirely depend on EBV's functions: Wp-restricted noncanonical lymphoma cells are intermediate, and canonical BL cells are least affected by ridding the cells of EBV. The authors conclude that the dependence on EBV of the different classes of EBV-positive lymphoma correlates with the extent of viral gene expression. Their findings strongly suggest that viral gene expression in lymphomas evolves from in vivo selection for specific viral functions to support tumorigenesis and evade selective pressure imposed by the immune system (see figure). Thus, viral gene expression does not reflect different viral programs of latent gene expression as thought previously5 but the cells are selected in vivo to acquire compensating cellular mutations that reduce their dependence on the virus. Ultimately, EBV-negative B-cell lymphomas might stem from cells that have gone even further in vivo and evolved to become entirely independent of the virus.
A hypothesis for EBV-induced lymphomagenesis. EBV transforms B lymphocytes, providing cells with much potentially oncogenic information. However, the viral genes these EBV-positive “proto” tumor cells express are immunogenic, placing the cells under strong negative selection by the immune system. In response, tumor cells evolve to express fewer viral genes by gaining cellular mutations that replace the functions of viral oncogenes. Different tumor cells express distinct sets of latent viral genes reflecting their in vivo evolution away from dependence on the virus and toward dependence on cellular mutations. The lengths of the lines for each tumor cell line reflect the hypothesized extent of this evolution. See the complete figure in the article beginning on page 1977.
A hypothesis for EBV-induced lymphomagenesis. EBV transforms B lymphocytes, providing cells with much potentially oncogenic information. However, the viral genes these EBV-positive “proto” tumor cells express are immunogenic, placing the cells under strong negative selection by the immune system. In response, tumor cells evolve to express fewer viral genes by gaining cellular mutations that replace the functions of viral oncogenes. Different tumor cells express distinct sets of latent viral genes reflecting their in vivo evolution away from dependence on the virus and toward dependence on cellular mutations. The lengths of the lines for each tumor cell line reflect the hypothesized extent of this evolution. See the complete figure in the article beginning on page 1977.
It is clear now that EBV contributes selectively to the survival and proliferation of different stages of B-cell lymphomas. In canonical BL cells, the likely viral candidates are EBNA1, BHRF1, and EBV's noncoding RNAs. For example, EBNA1 regulates few viral genes and hundreds of cellular genes at the transcriptional level but its role in preventing apoptosis in EBV-positive lymphomas, which are the focus of this article, remains to be demonstrated.7,8
It is still debatable whether BHRF1, a viral homologue and antiapoptotic member of the BCL-2 family, is expressed in BL cells.3 The BHRF1 gene product has been found to interfere with the proapoptotic Bim protein, preventing apoptosis in newly infected human B cells, and is a likely viral contribution in all EBV-positive lymphomas.1
Interesting and promising candidates are EBV's miRNAs that may play a decisive role in lymphomagenesis. Presumably, they fine-tune the expression of many hundreds of cellular target genes with mostly unknown functions9 but a recent report suggests that this virus' 44 miRNAs might directly contribute to cellular survival, promotion of cell-cycle entry, and proliferation of human B cells invitro.10
The findings by Vereide and Sugden do not provide the ultimate explanations but the implication of their findings is clear. The induced loss of EBV from canonical BLs, which have progressed to depend on few viral genes only, will provide a promising assay to identify those genes that complement cellular survival and/or proliferation in the absence of viral functions. The smart approach by these authors has gone a long way in revealing this fundamental option, which will have important basic and clinical implications in the future.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal