Abstract
Lenalidomide combined with dexamethasone is an effective treatment for refractory/relapsed multiple myeloma (MM). Lenalidomide stimulates natural killer (NK) cells and enhances antitumor responses. We assessed NK cell number and function in 25 patients with MM participating in a clinical trial of lenalidomide and dexamethasone. NK cell numbers increased from a mean of 2.20 ± 0.05 × 105/mL (baseline) to a mean of 3.90 ± 0.03 × 105/mL (cycle 6; P = .05); however, in vitro NK-cell–mediated cytotoxicity decreased from 48.9% ± 6.8% to 27.6% ± 5.1% (P = .0028) and could not be rescued by lenalidomide retreatment. Lenalidomide increased normal donor NK-cell cytotoxicity in vitro from 38.5% to 53.3%, but this was completely abrogated by dexamethasone. Dexamethasone suppression of NK cell–mediated cytotoxicity was partially reversed by a 3-day washout, but these cells remained refractory to lenalidomide-induced enhanced function. Lymphocyte subset depletion experiments revealed that lenalidomide's enhancement of NK cell–mediated cytotoxicity was mediated by CD4+ T-cell production of interleukin 2 and that dexamethasone acted by suppressing interleukin-2 production. Similarly, the reduced ability of NK cells from patients with MM to respond to lenalidomide was also due to impaired CD4 T-cell function. Our findings indicate that lenalidomide immunostimulatory effects on patient NK cells are severely blunted by concurrent dexamethasone administration.
Introduction
Multiple myeloma (MM) is characterized by the uncontrolled proliferation of monoclonal plasma cells in the bone marrow (BM) and accumulation of monoclonal paraprotein in the serum of the majority of affected patients.1
There is evidence that MM, at least in its early stages, is under the control of innate and adaptive immune responses,2-9 which are ultimately subverted by the production of plasma cell and BM-derived immunosuppressive cytokines including interleukin 6 (IL-6), transforming growth factor β (TGF-β), insulin-like growth factor 1 (IGF-1), vascular endothelial growth factor (VEGF), and tumor necrosis factor α (TNF-α).10 The disease-associated immunoparesis of MM is further compounded by the immunosuppressive effects of anti-MM therapy.10
Conventional MM therapy has used alkylator or anthracycline-based chemotherapy in combination with the corticosteroids dexamethasone or prednisolone. Increasingly, nonchemotherapy agents such as the immunomodulatory (IMiD) drugs thalidomide and lenalidomide or the proteasome inhibitor Bortezomib have been incorporated into induction regimens commonly to replace chemotherapy agents, but are still used in combination with high-dose corticosteroids such as dexamethasone.11,12
The anti-MM effect of immunomodulatory drugs is thought to be mediated by the combined effects of tumor necrosis factor α inhibition, direct inhibition of plasma cell proliferation, suppression of angiogenesis, and promotion of T-cell costimulation,13-16 and disruption of adhesion between malignant plasma cells and BM stroma.17-19
Lenalidomide also shows substantial capacity to activate natural killer (NK) cell cytotoxicity and NK cell–driven antibody-dependent cell-mediated cytotoxicity (ADCC).20,21 In vitro studies have shown that peripheral blood mononuclear cells (PBMCs) are required for lenalidomide-induced NK cell function, and this is achieved indirectly through activation of nuclear factor of activated T cell 2 (NFAT2) in T cells.22
The NK-stimulatory capacity of lenalidomide is of particular interest, as NK cells show cytotoxicity against malignant plasma cells23 via a natural cytotoxic receptor (NCR)– and NKG2D-dependent mechanism.24 More recent studies show that NK cell–mediated killing of plasma cells is DNAX accessory molecule 1– (DNAM-1–), natural killer group 2D (NKG2D)–, and NKp46-dependent.8,25 Intriguingly, the anti-MM drugs bortezomib, melphalan, and doxorubicin all up-regulated NKG2D and DNAM-1 ligands on MM cells and rendered them more susceptible to NK cell–mediated killing.8 These findings suggest that part of the therapeutic efficacy of lenalidomide may be via the promotion of NK cell–mediated responses against malignant plasma cells.
Until recently, the impact of dexamethasone on lenalidomide-enhanced NK cell function had not been assessed. In normal donor PBMCs, dexamethasone antagonized the stimulatory capacity of lenalidomide on both NK cells and T cells in vitro.26 However, the effect of dexamethasone on lenalidomide activation of NK cells in patients with MM has not been specifically analyzed.
Here, we assessed the function of NK cells from patients with relapsed/refractory MM who have received combination lenalidomide and dexamethasone therapy. We show that while the numbers of circulating NK cells increase after treatment with lenalidomide and dexamethasone, the cytotoxic capacity of NK cells becomes progressively and severely impaired due to dexamethasone-induced suppression of IL-2 production from CD4+ T cells. In summary, this study provides the first direct evidence that dexamethasone therapy is profoundly antagonistic to the immunostimulatory capacity of lenalidomide in patients with MM.
Methods
Reagents
Lenalidomide was provided by Celgene and dissolved in dimethylsulphoxide (DMSO) to a stock concentration of 10mM and stored at −20°C. Dexamethasone was obtained at a stock concentration of 10mM.
Patient samples and processing
Ethics approval from the Peter MacCallum Cancer Centre human ethics committee was obtained for the acquisition of peripheral blood from normal donors and MM patients (Peter MacCallum Cancer Centre Ethics protocol number 07/04). As part of a prospective clinical phase 2 trial of low-dose lenalidomide (Revlite), patients with relapsed and refractory MM were treated with lenalidomide (Revlimid, Celgene), 15 mg per oral daily, on days 1-21, and dexamethasone, 20 mg by mouth daily, on days 1-4, 9-12, and 17-20 every 28 days. This trial specifically targeted older (> 65 years) patients or those with pre-existing renal impairment who are less likely to tolerate full-dose lenalidomide or dexamethasone, hence the lower dosing schedule than standard. After 4 treatment cycles, dexamethasone was reduced to 20 mg on days 1-4 every 28 days, and lenalidomide and dexamethasone treatment continued until disease progression. Details of the trials are available at www.clinicaltrials.gov.au. Anticoagulated whole blood (50 mL) was collected on day 1 of cycles 1 (baseline), 2, 4, 6, and 9. PBMCs were isolated and cryopreserved until use for phenotypic and functional studies.
Phenotypic analysis
PBMCs from MM patients or from healthy donors were resuspended in cold phosphate-buffered saline and stained with Alexa350-CD3, fluorescein isothiocyanate (FITC)–CD4, phycoerythrin-Cy7 (PECy7)-CD8, FITC-CD16, PerCpCy5.5-CD19, allophycocyanin (APC)-CD25, APC-Cy7-CD45, and PE-CD56 for 20 minutes on ice. The cells were then washed and analyzed on the LSR II fluorescence-activated cell sorter (FACS; BD Biosciences). To obtain total CD4 T (CD3+CD4+) and NK cell (CD3−CD16+CD56+) numbers per milliliter of peripheral blood, the percentage of CD4 T or NK cells was multiplied by the total lymphocyte count, which was established by the Advia Cell Count instrument (Bayer Diagnostics).
Cell cultures
For functional studies, PBMCs from MM patients or healthy donors were cultured in RPMI-1640 supplemented with 10% fetal calf serum and 20 U/mL of IL-2. Where specified, cells were also treated with either 10μM lenalidomide, 10μM dexamethasone, both lenalidomide and dexamethasone at 10μM, or the addition of high-dose IL-2 at 500 U/mL. The concentration of lenalidomide used was based on previous studies,22 and the concentration of dexamethasone used was based on our titration experiments (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All cells were harvested on day 3 and used for cytotoxicity assays. The NK cell–sensitive K562 cell line was cultured in RPMI-1640 and 10% fetal calf serum, while the MM cell line U266 was cultured in RPMI-1640 supplemented with 10% fetal calf serum, HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and Glutamax.
Chromium release assays
A standard 4-hour chromium release assay was used to assess NK cell function. Briefly, 2 × 106 target cells (K562 and U266) were labeled with 50 μCi 51Cr for 90 minutes at 37°C. The cells were washed 3× in RPMI-1640 and plated at 1 × 104 per 50 μL in 96-well V-shaped plates. Effector cells (either untreated or drug-treated PBMCs) were plated at 5 × 105 per 50 μL into wells containing targets, to constitute an effector-to-target ratio of 50:1. Where specified, effector-to-target ratios were diluted down to 25, 12.5, and 6.25. Target cells with only media were used for background death (spontaneous release), while target cells in 2M HCl were used for total lysis. The plates were incubated at 37°C for 4 hours, and then read on a beta counter instrument. Specific lysis was calculated as: ([sample well − spontaneous] / [total lysis − spontaneous]) × 100.
Sorting and receptor blockade
To determine which specific subset of T cells was responsible for mediating lenalidomide-induced NK cell activation, healthy donor PBMCs were stained by direct method with the following antibodies: anti-CD3 Pacific Blue, anti-CD4 PECy7, anti-CD8 APC Cy7, anti-CD16 FITC, and anti-CD56 PE. The cells were then sorted into CD4 T cells (CD3+CD4+), CD8 T cells (CD3+CD8+), and NK cells (CD3−CD16+CD56+) with purity greater than 99%. Each subset was then cultured alone or in combination with sorted NK cells (at 1:1 or 5:1 CD4:NK ratio) for 3 days as described in “Cell cultures” without any drug treatment or with 10μM lenalidomide. Where specified, sorted NK cells were preblocked with anti–IL-2 receptor-α antibody at 10 μg per 1 × 106 cells for 20 minutes at room temperature. The preblocked NK cells were then cocultured with CD4 T cells and 10μM lenalidomide. On day 3, the cells were used as effectors in chromium release assays described above.
Proliferation assay
Healthy donor PBMCs were treated with drug combinations described under the Cell cultures section for 3 days. At day 3, the cells were washed twice with culture media to remove drugs and then stained with 2.5μM carboxyfluorescein diacetate succinimidyl ester (CFSE) per 1 × 106 cells. The cells were subsequently stimulated with 1 μg muromonab-CD3 (or Orthoclone; OKT-3) and 0.5 μg CD28/49d per 1 × 106 cells for 5 days. At day 5, the stimulated cells were labeled with anti-CD3 Pacific Blue, anti-CD4 APC, anti-CD8 PerCP, anti-CD16 FITC, and anti-CD56 PE and resuspended in 200μL FACS flow count beads to determine absolute cell numbers per proliferation cycle on the LSR II FACS. For recovery experiments assessing the ability of PBMCs to proliferate after removing dexamethasone, PBMCs were treated with 10μM dexamethasone for 3 days. At day 3 the cells were washed twice to remove the drug, then cultured in media (RPMI-1640 with 20 U/mL IL-2) for a further 3 days, either untreated or with 10μM lenalidomide. On day 6, the cells were stimulated for 5 days as described above.
Cytokine bead array
Where indicated, supernatants from cell cultures were harvested and frozen at −80°C for cytokine bead array analysis via the Luminex-100 instrument (Luminex), according to the manufacturer's instructions. Briefly, 25 μL of supernatants were mixed with 25 μL of premixed cytokine beads (interferon [IFN]–γ, IL-2, IL-4, IL-6, and IL-10) in a 96-well membrane plate for 1 hour. The plates were then washed twice with wash buffer, and 25 μL of detection antibodies were added, followed by 25 μL of streptavidin-PE. The plates were read on the Luminex machine for cytokine levels and expressed as picograms per milliliter after standardization.
Statistical analysis
All data were analyzed using GraphPad Prism software Version 5 and calculated via the 2-tailed Student t test, where significance was defined as P ≤ .05. All experimental data are shown as mean ± SE in the text as well as in the figures.
Results
Myeloma patient NK cells increase in number but lose cytotoxic function after lenalidomide and dexamethasone therapy
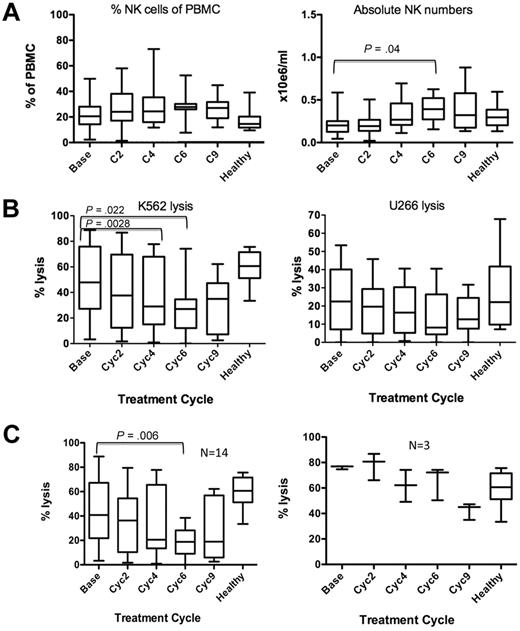
All patients (n = 25) were responding to lenalidomide-dexamethasone (Len-Dex) treatment at the time of participation in this correlative study (immunofixation negative complete remission, 3 patients; very good partial remission, 4 patients; partial remission, 15 patients; stable disease, 3 patients). At baseline, peripheral blood NK cell numbers from MM patients (n = 25) were not significantly different to age-matched healthy donors (n = 10), 0.22 ± 0.02 × 106/mL versus 0.3 ± 0.04 × 106/mL, respectively, P = .15 (Figure 1A). Nor did any individual patients show changes in NK cell frequency in the peripheral blood (PB) during therapy. However, patient PBMC cytotoxicity against a NK cell-only sensitive cell line, K562, progressively decreased with treatment (Figure 1B). At baseline, NK cell cytotoxicity from patients (n = 17) was not significantly different compared with that of healthy donors (48.9% ± 6.8% vs 58.0% ± 5.4% K562 cell lysis, respectively, P = .44). However, after 6 treatment cycles, NK cell cytotoxicity from patients was significantly reduced (Figure 1B, 27.6% ± 5.1%, P = .002). Of the 17 patients analyzed, 14 had decreased NK killing of K562 by cycle 6 of therapy, whereas NK killing in 3 patients remained unchanged (Figure 1C). No patients showed an increase in NK-mediated K562 killing during the course of therapy. Lysis of the MM cell line U266 remained relatively low compared with K562 lysis but also declined throughout treatment. This observed impairment in NK cell function suggested that there was an antagonistic mechanism occurring in vivo against lenalidomide's immunomodulatory activity.
Lenalidomide and dexamethasone therapy in MM patients increased NK cell number, but their NK cell function is impaired. (A) MM patient (n = 25) NK cell frequency (left panel) and absolute numbers per milliliter (right panel) of PBMCs were calculated. (B) Patient NK cytotoxicity was assessed against a NK-only sensitive cell line K562 (n = 17; left panel) and a MM cell line U266 (n = 13; right panel) at an effector-to-target ratio of 50:1. (C) The number of patients with decreased versus unchanged NK killing of K562 over the course of therapy. Healthy donor PBMCs were included as controls (n = 10).
Lenalidomide and dexamethasone therapy in MM patients increased NK cell number, but their NK cell function is impaired. (A) MM patient (n = 25) NK cell frequency (left panel) and absolute numbers per milliliter (right panel) of PBMCs were calculated. (B) Patient NK cytotoxicity was assessed against a NK-only sensitive cell line K562 (n = 17; left panel) and a MM cell line U266 (n = 13; right panel) at an effector-to-target ratio of 50:1. (C) The number of patients with decreased versus unchanged NK killing of K562 over the course of therapy. Healthy donor PBMCs were included as controls (n = 10).
The NK cell–stimulatory effect of lenalidomide is abrogated by dexamethasone
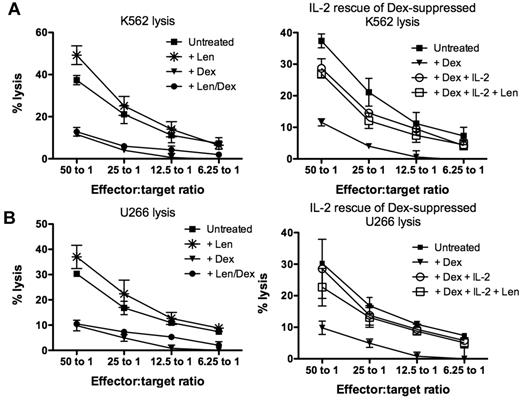
To assess the isolated effect of lenalidomide and dexamethasone on NK cell cytotoxicity, healthy donor PBMCs were maintained in culture for 3 days with single agent lenalidomide at 1μM or 10μM, dexamethasone at 1μM or 10μM, or lenalidomide and dexamethasone in combination. Similar data were obtained when drugs were used at 1μM (supplemental Figure 2) or 10μM (Figure 2). The addition of 10μM lenalidomide significantly increased K562 lysis from 38.6% ± 2.7% (untreated) to 53.3% ± 5.3% (P = .03; Figure 2A left panel) and U266 lysis from 26.3% ± 3.4% (untreated) to 35.9% ± 3.1% (P = .06; Figure 2B left panel). Conversely, NK cell impairment was clearly evident with dexamethasone-treated cells, which significantly reduced K562 and U266 lysis to 8.7% ± 1.9% (P = .0001) and 6.1% ± 2.0% (P = .0005), respectively. When PBMCs were treated with the combination of lenalidomide and dexamethasone, NK cell function was still significantly impaired (P = .0001 for K562 lysis and P = .0005 for U266 lysis), implying dominance of the suppressive effects of dexamethasone over the stimulation from lenalidomide. However, the addition of high-dose IL-2 at 500 U/mL rescued the dexamethasone-suppressed NK cytotoxicity of K562 to 29.3% ± 2.1% (P = .0035) and U266 to 22.4% ± 6.4% (P = .023), although this was still below untreated levels (Figure 2 right panels). The addition of lenalidomide with high-dose IL-2 had no additive effects on the rescue of dexamethasone-induced NK cell dysfunction compared with IL-2 only–treated cultures (P = .53). Importantly, dexamethasone-induced suppression of NK cell function was dose-dependent (supplemental Figure 1). Dexamethasone concentration higher than 0.1μM significantly impaired lenalidomide-induced NK cell cytotoxicity. When NK cell function was assessed in the presence of dexamethasone ranging from 0.01μM to 0.001μM, there was no significant impairment to NK cell function compared with lenalidomide-treated samples.
Dexamethasone antagonizes the immunomodulatory effect of lenalidomide. (A) Healthy donor PBMCs (n = 3) were treated with lenalidomide (*), dexamethasone (▾), lenalidomide and dexamethasone in combination (●), or no drugs (■) and used as effectors against K562 cells (left panel; n = 6). To assess whether dexamethasone-induced NK cell suppression could be rescued, PBMCs were cultured with dexamethasone in the presence of high-dose IL-2 at 500 U/mL (○) or both high-dose IL-2 and lenalidomide (□; right panel). (B) NK cell–mediated cytotoxicity against U266 after drug treatment was also assessed under the same conditions described above. These data are representative of 3 different experiments.
Dexamethasone antagonizes the immunomodulatory effect of lenalidomide. (A) Healthy donor PBMCs (n = 3) were treated with lenalidomide (*), dexamethasone (▾), lenalidomide and dexamethasone in combination (●), or no drugs (■) and used as effectors against K562 cells (left panel; n = 6). To assess whether dexamethasone-induced NK cell suppression could be rescued, PBMCs were cultured with dexamethasone in the presence of high-dose IL-2 at 500 U/mL (○) or both high-dose IL-2 and lenalidomide (□; right panel). (B) NK cell–mediated cytotoxicity against U266 after drug treatment was also assessed under the same conditions described above. These data are representative of 3 different experiments.
NK cells recover function after removing dexamethasone, but remain refractory to lenalidomide
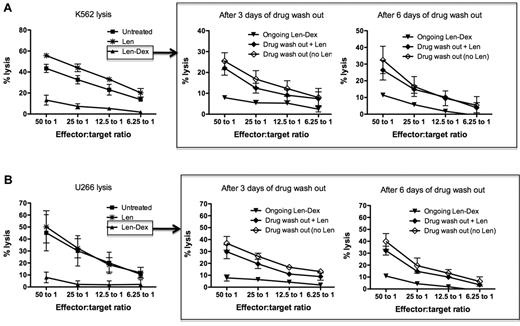
We have already established that lenalidomide amplified NK cell killing of K562 and U266, while the addition of dexamethasone abrogated NK cell cytotoxicity. The following experiments assessed whether dexamethasone-induced NK cell suppression was transient and reversible. To do this, healthy donor PBMCs were first treated with lenalidomide and dexamethasone in combination for 3 days. Subsequently, the drugs were washed out and the cells then cultured for a further 3 or 6 days, either in the presence or absence of lenalidomide, to determine whether lenalidomide would assist in NK cell recovery. This study had 2 important findings. First, after Len-Dex washout, NK cell function naturally recovered; this occurred within 3 days and was maintained for a further 3 days. Thus, after 3 days of drug washout, NK cell cytotoxicity recovered against both K562 (25.4% ± 4.0%) and U266 (36.8% ± 5.7%). This was significantly higher than ongoing Len-Dex treatment (P = .04 and P = .02 for K562 and U266 killing, respectively [Figure 3A-B middle panels]). Secondly, the addition of Len to the drug-washout cells did not have any additive effect on the recovery of NK cell killing. Thus, NK cell cytotoxicity of K562 and U266 3 days after drug washout was 25.4% ± 4.0% and 36.8% ± 5.7%, respectively. This was not significantly different to NK cytotoxicity against K562 and U266 when lenalidomide was added after drug washout (22.0% ± 3.3% for K562, P = .786 and 29.7% ± 5.8% for U266, P = .642). Hence we undertook further studies to investigate precisely how lenalidomide enhanced NK cell–mediated cytotoxicity.
NK cell function recovers after dexamethasone removal, but the same cells no longer respond to the immunostimulatory effect of lenalidomide. (A) Healthy donor PBMCs (n = 3) were cultured with no drug treatment (■), lenalidomide (*), or lenalidomide and dexamethasone (▴), and cytotoxicity was assessed against K562 at day 3 (left panel). To assess if NK cell function could recover after drug removal, the treated PBMCs were washed to remove drugs, then maintained in culture either without lenalidomide (♢) or with lenalidomide (♦). Chromium release assays using K562 as targets were performed 3 and 6 days after the drug washout (middle and right panels; n = 3). As a negative control, washed PBMCs were also treated with ongoing lenalidomide and dexamethasone (▾). (B) The same NK cell recovery experiment was repeated using U266 as target cells. Figures are pooled data from 3 separate experiments.
NK cell function recovers after dexamethasone removal, but the same cells no longer respond to the immunostimulatory effect of lenalidomide. (A) Healthy donor PBMCs (n = 3) were cultured with no drug treatment (■), lenalidomide (*), or lenalidomide and dexamethasone (▴), and cytotoxicity was assessed against K562 at day 3 (left panel). To assess if NK cell function could recover after drug removal, the treated PBMCs were washed to remove drugs, then maintained in culture either without lenalidomide (♢) or with lenalidomide (♦). Chromium release assays using K562 as targets were performed 3 and 6 days after the drug washout (middle and right panels; n = 3). As a negative control, washed PBMCs were also treated with ongoing lenalidomide and dexamethasone (▾). (B) The same NK cell recovery experiment was repeated using U266 as target cells. Figures are pooled data from 3 separate experiments.
Lenalidomide-enhanced NK cell cytotoxicity is dependent on CD4+ T cells and is mediated by IL-2
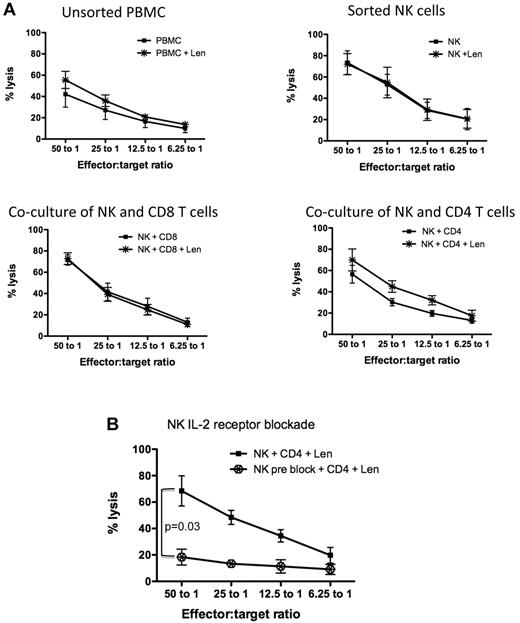
While it has been shown that lenalidomide-induced NK cell activation is IL-2 dependent,22 the specific subset of cells responsible for mediating this effect has not been elucidated. Healthy donor PBMCs were FACS-sorted into NK cells (CD3−CD16+CD56+), CD4 (CD3+CD4+), and CD8 (CD3+CD8+) T-cell subsets. Each individual subset was cultured alone or cocultured with NK cells (at a 1:1 ratio) for 3 days in either the presence or absence of lenalidomide. Cytotoxicity assays using K562 cell line as targets revealed that lenalidomide alone had no direct effect on the cytotoxicity capacity of sorted NK cells (Figure 4A top right panel). When NK cells were cocultured with either CD4+ (Figure 4A bottom right panel) or CD8+ (Figure 4A bottom left panel) T-cell subsets at a 1:1 ratio, only in the presence of CD4+ T cells did lenalidomide enhance NK cell–mediated cytotoxicity (from 56.5% ± 8.3% without lenalidomide to 69.9% ± 10.3% with lenalidomide, P = .005 at 50:1 effector:target ratio). Similar data were shown using the MM cell line U266 as targets (supplemental Figure 3). A higher ratio of lenalidomide-treated CD4+ T cells to NK cells (5:1), which is more in keeping with the human PB CD4:NK ratio, was able to further enhance NK cell–mediated cytotoxicity of U266 (supplemental Figure 3) or K562 (supplemental Figure 4) cells.
Lenalidomide-induced NK cell function is mediated by CD4+ T cells and secretion of IL-2. (A) Healthy donor PBMCs (n = 5) were sorted for CD4, CD8, and NK cells. NK cell–mediated cytotoxicity against K562 was assessed on unsorted PBMCs, sorted NK cells, or NK cells cocultured with CD8+ T cells or CD4+ T cells. These cultures were treated without (■) or with lenalidomide (*) for 3 days. (A) Pooled data from 5 separate experiments. (B) To assess whether lenalidomide-mediated NK cell function by CD4+ T cells was IL-2–driven, sorted NK cells were blocked with IL-2 receptor-α antibodies, then cocultured with sorted CD4+ T cells and lenalidomide ( ). (B) Pooled data from 4 separate experiments (4 normal donors).
). (B) Pooled data from 4 separate experiments (4 normal donors).
Lenalidomide-induced NK cell function is mediated by CD4+ T cells and secretion of IL-2. (A) Healthy donor PBMCs (n = 5) were sorted for CD4, CD8, and NK cells. NK cell–mediated cytotoxicity against K562 was assessed on unsorted PBMCs, sorted NK cells, or NK cells cocultured with CD8+ T cells or CD4+ T cells. These cultures were treated without (■) or with lenalidomide (*) for 3 days. (A) Pooled data from 5 separate experiments. (B) To assess whether lenalidomide-mediated NK cell function by CD4+ T cells was IL-2–driven, sorted NK cells were blocked with IL-2 receptor-α antibodies, then cocultured with sorted CD4+ T cells and lenalidomide ( ). (B) Pooled data from 4 separate experiments (4 normal donors).
). (B) Pooled data from 4 separate experiments (4 normal donors).
To confirm that lenalidomide-induced NK cell function through CD4+ T cells was IL-2-dependent, FACS-sorted NK cells were pretreated with anti-IL-2 receptor-α–blocking antibodies. The IL-2 receptor-α preblocked NK cells were then cocultured with CD4+ T cells with lenalidomide. The inability of NK cells to internalize IL-2 resulted in inhibition of NK cell cytotoxicity (Figure 4B), P = .03. In response to these findings, we postulated that the NK cell dysfunction induced by dexamethasone was due to impairment of CD4+ T cells, in particular their secretion of IL-2, and furthermore that lenalidomide could not rescue this dexamethasone-induced CD4+ T-cell impairment, resulting in ongoing NK cell dysfunction. Given the dependence of NK cell activation on IL-2 signaling, we next explored whether Len treatment of normal donor PBMCs altered NK cell IL-2Rα expression. Lenalidomide (10μM) up-regulated the IL-2Rα (CD25) on NK cells (supplemental Figure 5) from 3 normal donors. Taken together, these data indicate that lenalidomide induces CD4 T-cell secretion of IL-2, which binds to the up-regulated NK cell IL-2Rα, leading to enhanced NK-mediated killing of targets.
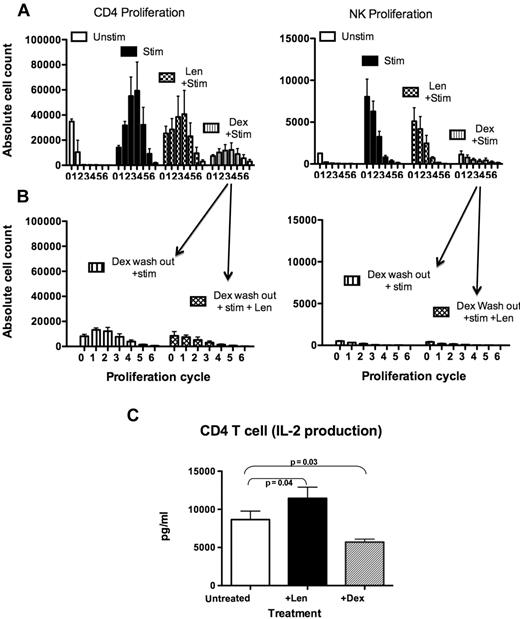
Dexamethasone impairs the ability of CD4 T cells to proliferate in response to stimulation and secretion of IL-2
We next investigated whether treating healthy donor PBMCs with lenalidomide or dexamethasone would affect the ability of CD4+ T cells to respond to anti-CD3 and anti-CD28 stimulation by proliferation and cytokine secretion. Stimulated healthy donor CD4+ T cells proliferated for 6 cycles (2.3 ± 0.2 × 105 total cells vs 0.4 ± 0.1 × 105 unstimulated, P = .032) within 5 days, as assessed by CFSE staining (Figure 5A left panel). NK cells also proliferated in stimulated cultures (0.18 ± 0.03 × 105 total cells vs 0.01 ± 0.001 × 105 for unstimulated, P = .056]; Figure 5A right panel), most likely through enhanced IL-2 production from stimulated CD4 T cells (supplemental Figure 6A). Stimulation of PBMCs pretreated with lenalidomide did not increase CD4 T-cell proliferation above the maximally stimulated controls (P = .1), but they were still able to proliferate (1.7 ± 0.6 × 105 total cells). In contrast, dexamethasone-treated PBMCs showed a marked impairment in proliferation in response to stimulation (0.6 ± 0.2 × 105 total cells, P = .001). To assess if CD4+ T and NK cells could recover and respond to stimulation after removal of dexamethasone, healthy donor PBMCs were treated with dexamethasone, followed by drug washout, and the cells were maintained in culture for a further 3 days either with or without lenalidomide. The cells were then stimulated by anti-CD3 and anti-CD28 and proliferation assessed 5 days later. Both CD4+ T cells and NK cells were unable to proliferate in response to stimulation even after removal of dexamethasone (P = .97 for CD4 T cells and P = .55 for NK cells; Figure 5B). Addition of lenalidomide did not rescue dexamethasone-induced suppression of CD4+ T or NK cell proliferation (P = .44 and P = .33, respectively). Sorted CD4+ T cells treated with dexamethasone also secreted lower levels of IL-2 compared with untreated (P = .03) or lenalidomide-treated cells (P = .0003; Figure 5C). In addition, sorted CD4+ T cells treated with lenalidomide secreted significantly higher levels of IL-2 than untreated cells (P = .04; Figure 5C). Because these results demonstrated that dexamethasone impaired CD4+ T cell-function in healthy donor PBMCs, we next examined whether NK cell dysfunction in MM patients was also due to loss of CD4+ T-cell function.
Dexamethasone suppressed CD4+ T-cell proliferation and IL-2 secretion. (A) To assess whether drug treatment influences the ability of cells to proliferate in response to stimulation, healthy donor PBMCs (n = 3)were treated with lenalidomide, dexamethasone, or no drugs. The cells were then labeled with CFSE and stimulated with OKT-3 and anti-CD28/49d (as described in “Methods”). Proliferation was assessed and expressed as absolute cell numbers per division (proliferation cycles 0-6; data are pooled from 3 experiments). (B) To assess whether NK and CD4+ T cells could still proliferate after removal of dexamethasone, PBMCs (n = 3) were treated with dexamethasone for 3 days, followed by dexamethasone washout. The cells were cultured for a further 3 days either with no drugs or with lenalidomide, then stimulated and labeled with CFSE (data are pooled from 3 separate experiments). (C) Supernatants from sorted CD4+ T cells treated with either lenalidomide or dexamethasone for 3 days were collected to assess IL-2 production by cytokine bead array (n = 9).
Dexamethasone suppressed CD4+ T-cell proliferation and IL-2 secretion. (A) To assess whether drug treatment influences the ability of cells to proliferate in response to stimulation, healthy donor PBMCs (n = 3)were treated with lenalidomide, dexamethasone, or no drugs. The cells were then labeled with CFSE and stimulated with OKT-3 and anti-CD28/49d (as described in “Methods”). Proliferation was assessed and expressed as absolute cell numbers per division (proliferation cycles 0-6; data are pooled from 3 experiments). (B) To assess whether NK and CD4+ T cells could still proliferate after removal of dexamethasone, PBMCs (n = 3) were treated with dexamethasone for 3 days, followed by dexamethasone washout. The cells were cultured for a further 3 days either with no drugs or with lenalidomide, then stimulated and labeled with CFSE (data are pooled from 3 separate experiments). (C) Supernatants from sorted CD4+ T cells treated with either lenalidomide or dexamethasone for 3 days were collected to assess IL-2 production by cytokine bead array (n = 9).
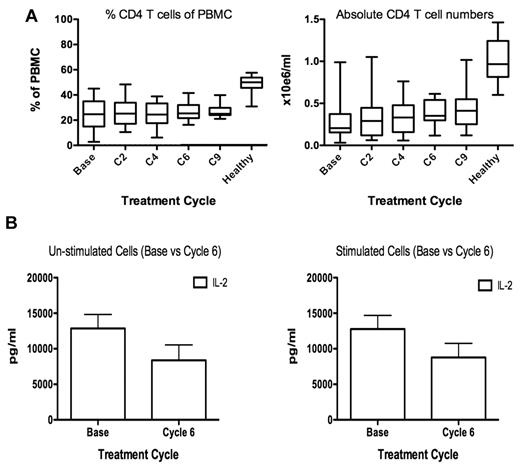
Myeloma patient CD4+ T cells secrete lower levels of IL-2 after lenalidomide and dexamethasone therapy
At baseline, MM patients had significantly lower absolute numbers of CD4+ T cells compared with healthy donors (0.21 vs 0.94 × 106/mL, respectively, P < .001), and the CD4+ T-cell numbers did not recover throughout treatment (Figure 6A). Since NK function in these patients was most significantly impaired at cycle 6 of treatment, we compared the level of IL-2 production from patient PBMCs collected at baseline and cycle 6. Production of IL-2 was lower in cycle 6 compared with baseline for unstimulated PBMCs, P = .10, or stimulated PBMCs, P = .03 (Figure 6B). To assess whether PBMCs from patients at cycle 6 were able to produce more cytokines in response to stimulation, PBMCs were activated by T-cell receptor (TCR) stimulation for 3 days. Patient PBMCs did not produce significantly more IL-2 in response to TCR stimulation (Figure 6B right panel) compared with unstimulated cells (Figure 6B left panel), and this was true for patient PBMCs collected at baseline as well as cycle 6. In contrast, stimulated healthy donor PBMCs produce significantly more IL-2 than untreated PBMCs (P = .0031; supplemental Figure 6A). The levels of IL-2 production from patient PBMC collected at cycle 6 were similar to dexamethasone-treated healthy donors (supplemental Figure 7A). In contrast to the data for IL-2, IFN-γ and IL-6 secretion was enhanced after stimulation of cycle 6 PBMCs compared with unstimulated cells, P = .006 and P = .02, respectively (supplemental Figure 6C).
Myeloma patient CD4+ T-cell numbers are unchanged during therapy, but their ability to secrete IL-2 is impaired. (A) Twenty-five MM patient PBMC CD4+ T-cell frequency (left panel) and absolute number per milliliter was calculated (right panel). (B) To assess whether NK cell dysfunction at cycle 6 of treatment correlated with reduced IL-2 production, PBMCs from 11 MM patients were collected at baseline and cycle 6, and IL-2 secretion was assessed by cytokine bead array on culture supernatant (left panel). To assess the ability of MM patient PBMCs to respond to stimulation, the cells were stimulated with OKT-3 and anti-CD28/49d before assessing IL-2 production (right panel).
Myeloma patient CD4+ T-cell numbers are unchanged during therapy, but their ability to secrete IL-2 is impaired. (A) Twenty-five MM patient PBMC CD4+ T-cell frequency (left panel) and absolute number per milliliter was calculated (right panel). (B) To assess whether NK cell dysfunction at cycle 6 of treatment correlated with reduced IL-2 production, PBMCs from 11 MM patients were collected at baseline and cycle 6, and IL-2 secretion was assessed by cytokine bead array on culture supernatant (left panel). To assess the ability of MM patient PBMCs to respond to stimulation, the cells were stimulated with OKT-3 and anti-CD28/49d before assessing IL-2 production (right panel).
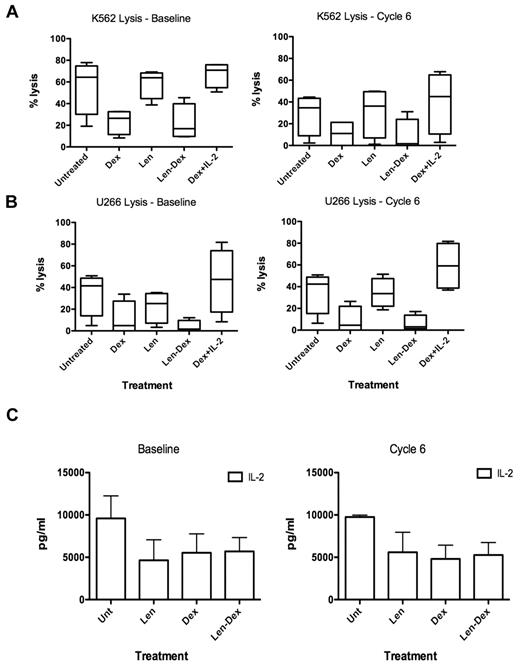
Dexamethasone prevents lenalidomide-induced IL-2 production and NK cell function in MM patients
We next examined whether lenalidomide enhanced IL-2 production and, indirectly, NK cell function in MM patient cells taken direct ex vivo. Myeloma patient PBMCs were collected at baseline and cycle 6 and cultured for 3 days with lenalidomide, dexamethasone, or lenalidomide and dexamethasone in combination. Unlike healthy donors (Figure 2), patient NK cell–mediated cytotoxicity from baseline and cycle 6 against K562 or U266 was not enhanced by lenalidomide (Figure 7A-B). Dexamethasone severely inhibited NK cell function (baseline P = .02, cycle 6 P = .055), while high dose IL-2 reversed this effect (Figure 7A-B), as seen with healthy donors (Figure 2). We postulated that the inability of lenalidomide to enhance MM patient NK cell function was due to the inability of the patients' CD4+ T cells to secrete IL-2 in response to lenalidomide due to prolonged exposure to dexamethasone in vivo. Analysis of culture supernatants revealed that IL-2 production was not enhanced by lenalidomide (Figure 7C) at baseline or cycle 6. None of the other cytokines analyzed, including IFN-γ, were enhanced after lenalidomide treatment of PBMCs ex vivo (supplemental Figure 7B-C).
Myeloma patient NK cell function cannot be enhanced by ex vivo treatment with lenalidomide. (A) Patient PBMCs (n = 4) were collected at both baseline and cycle 6 and treated with dexamethasone, lenalidomide, both lenalidomide and dexamethasone, dexamethasone with high-dose IL-2 (500 U/mL), or no drugs. The PBMCs were used as effectors at 50:1 target ratio against K562 as target cells, and (B) U266 targets. (C) To assess why lenalidomide could not enhance NK function, supernatants from the experiments described above were collected and assessed for IL-2 by cytokine bead array. Data are pooled from 4 separate experiments.
Myeloma patient NK cell function cannot be enhanced by ex vivo treatment with lenalidomide. (A) Patient PBMCs (n = 4) were collected at both baseline and cycle 6 and treated with dexamethasone, lenalidomide, both lenalidomide and dexamethasone, dexamethasone with high-dose IL-2 (500 U/mL), or no drugs. The PBMCs were used as effectors at 50:1 target ratio against K562 as target cells, and (B) U266 targets. (C) To assess why lenalidomide could not enhance NK function, supernatants from the experiments described above were collected and assessed for IL-2 by cytokine bead array. Data are pooled from 4 separate experiments.
Dexamethasone induced down-regulation of NK-activating receptors
The activation of NK cells not only requires IL-2-mediated signaling, but also the correct balance of NK activating and inhibitory receptors interacting with their cognate ligands on target cells. We examined whether this balance of NK receptors was affected by treatment of NK cells with dexamethasone and lenalidomide. To do this, NK receptors important for NK-mediated MM killing8 were assessed on NK cells from untreated, dexamethasone-, or Len-Dex–treated normal donor PBMCs (supplemental Figure 8). These results showed that NK cells constitutively express DNAM-1, NKG2D, and Nkp46. In addition, 1μM dexamethasone did not alter DNAM-1 expression (data not shown) but down-regulated both NKG2D and Nkp46, and 1μM lenalidomide could not rescue their expression.
Discussion
The use of lenalidomide and dexamethasone in combination is synergistic and results in substantial rates of clinical responses in MM patients, even in the relapsed or refractory setting.12,17,27-29 This clinical efficacy is likely due to the synergistic effects of this drug combination on tumor suppressor gene expression and caspase activation, and its wide range of mechanisms of action that limit plasma cell cross resistance.16,17,26 One of the proposed effector mechanisms of lenalidomide is its ability to stimulate and promote antitumor immune responses.22 However, the clinical impact of the combination of Len-Dex on the innate immunity of patients with MM has, to date, remained unclear.
NK cells are known to be capable of exerting an anti-MM effect.23,24 Changes in NK cell receptor ligand expression on MM cells and, hence, their resistance to NK cell–mediated killing may underlie the development of more proliferative and refractory plasma cell clones as MM progresses.24,25 Recently, the sensitivity of MM plasma cells to NK cell–mediated cytotoxicity has been shown to be enhanced by pretreatment with melphalan or bortezomib.8 These studies strongly suggest that NK cells may be potent effectors against MM, providing that their cytotoxic capacity can be fully harnessed. New drug therapies including proteasome inhibitors and agents with immunomodulatory activity may provide a means to promote NK cell–mediated control over MM; however, the routine use of the potently immunosuppressive corticosteroid dexamethasone may substantially limit this aspect of their action.
In a systematic analysis of the lymphocyte subset numbers and function in a cohort of patients treated with lenalidomide and low-dose dexamethasone, we observed that there was a progressive and profound reduction in the function of NK cells over the course of therapy. Our findings suggest that any ongoing disease control that might have been mediated by the enhancement of NK cell function by lenalidomide will have been severely abrogated by the concurrent use of dexamethasone. Even more concerning is the apparent permanence of the dexamethasone-induced NK cell defect, which prevents subsequent enhancement of NK cell function with lenalidomide treatment. This was shown in both healthy donors and MM patients. In this study, the effects of Len-Dex therapy on NK cell function were examined largely by cytotoxicity assays and changes in NK receptor expression. These studies confirmed that in both patients and healthy donors, the major mediators of NK cell function, IL-2 and activating receptors are both significantly suppressed by dexamethasone. Furthermore, these suppressive effects could not be reversed by lenalidomide.
Our findings indicate that if the lenalidomide immunomodulatory activity is to be promoted, then the dexamethasone-induced NK cell dysfunction must be reversed or avoided. Importantly, our titration experiments revealed that at dexamethasone concentrations below 100nM, NK cells remain sensitive to the stimulating effect of lenalidomide. A clinical dose of 40 mg/d of dexamethasone results in a maximum concentration (Cmax) of 300nM, well above the 100nM concentration capable of blocking the immunostimulatory effects of lenalidomide (personal communication from G. Prentice, K. Lynch, S. Howlett, Celgene Think Tank e-bulletin, issue 1, August 2009). We propose that a dose of dexamethasone substantially lower than 40 mg/day, or dosed less frequently, may preserve the antiproliferative and apoptotic synergy with lenalidomide, while still allowing lenalidomide to promote CD4+ T-cell and NK cell activation. Indeed, improved overall survival and reduced treatment-related mortality has been reported using a lower dose of dexamethasone in combination with Len in patients with newly diagnosed MM.30
Based on our data, future clinical trials using immunomodulators or other immunotherapy agents in the treatment of MM should consider minimizing the doses of corticosteroids, even omitting them completely in later cycles in responding patients. This may increase the likelihood of a responsive immune system to immunomodulatory compounds and hence enhance its ability to eradicate minimal residual disease.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Health and Medical Research Council (NHMRC) of Australia, Program Grant no. 454569, and the Peter MacCallum Cancer Center Morris Family Grant. This study was also supported (in part) by research funding from Celgene Corp. to H.Q. M.J.S. was supported by a NHMRC Senior Principal Research Fellowship and the NHMRC Australian Fellowship. J.A.T. was supported by NHMRC Senior Principal Research Fellowships. H.Q. was supported by the Vincent Fairfax Scholarship from the Royal College of Australian Physicians.
Authorship
Contribution: A.K.H., H.Q., and T.T. performed experiments; A.K.H. and H.Q. contributed to the direction of the work and the writing of the manuscript; H.Q. and H.M.P. initiated the clinical trial, from which all patient samples used in this manuscript were derived; J.A.T., S.J.H., and M.J.S. contributed to scientific direction and writing of the manuscript; and P.N. and D.S.R. directed the research and the manuscript.
Conflict-of-interest disclosure: H.M.P. is on the advisory board for Celgene Corp, for which he has received honoraria. The remaining authors declare no competing financial interests.
Correspondence: Paul Neeson, Cancer Immunology Program, Peter MacCallum Cancer Centre, East Melbourne, Victoria 3002, Australia; e-mail: paul.neeson@petermac.org.
References
Author notes
A.K.H. and H.Q. contributed equally to this work and share first authorship.
P.N. and D.S.R. contributed equally to this work as senior authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal