Abstract
CD40L on CD4+ T cells plays a vital role in the activation of antigen-presenting cells, thus catalyzing a positive feedback loop for T-cell activation. Despite the pivotal juxtaposition of CD40L between antigen-presenting cells and T-cell activation, only a T-cell receptor stimulus is thought to be required for early CD40L surface expression. We show, for the first time, that CD40L expression on peripheral blood CD4+ T cells is highly dependent on a cell-cell interaction with CD14hiCD16− monocytes. Interactions with ICAM-1, LFA-3, and to a lesser extent CD80/CD86 contribute to this enhancement of CD40L expression but are not themselves sufficient. The contact-mediated increase in CD40L expression is dependent on new mRNA and protein synthesis. Circulating myeloid dendritic cells also possess this costimulatory activity. By contrast, CD14loCD16+ monocytes, plasmacytoid dendritic cells, B-cell lymphoma lines, and resting, activated, and Epstein-Barr virus–immortalized primary B cells all lack the capacity to up-regulate early CD40L. The latter indicates that a human B cell cannot activate its cognate T cell to deliver CD40L-mediated help. This finding has functional implications for the role of biphasic CD40L expression, suggesting that the early phase is associated with antigen-presenting cell activation, whereas the late phase is related to B-cell activation.
Introduction
CD40 ligand (CD40L; CD154) is an inducible costimulatory molecule involved in promoting B- and T-cell responses, and the consequences of human CD40L deficiency are readily apparent in the X-linked form of the hyper-IgM syndrome.1 CD40L is absent or present at low levels on the surface of circulating CD4+ T cells, whereas its cognate receptor, CD40, is constitutively expressed on the surface of B cells, monocytes, dendritic cells (DCs), endothelial cells, and several other cell types.2-5 On B cells, CD40/CD40L interactions initiate a program of B-cell activation, Ig secretion, isotype switching, and B-cell memory formation. Through the up-regulation of major histocompatibility complex class II and costimulatory ligands on antigen-presenting cells, this interaction also plays a critical role in activating T cells and promoting Th1 differentiation by inducing interleukin-12 (IL-12) production.6-10
It has long been established that CD40L is rapidly expressed on the majority of CD4+ T cells on activation but returns to near-baseline levels by 24 hours.11 More recently, it has been reported that a second peak of CD40L expression at 48 hours follows the nadir at 24 hours.12,13 Although the kinetics of biphasic CD40L expression are identical in human and mouse, it appears that the mechanisms that regulate late-phase expression differ. In the mouse, IL-4 and IL-12 counterregulate the late phase of CD40L expression, with IL-4 inhibiting and IL-12 promoting expression.13 By contrast, late-phase human CD40L expression is CD28/IL-2–dependent.14 The biologic impact of biphasic CD40L expression has been investigated in several systems. For example, whereas early-phase CD40L expression promotes B-cell differentiation and antibody secretion in the mouse, sustained expression inhibits these same processes.15-19 By contrast, early CD40L expression is not sufficient to induce human IL-12p70, which requires both early and late CD40L expression.12 In addition, constitutive expression of CD40L in transgenic or bone marrow chimeric mice results in a high frequency of T-cell lymphoproliferative abnormalities.20,21 Collectively, these findings demonstrate that the regulated expression of CD40L is crucial to its normal physiologic function.
Although there is little surface expression of CD40L on circulating human or mouse CD4+ T cells, CD40L mRNA is readily detected in unstimulated mouse, but not human, CD4+ T cells.22-27 This suggests a fundamental difference in the regulation of CD40L expression between human and mouse. It has been proposed that the apparent absence of surface CD40L on resting mouse CD4+ T cells is not the result of a lack of CD40L expression but rather to tonic CD40L-CD40 interactions that induce down-regulation of the ligand.28 This premise is based on the observation that naive CD4+ T cells in the CD40 knockout mouse constitutively expresses surface CD40L.28,29 In the mouse, preformed mRNA presumably accounts for constitutive CD40L expression and may also contribute to its rapid up-regulation on T-cell activation.13,28,30,31 It has also been reported that, in lupus prone mouse strains, resting CD4+ T cells contain an intracellular pool of CD40L protein that contributes to its rapid surface expression on activation.32 And in human tonsilar CD4+ T cells, preformed intracellular CD40L protein is reported to be the source of surface CD40L in the first 2 hours after T-cell activation.33
In human and mouse, induction of early CD40L expression appears to require only a T-cell receptor (TCR) signal.13,34 One exception to this generalization is the report that early CD40L expression on phytohemagglutinin-activated human CD4+ T cells is enhanced by CD2 interactions with LFA-3 on vascular endothelial cells.35 Here we report on the regulation of early CD40L expression in peripheral blood mononuclear cells (PBMCs) and isolated CD4+ T cells. We find that neither CD40L protein nor CD40L mRNA is constitutively expressed in PBMC cultures or isolated CD4+ T cells to any significant extent. Although the induction of early CD40L requires a TCR stimulus, it is highly dependent on cell-cell contact with CD14hi monocytes. This costimulation of CD40L expression on CD4+ T cells by monocytes is distinct from CD2/LFA-3, ICAM-1/LFA-1, and CD80/CD86/CD28 interactions. This is the first report of a monocyte costimulatory activity impacting CD40L expression that probably plays an important role in regulating T- and B-cell activation and differentiation.
Methods
Subjects
Buffy coats and elutriated cells were from subjects belonging to the National Institutes of Health adult donor pool (IRB #99-CC-0168). Specimens were processed and cultured within 18 hours of collection.
Cell lines
The human monocytic lines U937, THP-1, K562, and HL-60 were from ATCC. The MonoMac6 line was kindly provided by Loems Ziegler-Heitbrock and Kathleen Clouse. Epstein-Barr virus (EBV)–transformed B cells were generated using supernatant from the B95–8 line (ATCC #CRL 1612) as described in the ATCC protocol. Ramos and Raji B cell lines were kindly provided by Art Shaffer.
Cell purifications
PBMCs and CD4+ T-cell isolations were performed as previously described.14 Elutriated monocytes were further purified by negative selection according to the manufacturer's directions (StemCell Technologies). CD16+CD14lo and CD16−CD14hi monocytes were then isolated by positive selection using microbeads (Miltenyi Biotec) on an AutoMACS (Miltenyi Biotec). Circulating DCs were enriched from elutriated monocytes by negative selection using microbeads (Miltenyi Biotec) and then fractionated on a FACSAria into myeloid (CD1c+CD303−) and plasmacytoid (CD1c−CD303+) subsets. Purified B cells were isolated by negative selection using microbeads (Miltenyi Biotec). All cell fractions were more than or equal to 95% pure.
Cell-culture reagents
Complete media (R10) consisting of RPMI 1640 (Biosource), 10% fetal bovine serum (Hyclone), and supplements was as previously described.14 Actinomycin D (5 μg/mL), cycloheximide (50 μg/mL), and dichlororibofuranosyl benzimidazole (200μM) were from Calbiochem. Lymphocyte separation media was from Mediatech. Ficoll-Paque was from GE Healthcare.
Antibodies in PBMC cultures
Antibodies to CD28 (clone 28.2), CD80 (clone L307.4), CD86 (clone IT2.2), and CD2 (clone RPA2.10) were from BD Biosciences PharMingen, as were the isotype controls. Antibody to CD18 (clone TS1/18) was from BioLegend. Antibody to CD3 (OKT3) was from OrthoBiotech. Blocking anti-CD40 antibody (clone G28.5) was a kind gift from Andreas Thiel or purified from the hybridoma (ATCC #HB-9110).
Cell stimulations
Plates (Corning Life Sciences) were coated overnight at room temperature with 2 μg/mL OKT3 in phosphate-buffered saline. PBMCs in R10 (1 × 106 cells/mL) were added to flat-bottom 24-well (2 mL) or 96-well (200 μL) plates with or without soluble anti-CD28 antibody at 2 μg/mL. CD4+ T cells were plated alone or after reconstitution with monocytes, monocyte lines, monocyte-derived DCs, primary DCs, activated primary B cells, or B cell lines in the same proportion as present in autologous PBMCs (unless indicated otherwise) and stimulated in an identical fashion. B cells were activated by incubating for 3 days in 0.1 mg/mL anti-CD40 (R&D Systems) and 0.2 ng/mL rIL-21 (Biosource). Monocyte-derived DCs were generated from purified CD16+CD14lo and CD16−CD14hi monocytes by culturing in granulocyte-macrophage colony-stimulating factor and IL-4 (R&D Systems, 50 ng/mL each) for 5 days. Reconstituted CD4+ T-cell cultures were stimulated in the presence of 5 μg/mL of the anti-CD40 blocking antibody G28.5 to prevent masking of CD40L expression.36
Flow cytometric analysis
Flow cytometric analysis was performed according to standard protocols. Conjugated anti-CD4 (allophycocyanin), anti-CD40L (phycoerythrin), and isotype control antibodies were from BD Biosciences PharMingen. Conjugated antibodies to CD303 (allophycocyanin), CD16 (phycoerythrin), and CD1c (fluorescein isothiocyanate) were from Miltenyi Biotec. Cells were stained according to the manufacturer's suggestions or our antibody titration data. Viaprobe (BD Biosciences) was added to gate out dead cells. Intracellular staining was accomplished by a 30-minute fixation with Cytofix/Cytoperm reagent (BD Biosciences PharMingen), followed by washing and staining in BD Perm-wash buffer. Stained cell populations were analyzed on a FACSCalibur flow cytometer (BD Biosciences). Color compensations were made with each round of staining. Data were analyzed using CellQuest Classic (BD Biosciences) or FlowJo software Version 8.3.3 (TreeStar) and expressed as percentage CD40L+ cells after gating on CD4+ lymphocytes or CD4+ T cells.
Northern blot analysis
Purified CD4+ T cells were stimulated with plate-bound anti-CD3 antibody with or without U937 cells (2:1) for 6 hours. To normalize the total amount of RNA in each sample, after stimulation the same number of U937 cells was added to those samples that had been stimulated in the absence of U937 cells as was present in those cultures stimulated with U937 cells. RNA was prepared using the RNeasy Micro Kit (QIAGEN), concentrated using Micron Ultracel YM-100 filters (Millipore), resolved on a formaldehyde-agarose gel, and transferred to Hybond-N+ (GE Healthcare) followed by UV cross-linking in a Stratagene 2400. The CD40L cDNA probe was labeled with 32P-dCTP (PerkinElmer Life and Analytical Sciences) using the Prime-it-II kit (Stratagene). After membrane blocking, the probe was hybridized in DIG Easy Hyb Granules solution (Roche Diagnostics) at 65°C overnight in the rotating chamber of a model 310 air-heated incubator (Robbins Scientific). Afterward, the membrane was washed twice for 10 minutes with 20 mL 2 times saline sodium citrate buffer at room temperature, twice with 20 mL 2 times saline sodium citrate buffer plus 0.1% sodium dodecyl sulfate at 65°C, and twice with 20 mL 0.2 times saline sodium citrate buffer plus 0.1% sodium dodecyl sulfate at 65°C. The membrane was imaged using a Molecular Dynamics Storm Model 860 instrument (GE Healthcare)with Image Quart Version 1.2 software.
Quantitative RT-PCR
Quantitative reverse-transcribed polymerase chain reaction (RT-PCR) was performed as described previously except that PCR Buffer II (Applied Biosystems) and Rox dye (Invitrogen) were substituted for TaqMan Buffer A.37 In addition, the PCR step was performed at 60°C. Predeveloped human CD40L and rRNA primer/probe sets were purchased from Applied Biosystems. rRNA was quantitated using a standard curve generated with total RNA from PBMCs in a fashion identical to that described earlier.38 CD40L mRNA was quantitated using an absolute RNA standard curve generated with cRNA transcribed from a cDNA template using T7 RNA polymerase according to the manufacturer's directions (Ambion). All samples were run in triplicate and CD40L mRNA was normalized to ng of rRNA.
Results
Preformed intracellular protein does not contribute to CD40L surface expression
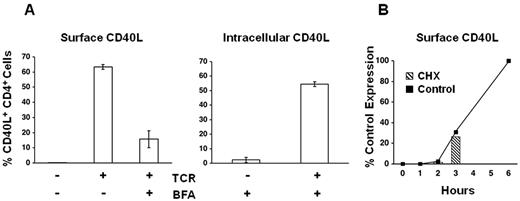
We have previously shown that, in humans, a low level of CD40L is expressed on a small population (< 10%) of circulating memory CD4+ T cells.14 In the mouse, tonic CD40L-CD40 interactions between circulating CD4+ T cells and B cells (or monocytes) in peripheral blood have been shown to mask CD40L expression on most T cells. To test whether this interaction might result in an apparent lack of CD40L surface expression on circulating human T cells, we cultured purified CD4+ T cells without activating them in the presence and absence of CD40+ cells for intervals from 1 to 96 hours. In contrast to mouse CD4+ T cells, which spontaneously up-regulate surface CD40L under these conditions (ie, in the absence of CD40+ cells), human CD4+ T cells did not show any change in their level of surface CD40L (data not shown). Having found that tonic CD40L-CD40 interactions do not mask constitutive expression of CD40L on the surface of circulating human CD4+ T cells, we performed intracellular staining on freshly isolated PBMCs to examine the potential contribution of preformed CD40L protein to its rapid surface expression on activation. To confirm that we could readily detect intracellular CD40L by flow cytometry, as a control, brefeldin A (BFA) was added to TCR-stimulated PBMC cultures at 2 hours, and cells were harvested at 6 hours for staining of surface and intracellular CD40L. As expected, surface staining of CD40L is markedly inhibited on activated CD4+ T cells treated with BFA, whereas intracellular CD40L is readily detected in the same cells (Figure 1A). In contrast to activated cells treated with BFA, intracellular CD40L staining in unstimulated cells is minimal (Figure 1A). These findings demonstrate that, unlike the mouse, unstimulated human CD4+ lymphocytes do not contain an intracellular pool of CD40L. To corroborate this conclusion, the dependence of CD40L surface expression on new protein synthesis was tested using the translational inhibitor cycloheximide (CHX). To help discriminate between global inhibition of T-cell activation and inhibition of CD40L translation per se, CHX was added to parallel cultures at the onset of stimulation (0 hour) or at 1, 2, or 3 hours after stimulation (Figure 1B). Comparison of the CHX-treated cultures stained for CD40L expression at 6 hours, to the level of CD40L expression at 0, 1, 2, and 3 hours in control cultures stimulated without CHX, reveals that there is no increase in CD40L expression after the addition of CHX.
Preformed intracellular CD40L does not contribute to surface expression. (A) PBMCs were left unstimulated or TCR stimulated for 6 hours as indicated. After 2 hours of stimulation, BFA was added to the samples as indicated. Cells were stained for surface CD40L or for intracellular CD40L after fixation and permeabilization. (B) PBMCs were plated in the absence (Control) or presence of a translational inhibitor (CHX). Control samples were harvested and stained for CD40L at the indicated times (solid line). CHX was added to the treated cultures at 0, 1, 2, or 3 hours, and the CHX-treated samples were harvested and stained for CD40L at 6 hours (hatched columns; undetectable in 0 and 1 hour samples). CD40L expression is plotted relative to the 6-hour control (100%).
Preformed intracellular CD40L does not contribute to surface expression. (A) PBMCs were left unstimulated or TCR stimulated for 6 hours as indicated. After 2 hours of stimulation, BFA was added to the samples as indicated. Cells were stained for surface CD40L or for intracellular CD40L after fixation and permeabilization. (B) PBMCs were plated in the absence (Control) or presence of a translational inhibitor (CHX). Control samples were harvested and stained for CD40L at the indicated times (solid line). CHX was added to the treated cultures at 0, 1, 2, or 3 hours, and the CHX-treated samples were harvested and stained for CD40L at 6 hours (hatched columns; undetectable in 0 and 1 hour samples). CD40L expression is plotted relative to the 6-hour control (100%).
Diminished CD40L expression in isolated human CD4+ T-cell cultures on activation
Unexpectedly, we observed that when purified CD4+ T cells and the PBMCs from which they were isolated were activated in parallel under identical conditions, there was markedly less CD40L expressed in the isolated CD4+ T-cell culture than in the autologous PBMC culture (Figure 2A). This was the case for both early, CD28-independent and late, CD28-dependent CD40L expression. Increasing TCR signal strength beyond what was found to be optimal in PBMC cultures had no impact on the level of CD40L expression in isolated CD4+ T-cell cultures (data not shown).
CD40L expression is diminished in CD4+ T-cell and lymphocyte cultures. PBMCs were plated at 1 × 106 cells/mL. Autologous CD4+ T cells (> 95% purity) or elutriated lymphocytes were plated at the same density as that present in unfractionated PBMCs. (A) CD4+ T cells and PBMCs were stimulated in an identical fashion as indicated. Cells were harvested and stained for CD40L expression at the indicated times. Data are mean ± SE of 6 donors. (B) Purified CD4+ T cells were plated in conditioned media harvested from 6-hour-stimulated PBMCs. Cultures were left unstimulated or TCR stimulated for 6 hours. Autologous PBMCs were cultured in an identical fashion. Cells were harvested and stained for CD40L expression. Results from a single donor are shown. (C) Lymphocytes and PBMCs were stimulated in an identical fashion as indicated. Cells were harvested and stained for CD40L expression at the indicated times. Data are mean ± SE of 3 donors.
CD40L expression is diminished in CD4+ T-cell and lymphocyte cultures. PBMCs were plated at 1 × 106 cells/mL. Autologous CD4+ T cells (> 95% purity) or elutriated lymphocytes were plated at the same density as that present in unfractionated PBMCs. (A) CD4+ T cells and PBMCs were stimulated in an identical fashion as indicated. Cells were harvested and stained for CD40L expression at the indicated times. Data are mean ± SE of 6 donors. (B) Purified CD4+ T cells were plated in conditioned media harvested from 6-hour-stimulated PBMCs. Cultures were left unstimulated or TCR stimulated for 6 hours. Autologous PBMCs were cultured in an identical fashion. Cells were harvested and stained for CD40L expression. Results from a single donor are shown. (C) Lymphocytes and PBMCs were stimulated in an identical fashion as indicated. Cells were harvested and stained for CD40L expression at the indicated times. Data are mean ± SE of 3 donors.
Although reduced levels or the absence of cytokines, such as IL-2 and IL-12, might explain diminished late-phase expression in human CD4+ T-cell cultures, early-phase expression is reportedly independent of cytokine signaling. To broadly test for the contribution of soluble factors to early CD40L expression, we activated purified CD4+ T cells in conditioned media harvested from PBMC cultures that had been stimulated for 16 hours. As shown in Figure 2B, conditioned media could not restore early CD40L expression in isolated CD4+ T-cell cultures. The same result was obtained using conditioned media from PBMCs that had been stimulated 2, 4, or 6 hours (data not shown). These results suggested that the purified CD4+ T-cell cultures lacked a cellular component present in PBMC cultures that is necessary for maximal CD40L expression. We initially tested this hypothesis by repeating the experiments with elutriated lymphocytes, which differ from unfractionated PBMCs in that they are depleted of monocytes and circulating DCs. As we observed for CD4+ T cells, elutriated lymphocytes could not fully up-regulate CD40L in the presence or absence of CD28 costimulation, suggesting that primary monocytes or DCs, not B cells, were the critical missing component in these cultures (Figure 2C).
Early CD40L expression is dependent on cell contact with monocytes
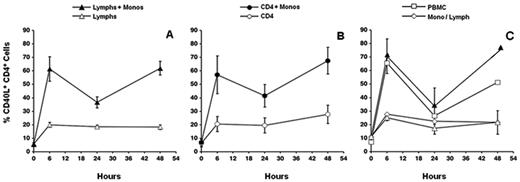
The inability of elutriated lymphocytes to fully express CD40L suggested that peripheral blood monocytes or DCs promote early CD40L expression. To directly test this, highly purified monocytes were added to elutriated lymphocytes from the same donor before stimulation. In these reconstituted cultures, CD40L was expressed at the same level as in unfractionated PBMCs (Figure 3A). Likewise, the addition of purified monocytes to purified CD4+ T-cell cultures restored CD40L expression, demonstrating that monocytes can directly promote early CD40L expression (Figure 3B). Previously published data from our laboratory permit us to conclude that early CD40L expression on naive and memory subsets of CD4+ T cells is equally dependent on monocytes.14 These reconstitution experiments, together with the inability of conditioned media to restore early CD40L expression, strongly suggested that cell-cell contact with monocytes was required for early CD40L expression in our PBMC cultures. We used a transwell cell-culture system to directly demonstrate this. Only when purified monocytes and CD4+ T cells were permitted to contact one another was early CD40L expression equal to that seen in stimulated PBMC cultures (Figure 3C). We also observed that monocytes purified by either negative or positive selection were equally capable of up-regulating CD40L expression. To determine whether CD40L costimulatory activity was present on circulating monocytes or induced on adhesion of monocytes to plates, monocytes were fixed in paraformaldehyde before reconstitution with CD4+ T cells. As shown in Figure 4, these fixed monocytes promoted CD40L expression as well as unfixed monocytes, demonstrating that monocyte CD40L costimulatory activity is constitutively expressed on the surface of circulating monocytes. Pretreatment of monocytes with a protease cocktail before fixation drastically diminished CD40L expression in reconstituted cultures (data not shown).
Monocytes restore early CD40L expression. (A) Elutriated lymphocytes or (B) purified CD4+ T cells (> 95% pure) were plated alone or reconstituted with purified monocytes at the same density as in unfractionated PBMCs. Cultures were stimulated with anti-TCR and anti-CD28 mAb. Cells were harvested and stained for CD40L expression at the indicated times. Data are mean ± SE of 4 donors. (C) Elutriated lymphocytes were plated alone in the insert of a transwell dish (Lymphs) or reconstituted with purified monocytes (Lymphs + Monos) before plating. Alternatively, lymphocytes were plated in the insert, and monocytes were plated in the bottom of the well (Mono/Lymph). As a control, PBMCs were plated in the insert. Cells were stimulated with anti-TCR and anti-CD28 mAb, harvested, and stained for CD40L expression at the indicated times. Data are mean ± SE of 2 donors.
Monocytes restore early CD40L expression. (A) Elutriated lymphocytes or (B) purified CD4+ T cells (> 95% pure) were plated alone or reconstituted with purified monocytes at the same density as in unfractionated PBMCs. Cultures were stimulated with anti-TCR and anti-CD28 mAb. Cells were harvested and stained for CD40L expression at the indicated times. Data are mean ± SE of 4 donors. (C) Elutriated lymphocytes were plated alone in the insert of a transwell dish (Lymphs) or reconstituted with purified monocytes (Lymphs + Monos) before plating. Alternatively, lymphocytes were plated in the insert, and monocytes were plated in the bottom of the well (Mono/Lymph). As a control, PBMCs were plated in the insert. Cells were stimulated with anti-TCR and anti-CD28 mAb, harvested, and stained for CD40L expression at the indicated times. Data are mean ± SE of 2 donors.
Fixed monocytes promote CD40L expression. Monocytes, or monocytes that had been fixed and washed, were reconstituted with autologous elutriated lymphocytes. Cells were plated at the same density as in unfractionated PBMCs and stimulated with anti-TCR mAb. Autologous PBMCs were cultured in parallel. Cells were harvested at 6 hours and stained for CD40L expression. Data are mean ± SE of 3 donors.
Fixed monocytes promote CD40L expression. Monocytes, or monocytes that had been fixed and washed, were reconstituted with autologous elutriated lymphocytes. Cells were plated at the same density as in unfractionated PBMCs and stimulated with anti-TCR mAb. Autologous PBMCs were cultured in parallel. Cells were harvested at 6 hours and stained for CD40L expression. Data are mean ± SE of 3 donors.
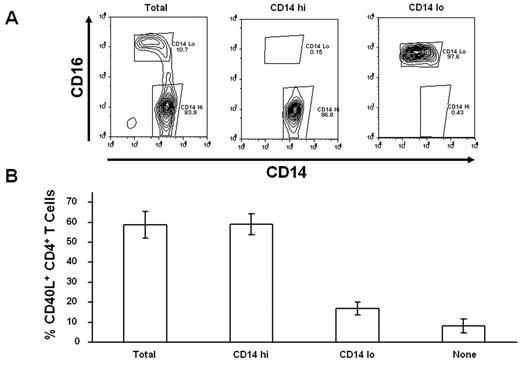
Peripheral blood monocytes are heterogeneous, with CD16−CD14hi and CD16+CD14lo cells constituting the 2 principal subpopulations. To determine whether both monocyte populations possess the capacity to costimulate CD40L expression, we fractioned monocytes into CD16+ and CD16− populations and compared the CD40L costimulatory activity of these purified populations to bulk monocytes on a per-cell basis (Figure 5A). Although in this instance we added CD16+CD14lo monocytes to T cells at a much higher ratio than present in PBMCs, it is clear that CD40L costimulatory activity is predominately associated with the CD16−CD14hi monocyte subset (Figure 5B).
CD14hi monocytes selectively costimulate CD40L expression. (A) Elutriated monocytes (Total) were initially fractionated into CD16+ and CD16− populations by magnetic bead sorting, and then CD14hi cells were further selected from the CD16− population by positive selection on magnetic beads. (B) Purified CD4+ T cells were stimulated with anti-TCR mAb for 6 hours without monocytes (None) or after reconstitution with elutriated monocytes (Total), CD14hiCD16−, or CD14loCD16+ purified monocyte subsets. Percentage CD40L+CD4+ T cells are expressed relative to the cultures reconstituted with total monocytes. Data are mean ± SE of 3 donors.
CD14hi monocytes selectively costimulate CD40L expression. (A) Elutriated monocytes (Total) were initially fractionated into CD16+ and CD16− populations by magnetic bead sorting, and then CD14hi cells were further selected from the CD16− population by positive selection on magnetic beads. (B) Purified CD4+ T cells were stimulated with anti-TCR mAb for 6 hours without monocytes (None) or after reconstitution with elutriated monocytes (Total), CD14hiCD16−, or CD14loCD16+ purified monocyte subsets. Percentage CD40L+CD4+ T cells are expressed relative to the cultures reconstituted with total monocytes. Data are mean ± SE of 3 donors.
CD40L costimulatory activity is present on primary and induced DCs
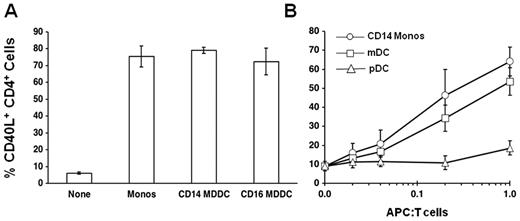
CD16+ monocytes are thought to be more mature than the CD16− subset; thus, it was surprising that they lack the capacity to up-regulate CD40L expression. We therefore tested the ability of DCs derived from these 2 monocyte subsets to promote CD40L expression. After 5 days of culture in granulocyte-macrophage colony-stimulating factor and IL-4, DCs derived from both monocyte subsets were equally capable of costimulating CD40L expression on CD4+ T cells (Figure 6A). We then asked whether primary plasmacytoid and myeloid DCs isolated directly from the circulation could costimulate CD40L expression. When reconstituted with CD4+ T cells at a 1:1 ratio, myeloid, but not plasmacytoid, DCs were as effective as CD14hi monocytes in promoting CD40L expression (Figure 6B). Although monocytes are present in PBMCs at approximately a 1:1 ratio with CD4+ T cells, circulating DCs constitute less than 2% of PBMCs and thus would not contribute to the costimulation of CD40L expression in a PBMC culture (Figure 6B).
Monocyte-derived and primary DCs costimulate CD40L expression. (A) Purified CD14hiCD16− and CD14loCD16+ monocytes were cultured for 5 days in granulocyte-macrophage colony-stimulating factor and IL-4 to generate CD14hi monocyte-derived DCs (CD14 MDDC) and CD14lo CD16+ monocyte-derived DCs (CD16 MDDC). Purified CD4+ T cells were stimulated with anti-TCR mAb for 6 hours without monocytes (None) or after reconstitution (1:1) with CD14hiCD16− monocytes, CD14 MDDC, or CD16 MDDC. Cells were harvested at 6 hours and stained for CD40L expression. Data are mean ± SE (n = 3). (B) Purified CD4+ T cells were stimulated with anti-TCR mAb for 6 hours without antigen-presenting cells (0.0) or after reconstitution with increasing numbers of CD14hi monocytes (CD14 Monos), myeloid DCs (mDC), or plasmacytoid DCs (pDC). The ratio of antigen-presenting cells to CD4+ T cells is shown along the x-axis. Monocytes are present in PBMCs at approximately a 1:1 ratio with CD4+ T cells, whereas circulating DCs constitute less than 2% (0.02) of PBMCs. Cells were harvested at 6 hours and stained for CD40L expression. Data are mean ± SE (n = 3).
Monocyte-derived and primary DCs costimulate CD40L expression. (A) Purified CD14hiCD16− and CD14loCD16+ monocytes were cultured for 5 days in granulocyte-macrophage colony-stimulating factor and IL-4 to generate CD14hi monocyte-derived DCs (CD14 MDDC) and CD14lo CD16+ monocyte-derived DCs (CD16 MDDC). Purified CD4+ T cells were stimulated with anti-TCR mAb for 6 hours without monocytes (None) or after reconstitution (1:1) with CD14hiCD16− monocytes, CD14 MDDC, or CD16 MDDC. Cells were harvested at 6 hours and stained for CD40L expression. Data are mean ± SE (n = 3). (B) Purified CD4+ T cells were stimulated with anti-TCR mAb for 6 hours without antigen-presenting cells (0.0) or after reconstitution with increasing numbers of CD14hi monocytes (CD14 Monos), myeloid DCs (mDC), or plasmacytoid DCs (pDC). The ratio of antigen-presenting cells to CD4+ T cells is shown along the x-axis. Monocytes are present in PBMCs at approximately a 1:1 ratio with CD4+ T cells, whereas circulating DCs constitute less than 2% (0.02) of PBMCs. Cells were harvested at 6 hours and stained for CD40L expression. Data are mean ± SE (n = 3).
CD40L costimulatory activity is present on multiple monocytic cell lines
To further assess the developmental expression of the CD40L costimulatory activity on monocytes, we examined several monocytic cell lines that are representative of different stages of monocyte differentiation for their ability to costimulate early CD40L expression. We found that all of the tested lines promote early CD40L expression on primary CD4+ T cells as well as or better than primary peripheral blood monocytes (Table 1). Epithelial-derived cell lines could not promote CD40L expression (data not shown). Although our stimulations with elutriated lymphocytes indicated that primary B cells had no discernable effect on early CD40L expression, we also tested the ability of activated primary B cells, EBV-transformed primary B cells, and the Ramos and Raji B-cell lymphoma lines to promote early CD40L expression. As shown in Table 2, none of these B cells could stimulate early CD40L expression on CD4+ T cells. It is recognized that high levels of CD40 on the surface of accessory cells can mask the expression of CD40L. Thus, it is possible that the capacity of these various B cells to promote CD40L expression could be obscured by the presence of some unrecognized inhibitory molecule. To address this question, we performed mixing experiments to test whether activated primary B cells could inhibit early CD40L expression in TCR-stimulated cultures of CD4+ T cells and CD14hi monocytes. In 4 donors, we observed no difference (P = .13) in CD40L expression in the presence and absence of activated primary B cells.
Costimulation of early CD40L expression by monocytic cells
| Cell type . | Classification . | % CD40L+ CD4 T cells, mean (SE) . |
|---|---|---|
| Monocyte | Primary | 54 (4) |
| U937 | Histiocytic lymphoma | 67 (4) |
| THP1 | Acute monocytic leukemia | 55 (2) |
| K562 | Chronic myelogenous leukemia | 44 (0.2) |
| MonoMac6 | Mature monoblastic leukemia | 61 (10) |
| HL-60 | Promyelocytic leukemia | 73 (6) |
| Cell type . | Classification . | % CD40L+ CD4 T cells, mean (SE) . |
|---|---|---|
| Monocyte | Primary | 54 (4) |
| U937 | Histiocytic lymphoma | 67 (4) |
| THP1 | Acute monocytic leukemia | 55 (2) |
| K562 | Chronic myelogenous leukemia | 44 (0.2) |
| MonoMac6 | Mature monoblastic leukemia | 61 (10) |
| HL-60 | Promyelocytic leukemia | 73 (6) |
Costimulation of early CD40L expression by B cells
| Cell added . | Classification . | % CD40L+ CD4 T cells, mean (SE) . |
|---|---|---|
| None | — | 4 (1) |
| U937 | Monocyte (histiocytic lymphoma) | 59 (10) |
| B | Activated primary | 10 (2) |
| B | EBV transformed primary | 6 (1) |
| Ramos | Burkitt lymphoma line | 5 (3) |
| Raji | Burkitt lymphoma line | 10 (2) |
| Cell added . | Classification . | % CD40L+ CD4 T cells, mean (SE) . |
|---|---|---|
| None | — | 4 (1) |
| U937 | Monocyte (histiocytic lymphoma) | 59 (10) |
| B | Activated primary | 10 (2) |
| B | EBV transformed primary | 6 (1) |
| Ramos | Burkitt lymphoma line | 5 (3) |
| Raji | Burkitt lymphoma line | 10 (2) |
— indicates not applicable.
Monocyte CD40L costimulatory activity is distinct from LFA-3, ICAM-1, and CD80/CD86 interactions
It has been reported that CD2/LFA-3 interactions between phytohemagglutinin-activated human CD4+ T cells and vascular endothelial cells are important for the normal up-regulation of CD40L in that system. We assessed the contribution of this interaction to CD40L expression in PBMC cultures using a blocking antibody to CD2. Although this antibody inhibited a mixed lymphocyte reaction by more than 90%, we observed only a modest inhibition (20% ± 4%, n = 7) of CD40L expression in 6-hour-stimulated PBMCs. Although an agonistic anti-CD28 antibody does not enhance early CD40L expression, because CD28 is constitutively expressed on CD4+ T cells and variable levels of B7 expression are found on resting PBMCs, we also performed experiments with a combination of anti-CD80/CD86 blocking antibodies to determine the potential contribution of this pathway. Although these antibodies completely inhibited CD28-dependent CD40L expression, they only slightly inhibited (7% ± 1%, n = 5) expression at 6 hours.14
Because the monocytic lines in Table 1 all costimulate early CD40L expression, surface proteins that are differentially expressed on these cells probably do not account for their CD40L costimulatory activity (Table 3). For instance, HL-60 promotes CD40L expression more effectively than K562 cells but has much lower levels of ICAM-1, suggesting that interactions between LFA-1 (CD11a/CD18) and ICAM-1 are unlikely to promote up-regulation of CD40L. Nonetheless, to directly assess the impact of ICAM-1 on the induction of CD40L expression, we conducted additional comparisons of anti-CD2, anti-CD80/CD86, and anti-CD18 blocking monoclonal antibodies (mAbs) alone and in combination (Table 4). In these experiments, inhibition with anti-CD2 mAb was similar to what we had observed earlier; and although we saw somewhat greater inhibition with anti-CD80/CD86 mAbs than before, there was no difference (P = .33) between the ability of anti-CD80/CD86 and anti-CD2 mAbs to inhibit CD40L expression. Anti-CD18 mAb partially inhibited the ability of primary monocytes to promote CD40L expression more potently than the anti-CD80/CD86 or anti-CD2 mAbs. However, the combination of all 3 antibodies was not more effective than ant-CD18 alone (P = .18), indicating that, on primary monocytes, interactions with LFA-1 predominate over those of CD2 or CD80/CD86.
Expression of select surface markers on monocytic and B-cell lines
| Cell line . | Monos . | U937 . | THP1 . | K562 . | MonoMac6 . | HL-60 . | Ramos . |
|---|---|---|---|---|---|---|---|
| CD14 | 88 | 90 | 88 | 1 | 92 | 17 | ND |
| ICAM-1 | 98 | 99 | 94 | 99 | 94 | 13 | 99 |
| CD40 | 11 | 0 | 7 | 0 | 12 | 0 | 67 |
| Cell line . | Monos . | U937 . | THP1 . | K562 . | MonoMac6 . | HL-60 . | Ramos . |
|---|---|---|---|---|---|---|---|
| CD14 | 88 | 90 | 88 | 1 | 92 | 17 | ND |
| ICAM-1 | 98 | 99 | 94 | 99 | 94 | 13 | 99 |
| CD40 | 11 | 0 | 7 | 0 | 12 | 0 | 67 |
Values are percentage positive.
ND indicates not done.
Monocyte costimulation requires new mRNA synthesis
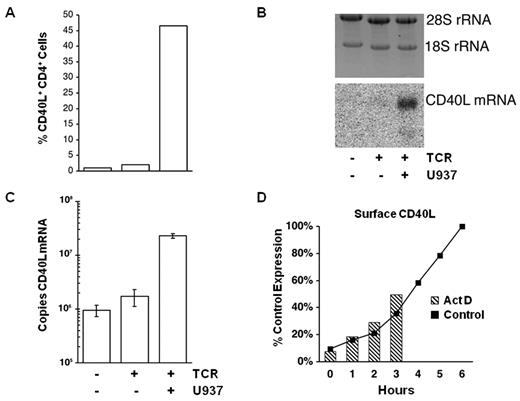
To investigate the mechanism(s) by which monocytes costimulate CD40L expression, we measured CD40L surface protein and CD40L mRNA in CD4+ T-cell cultures after 6 hours of activation in the presence and absence of monocytic cells (Figure 7). CD40L mRNA is not detectable in unstimulated CD4+ T cells by Northern blot analysis, and there is little increase in the level of CD40L mRNA in isolated CD4+ T cells stimulated with anti-CD3 antibody (Figure 7B). Strikingly, the same stimulation in the presence of monocytic (U937) cells dramatically increases the level of CD40L mRNA. Real-time RT-PCR was performed on the same samples analyzed by Northern blot to quantitate the differences in CD40L mRNA. This assay, which has no background signal and an absolute sensitivity of 10 000 copies of CD40L mRNA, reveals that unstimulated CD4+ T cells contain approximately 106 copies of CD40L mRNA per nanogram of rRNA. As shown in Figure 7C, CD40L mRNA in isolated CD4+ T-cell cultures is increased less than 2-fold by TCR stimulation, but the same cells express 20-fold more CD40L mRNA when stimulated in the presence of monocytic cells (CD40L mRNA is undetectable in U937 cells; data not shown). These results demonstrate that monocytes costimulate CD40L expression by increasing the amount of CD40L mRNA in CD4+ T cells. Transcriptional inhibitors were added to PBMC cultures to determine whether new mRNA synthesis is needed for monocytes to costimulate CD40L expression (Figure 7D). To help discriminate between global inhibition of T-cell activation and inhibition of CD40L transcription per se, inhibitors were added to parallel cultures at the onset of stimulation (0 hour) or at 1, 2, or 3 hours after stimulation. Comparison of the actinomycin D (Act D)–treated cultures, stained for CD40L expression at 6 hours, to the level of CD40L expression at 0, 1, 2, and 3 hours in control cultures stimulated without Act D, reveals that there is little increase in CD40L expression after the addition of Act D. The same results were obtained using dichlororibofuranosyl benzimidazole as an inhibitor.
Monocyte cell contact increases CD40L mRNA in CD4+ T cells. Purified CD4+ T cells were cultured alone or with U937 cells and stimulated for 6 hours as indicated at the bottom of the figure. (A) Flow cytometric analysis of CD40L surface expression. (B) RNA was isolated from an aliquot of the same cells shown in panel A. Equal amounts of total RNA from each sample were loaded per lane; and after electrophoresis, the gel was stained for 28S and 18S rRNA (top panel). The gel image was acquired on a Biorad Fluor-S MultiImager with Quantify One Version 4.2 software. Northern blot of the same gel probed for CD40L mRNA (bottom panel). The position of CD40L mRNA is indicated. (C) Results of quantitative RT-PCR for CD40L mRNA performed on an aliquot of the same RNAs used in the Northern blot. Data are mean ± SE for 3 replicates of each sample, expressed as the number of CD40L mRNA copies per nanogram of rRNA. Representative results from one of 4 donors are shown. (D) PBMCs were plated in the presence (Act D) or absence (Control) of a transcriptional inhibitor and stimulated with anti-TCR mAb. Control samples were harvested and stained for CD40L at the indicated times (solid line). Act D was added to the PBMC cultures at 0, 1, 2, or 3 hours, and the Act D–treated samples were harvested and stained for CD40L at 6 hours (hatched columns). CD40L expression is plotted relative to the 6-hour control (100%). Representative results from one of 4 donors are shown.
Monocyte cell contact increases CD40L mRNA in CD4+ T cells. Purified CD4+ T cells were cultured alone or with U937 cells and stimulated for 6 hours as indicated at the bottom of the figure. (A) Flow cytometric analysis of CD40L surface expression. (B) RNA was isolated from an aliquot of the same cells shown in panel A. Equal amounts of total RNA from each sample were loaded per lane; and after electrophoresis, the gel was stained for 28S and 18S rRNA (top panel). The gel image was acquired on a Biorad Fluor-S MultiImager with Quantify One Version 4.2 software. Northern blot of the same gel probed for CD40L mRNA (bottom panel). The position of CD40L mRNA is indicated. (C) Results of quantitative RT-PCR for CD40L mRNA performed on an aliquot of the same RNAs used in the Northern blot. Data are mean ± SE for 3 replicates of each sample, expressed as the number of CD40L mRNA copies per nanogram of rRNA. Representative results from one of 4 donors are shown. (D) PBMCs were plated in the presence (Act D) or absence (Control) of a transcriptional inhibitor and stimulated with anti-TCR mAb. Control samples were harvested and stained for CD40L at the indicated times (solid line). Act D was added to the PBMC cultures at 0, 1, 2, or 3 hours, and the Act D–treated samples were harvested and stained for CD40L at 6 hours (hatched columns). CD40L expression is plotted relative to the 6-hour control (100%). Representative results from one of 4 donors are shown.
Discussion
CD40L is rapidly expressed and promptly down-regulated after TCR stimulation.39-42 Down-regulation is triggered by interactions with CD40, principally on activated B cells.43,44 Unexpectedly, in CD40-deficient mice, circulating CD4+ T cells constitutively express surface CD40L.28,29 This prompted the suggestion that wild-type CD4+ T cells also constitutively express CD40L but that tonic interactions with circulating CD40+ cells down-regulate the ligand, resulting in an apparent lack of surface expression. Several observations in wild-type mice, such as the spontaneous expression of surface CD40L when unstimulated CD4+ T cells are cultured in the absence of CD40+ cells, and the presence of preformed CD40L mRNA and protein, support this notion.28 Although both circulating human and mouse CD4+ T cells express little or no surface CD40L, resting human cells do not contain preformed CD40L mRNA.22-27 Furthermore, humans with CD40 deficiency do not constitutively express CD40L.45 To reconcile these reports of mouse and human CD40L expression, we addressed the proposition that CD40L might be constitutively expressed on human CD4+ T cells. Although preformed CD40L protein has been reported in tonsillar CD4+ T cells, consistent with other reports, our results demonstrate that there is no intracellular pool of CD40L protein in circulating human CD4+ T cells.26,36,46 Correspondingly, we did not observe spontaneous CD40L surface expression on unstimulated CD4+ T cells cultured in the absence of CD40+ cells. This was corroborated by demonstrating that new CD40L surface expression on activated cells requires protein and mRNA synthesis. The low level of CD40L mRNA in unstimulated CD4+ T cells, which presumably encodes surface CD40L present on circulating memory cells, does not contribute to CD40L expression at 6 hours as there is no increase of CD40L when transcription is inhibited.14 These results demonstrate that homeostatic regulation of human CD40L expression is fundamentally different from the mouse.
In the mouse, naive and resting B cells tolerize rather than activate naive T cells.47-50 By contrast, activated B cells are uniformly reported to be effective antigen-presenting cells, and it has been directly shown that they promote CD40L expression whereas resting B cells do not.51,52 Our observation that circulating human B cells cannot promote early CD40L expression is consistent with reports in the mouse. However, the observation that activated or EBV-transformed human B cells cannot promote CD40L expression was unexpected. EBV-immortalized B cells are efficient antigen-presenting cells for T-cell activation as measured by both proliferation and cytokine production.53,54 Although some reports indicate that EBV-immortalized B cells are suboptimal antigen-presenting cells for certain antigens or isolated resting T cells, in our experimental system a maximal TCR stimulus is being delivered by anti-CD3 mAb.55,56 Thus, the failure of activated human B cells to promote CD40L expression cannot be the result of deficient expression of the costimulatory molecules usually associated with T-cell activation.57 CD40 mediated down-modulation also cannot account for the failure of B cells to promote CD40L expression as our experiments were performed in the presence of an anti-CD40 antibody that blocks interactions with CD40L.36,58 Similarly, the mixing experiments demonstrate that there are no other negative signals on B cells that might be inhibiting the induction of early CD40L. Collectively, the mixing experiments and the blocking experiments demonstrate that accessory molecules, such as CD2, LFA-1, and CD80/CD86, contribute to, but are not sufficient for, the monocyte-dependent enhancement of early CD40L expression. Results with the HL-60 monocytic line indicate that some cells can promote CD40L expression independently of LFA-1/ICAM-1 interactions. An important implication of these findings is that B-cell antigen-presenting cell accessory function is not sufficient to fully activate human CD4+ T cells. In essence, a human B cell cannot activate its cognate helper T cell to deliver CD40L-mediated help. Prior activation of the helper T cell, probably by a macrophage or DC, is needed to induce CD40L-dependent pathways in its cognate B cell. Thus, we favor a model for biphasic human CD40L expression that differs from what has been proposed in the mouse.13 We postulate that the early, CD28-independent phase of CD40L expression functions to activate monocyte/DCs and, as a consequence of adequate costimulation, the ensuing CD28-dependent late phase promotes B-cell activation and differentiation. This model, in contrast to the mouse version, also accounts for the IL-2-dependent nature of T-dependent humoral responses in humans, as well as the ability of IL-12 to promote human B-cell activation and antibody production.14,59-62
Although CD40L was originally associated with B-cell activation and differentiation, it was soon recognized that it plays a role in the activation of multiple antigen-presenting cells and also contributes to T-cell differentiation by inducing IL-12.63-66 Through its activation of antigen-presenting cells, CD40L participates in a potentially dangerous positive feedback loop for T-cell activation. Yet paradoxically, the induction of CD40L expression has been thought to require only a TCR signal.34,43 This report is the first demonstration that human CD40L expression is highly dependent on a costimulatory signal present on only a subset of antigen-presenting cells. Unexpectedly, this signal is delivered by the less mature CD16−CD14hi population of circulating monocytes rather than the proinflammatory CD16+CD14lo population.67 The developmental relationship of these 2 subpopulations is incompletely understood, but it is presumably monocyte-derived tissue macrophages or DCs that T cells would encounter in vivo. Our observation that both CD16+ and CD16− monocyte-derived DCs costimulate CD40L expression supports this presumption. In addition, circulating myeloid, but not plasmacytoid, DCs are as potent as monocytes in promoting early CD40L expression. However, they do not contribute to early CD40L expression in our PBMC culture system as their numbers are too few. As for resting B cells, the inability of plasmacytoid DCs to costimulate CD40L expression may contribute to their well-recognized capacity to toleraize T cells.68 Why CD16−CD14hi monocytes, which express other molecules associated with T-cell activation at relatively low levels, should have the capacity to promote early CD40L expression is unclear. We speculate that the constitutive expression of this costimulatory activity may reflect its early position in the T-cell activation cascade or an interaction with some other cell type that monocytes encounter as they differentiate into macrophages.
We have shown that CD40L expression is costimulated by cell surface proteins that are constitutively expressed on monocytes and that LFA-3, ICAM-1, and CD80/CD86 contribute to, but are not sufficient for costimulation. In a human vascular endothelial cell system, CD2/LFA-3 interactions promote CD40L expression on purified, phytohemagglutinin-activated CD4+ T cells by stabilizing the CD40L mRNA.35 Although we have not directly tested the potential contribution of CD40L mRNA stabilization, the ability of transcriptional inhibitors to block CD40L surface expression indicates that new CD40L mRNA synthesis is required. Blocking SLAM, which appears to negatively regulate the early expression of mouse CD40L, also does not effect human CD40L expression and cells from a patient with SLAM Associated Protein deficiency do not over express CD40L (data not shown). Based on the distribution, kinetics of expression, and biology of the known costimulatory molecules, we hypothesize that our observations represent a novel costimulatory activity.
The findings presented here indicate that CD40L function and its regulation are more complex than originally envisioned. Like IL-2, maximal CD40L expression is dependent on accessory signals; however, unlike IL-2, these signals are distinct at early and late times after TCR engagement. Our report reveals a previously unrecognized level of regulation governing CD40L expression that constrains the potential for a runaway positive feedback loop between antigen-presenting cell and T-cell activation. Furthermore, our results have important implications for humoral responses in humans as they show that in contrast to the mouse, activated human B cells are poor potentiators of CD40L expression and that tonic CD40-CD40L interactions are unlikely to contribute to the survival of circulating autoreactive B cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Rachel Ettinger, Stuart Tangye, and Giorgio Trinchieri for critical reading of the manuscript and Amy Klion for providing PBMCs from the XLP donor.
This work was supported by the Department of Health and Human Services (intramural funding).
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
National Institutes of Health
Authorship
Contribution: J.A.R. conceived and designed the experiments, analyzed and interpreted the data, and wrote the manuscript; J.T.S., S.C., J.S., H.A., P.Q.H., and Q.C. designed and performed experiments, collected data, and analyzed and interpreted results; and J.T.S., P.Q.H., and J.S. contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jack A. Ragheb, 29 Lincoln Dr, Bldg 29B, Rm 4G13, HFD-122, Bethesda, MD 20892; e-mail: jr50b@nih.gov.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal