Abstract
Idiotype vaccination for follicular lymphoma is primarily being developed as remission consolidation after chemotherapy. We investigated idiotype vaccination as primary intervention for treatment-naive indolent B-cell lymphoma and in a separate cohort as remission consolidation after chemotherapy to assess immunization-induced immune responses in relation to progression-free survival (German Clinical Trials Register, DRKS00000227). Twenty-one patients in each cohort received 6 intradermal injections of adjuvanted recombinant idiotype Fab fragment (FabId); 76% of patients in both groups developed anti-idiotype antibodies and/or cellular immunity as measured by enzyme-linked immunosorbent assay and interferon-γ ELISpot. In treatment-naive patients, only cellular responses correlated with superior progression-free survival (P < .002) and durable objective remissions (P = .04). Immunization-induced T cells recognized hypermutated or complementarity-determining region 3 epitopes. After remission consolidation immunization, induction of anti-idiotype antibodies correlated with progression-free survival. Low B-cell counts after rituximab therapy predicted for failure to develop anti-idiotype antibodies. These results are similar to published trials showing an association of humoral immunity with control of residual lymphoma. In contrast, effective immunity against untreated lymphoma appears to be dependent on idiotype-specific T cells. Sustained remissions in patients with vaccination-induced cellular immunity suggest clinical benefit and warrant a randomized comparison of this vaccine with expectant management for asymptomatic follicular lymphoma.
Introduction
Indolent B-cell lymphomas in advanced stages remain incurable with available therapies.1-3 The initiation of cytoreductive therapy immediately after diagnosis does not confer a survival advantage for asymptomatic patients compared with deferral of treatment until symptomatic progression.4,5 Given the unavoidable risk of undesirable side effects of conventional chemotherapy and antibody-based cytoreductive regimens, observation remains the recommended approach in this patient population despite improved survival rates with rituximab-based chemoimmunotherapy for follicular lymphoma (FL) patients who require treatment.2,4-6
The individual B-cell receptor, commonly referred to as the lymphoma “idiotype,” constitutes a unique structure and hence a tumor-specific antigen of a malignant B-cell clone. Immunization of indolent B-lymphoma patients against their tumor's idiotype can induce specific antitumor immune responses in vivo.7-11 Based on the assumption that active tumor immunotherapy has the best chance of providing clinical benefit in the clinical setting of minimal residual disease, when only residual tumor cells must be suppressed or eliminated, most clinical trials have investigated idiotype vaccination as remission consolidation after successful prior cytoreductive therapy. In this setting, FL patients who develop an anti-idiotype antibody response experience improved progression-free survival compared with patients who fail to develop a response to the vaccine.8,12,13 Retrospective analyses suggest that this benefit may translate into improved overall survival.14
Recently reported controlled randomized trials of idiotype vaccination for FL in clinical remission have, however, yielded conflicting results in intention-to-treat analyses: When patients were immunized after rituximab monotherapy or after conventional chemotherapy, progression-free survival was not significantly different in vaccination and control groups.12,15 In contrast, when immunization was restricted to patients in complete remission, patients who received an otherwise similar idiotype vaccine formulation had superior relapse-free survival compared with control immunization.16
The potential benefit of idiotype vaccination for lymphoma patients who have not received prior cytoreductive therapy has not yet been addressed systematically. Induction of an efficacious antitumor immune response before other interventions may represent an attractive approach to delay or prevent the necessity for conventional cytoreductive therapy in advanced-stage but asymptomatic indolent B-lymphoma patients. We have recently presented indirect, “reverse immunology” evidence for T cell–mediated immunosurveillance against the idiotype of follicular lymphomas.17 These results permit to speculate that subclinical T-cell reactivity that was insufficient to control lymphoma growth could be enhanced by active immunotherapy to exert clinically meaningful antilymphoma activity.
We report here the results of a phase II vaccination trial designed to demonstrate the immunologic efficacy of recombinant idiotype vaccination as upfront intervention for asymptomatic patients and for remission consolidation. To ease the technical challenge of manufacturing idiotype vaccines on a patient-individual basis, we have developed the expression of idiotype protein as Fab fragments (FabId) in Escherichia coli based on validated immunoglobulin clonality criteria.18,19 In a phase I trial, we have previously demonstrated the feasibility and tolerability of intradermal injection of FabId with the lipid-based adjuvant MF59 in conjunction with subcutaneous granulocyte-macrophage colony-stimulating factor.12
Methods
Patients
Adult patients with nonsecretory indolent B-cell lymphoma in Ann Arbor stage III or IV were eligible if fresh lymphoma tissue could be obtained without general anesthesia. Active infections and severe concomitant illness, active central nervous system involvement, and systemic antineoplastic therapy, including corticosteroids within 4 weeks before the first vaccination were exclusion criteria. Pregnant or lactating women and women with reproductive capacity not using contraception were ineligible. The trial enrolled patients at the University Medical Centers of Freiburg and Greifswald from 2003 to 2009 into 2 separate cohorts: group A consisted of patients who had not received any type of antineoplastic therapy and had no indications for cytoreductive therapy.20 Vaccinations were withheld for patients with evidence for spontaneous lymphoma regression. Patients in objective clinical remission according to Cheson criteria21 after cytoreductive therapy were eligible for vaccination as group B.
The trial protocol was approved by the Institutional Review Board at University Medical Center Freiburg and registered in the German Clinical Trials Register (no. DRKS00000227). All participants gave informed consent in accordance with the Declaration of Helsinki.
Vaccine production and vaccination schedule
Recombinant FabId vaccines were formulated with the adjuvant MF59 and purchased from CellGenix GmbH.10 FabId fragments were produced by anchored PCR cloning of consensus immunoglobulin (Ig) heavy and light chain transcripts from unfixed lymphoma tissue and expression in E coli.18 One vaccination consisted of subcutaneous injections of 250 μg sargramostim (Immunex) per day on 5 consecutive days at the same location. On day 2, 0.5 mg FabId/MF59 was injected intradermally at the sargramostim-conditioned site. Six vaccinations were given in 4-weekly intervals. Patients without lymphoma progression were offered additional maintenance vaccinations at 3-month intervals starting at week 36. Anti–HBs-negative patients received hepatitis B immunizations (Gen-HB-Vax; Aventis Pasteur) with the first, third, and sixth idiotype vaccination. Anti-HBs titers were measured at week 24.
A complete restaging was performed before the first vaccination.21 Disease sites were reassessed on weeks 8, 16, 24, 36, and 52. Subsequently, disease status was reevaluated every 3 months or at suspected relapse. Vaccinations were discontinued if clinical progression was documented. Adverse events were reported according to World Health Organization criteria.22
Immunologic studies
Serum IgG concentration and circulating CD4+, CD8+, and CD19+ lymphocytes were measured before vaccination by flow cytometry according to the institutional diagnostic standards. AntiFabId immune responses of freshly isolated peripheral blood mononuclear cells (PBMCs) were assessed by interferon-γ (IFN-γ) enzyme-linked immunospot (ELISpot) with a protocol validated by blinded interlaboratory testing (www.cimt.eu)23 and adapted for presentation of polypeptides by autologous dendritic cells.10 Only assays with 200 to 400 spots/2 × 104 cells in lectin controls were evaluated. Background spots obtained with dendritic cells without addition of antigen were subtracted. A cellular response to the vaccine idiotype was defined as a significant increase (P < .05 by 2-sided t test) of spots at week 24 and at least one additional postvaccination time point compared with prevaccination samples. The specificity of idiotype-reactive T cells was subsequently tested by ELISpot with control dendritic cells pulsed with allogeneic idiotype that were chosen to share the same VH family with the vaccine idiotype. For epitope mapping studies, idiotype peptides potentially presented by a patient's major histocompatibility complex class I alleles were identified by the SYFPEITHI algorithm (www.syfpeithi.de) and synthesized. Peptide-loaded (1 μg/mL) dendritic cells were used as stimulators in ELISpot assays with unexpanded PBMCs.
To measure FabId-induced proliferation, overnight-rested PBMCs were labeled with 0.5μM carboxyfluorescein succinimidyl ester and cultured with antigen-pulsed or unpulsed dendritic cells. Anti-CD3/CD28–coated beads (Dynal) or phytohemagglutinin (ICN Biomedicals) served as positive controls. After 6 days of stimulation, cells were stained with anti-CD4–allophycocyanin and anti-CD8–Pacific blue antibodies or appropriate isotype controls (all from eBioscience). Loss of carboxyfluorescein succinimidyl ester was analyzed for the lymphocyte gate and in CD4+ and CD8+ cells by flow cytometry.24
To distinguish T helper type 1 (Th1)- and Th2-dominated immune responses, secretion of interleukin-4, interleukin-5, IFN-γ, and tumor necrosis factor-α by FabId-stimulated peripheral blood cells was measured by cytokine bead arrays (BD Biosciences). More detailed information on T-cell assays according to the MIATA framework (www.miataproject.org; Version 1.0, modules 1-5) is available on request.25
Antibody responses to the vaccine were detected by enzyme-linked immunosorbent assay and were defined as a 4-fold titer increase compared with prevaccination serum10 at week 24 and at least one additional time point. To demonstrate binding of immune serum to tumor cells, IgG was isolated by negative selection (Melon Gel IgG purification kit; Thermo Fisher Scientific) from prevaccination and postvaccination serum and incubated with viable cells from tumor biopsies. After fixation, cells were stained with Vectashield/DAPI (Vector Laboratories), anti-CD19–Alexa 647, and anti-IgG–biotin/streptavidin–fluorescein isothiocyanate (both Invitrogen). The fraction of fluorescein isothiocyanate+ cells in the CD19+ population were analyzed by automated digital fluorescence microscopy (ScanR̂ High Content Screening Station; Olympus) with 20× UPLSAPO NA 0.75 air objective and an ORCA-AG camera (Hamamatsu). Images were acquired with Olympus ScanR̂ Acquisition Software Version 2.1.0.16 and analyzed with Olympus ScanR̂ Analysis Software Version 1.2.06).
Fc receptor polymorphism analysis
Sequence variations at position 158 of FcγRIIIa (CD16) were assessed by PCR-based restriction fragment length polymorphism analysis of genomic DNA.26
Statistical methods
The trial was designed according to the optimal 2-stage design27 to detect as primary endpoint an immune response rate of P1 ≥ 30% at week 24 against a spontaneous response rate of P0 = 5% with α and β errors of 10%. An immune response was defined as a cellular response, antibody response, or both. These criteria translate into patient number n1 = 7 and n = 21. Both patient groups (upfront immunization and remission consolidation) were analyzed independently.
Secondary endpoints were objective lymphoma responses, progression-free survival, treatment-free survival, and overall survival from the start of vaccination and from diagnosis. Progression-free survival was defined as the interval between start of vaccination and death from any cause or objective lymphoma progression according to Cheson criteria, whichever came first.21 Treatment-free survival was defined as the interval between start of vaccination and next cytoreductive therapy or death. Progression-free and treatment-free survival was estimated by the Kaplan-Meier method and compared from start of vaccination and in a landmark analysis28 from week 24 by log-rank test (Prism Version 5.0; GraphPad Software). Comparisons of laboratory data between patient groups were performed with the 2-tailed Mann-Whitney test. Analyses of experimental results from different time points were performed with Wilcoxon 2-tailed matched-pair test.
Results
Patient population and toxicity
In both groups, FL was the most common diagnosis. The majority of patients had additional disease-related risk factors beyond the advanced clinical stage and fell into intermediate-risk categories according to their disease-adapted prognostic scores (Tables 1 and 2). Four FL patients had objectively progressing disease at the start of vaccination. Two additional FL patients in “watch and wait” situation who had been scheduled for group A had evidence for spontaneous tumor regression and were not treated.
Clinical characteristics of patients: group A (watch and wait)
| . | . | All patients . | FL . | MCL . | nMZL . |
|---|---|---|---|---|---|
| n | 21 | 15 | 3 | 3 | |
| Median (range) age | years | 53 (30-78) | 44 (30-60) | 77 (56-78) | 68 (57-75) |
| Stage | III A | 10 | 9 | 0 | 1 |
| IV A | 11 | 6 | 3 | 2 | |
| Risk score, n | IPI 1/5: 13 | FLIPI 1/5: 5 | MIPI 5-6: 1 | IPI 1/5: 1 | |
| IPI 2-3/5: 8 | FLIPI 2/5: 8 | MIPI 6-7: 2 | IPI 2/5: 2 | ||
| FLIPI 3/5: 2 | |||||
| Median (range) time from diagnosis to first vaccination | months | 13 (5-105) | 13 (5-105) | 11 (7-15) | 18 (8-27) |
| Remission status | SD | 17 | 11 | 3 | 3 |
| PD | 4 | 4 | 0 | 0 | |
| FcγRIIIa genotype | 158FF | 9 | 6 | 2 | 1 |
| 158FV | 8 | 6 | 1 | 1 | |
| 158VV | 3 | 2 | 0 | 1 |
| . | . | All patients . | FL . | MCL . | nMZL . |
|---|---|---|---|---|---|
| n | 21 | 15 | 3 | 3 | |
| Median (range) age | years | 53 (30-78) | 44 (30-60) | 77 (56-78) | 68 (57-75) |
| Stage | III A | 10 | 9 | 0 | 1 |
| IV A | 11 | 6 | 3 | 2 | |
| Risk score, n | IPI 1/5: 13 | FLIPI 1/5: 5 | MIPI 5-6: 1 | IPI 1/5: 1 | |
| IPI 2-3/5: 8 | FLIPI 2/5: 8 | MIPI 6-7: 2 | IPI 2/5: 2 | ||
| FLIPI 3/5: 2 | |||||
| Median (range) time from diagnosis to first vaccination | months | 13 (5-105) | 13 (5-105) | 11 (7-15) | 18 (8-27) |
| Remission status | SD | 17 | 11 | 3 | 3 |
| PD | 4 | 4 | 0 | 0 | |
| FcγRIIIa genotype | 158FF | 9 | 6 | 2 | 1 |
| 158FV | 8 | 6 | 1 | 1 | |
| 158VV | 3 | 2 | 0 | 1 |
IPI indicates International Prognostic Index; FLIPI, Follicular Lymphoma International Prognostic Index; and MIPI, Mantle Cell Lymphoma International Prognostic Index.
Clinical characteristics of patients: group B (remission consolidation)
| . | . | All patients . | FL . | MCL . | LL . |
|---|---|---|---|---|---|
| n | 21 | 14 | 6 | 1 | |
| Median (range) age | years | 54 (29-70) | 51 (29-70) | 56 (44-64) | 37 |
| Stage | IIA* | 1* | 1* | 0 | 0 |
| IIIA | 6 | 5 | 1 | 0 | |
| IIIB | 2 | 2 | 0 | 0 | |
| IVA | 10 | 5 | 5 | 1 | |
| IVB | 2 | 1 | 1 | 0 | |
| Risk score: n | IPI 1/5: 10 | FLIPI 1/5: 4 | MIPI 5-6: 3 | IPI 2/5: 1 | |
| IPI 2-3/5: 9 | FLIPI 2/5: 7 | MIPI 6-7: 3 | |||
| IPI 4-5/5: 2 | FLIPI 3-4/5: 3 | ||||
| Last systemic therapy | CHOP | 2 | 2 | 0 | 0 |
| R-CHOP | 6 | 6 | 0 | 0 | |
| F | 1 | 0 | 1 | 0 | |
| FC | 2 | 1 | 0 | 1 | |
| FC-R | 2 | 2 | 0 | 0 | |
| Auto-SCT | 5 | 0 | 5 | 0 | |
| R mono | 2 | 2 | 0 | 0 | |
| Chloramb | 1 | 1 | 0 | 0 | |
| Remission status | First CR | 11 | 6 | 5 | 0 |
| First PR | 3 | 1 | 1 | 1 | |
| Second CR | 2 | 2 | 0 | 0 | |
| Second PR | 4 | 4 | 0 | 0 | |
| Fifth PR | 1 | 1 | 0 | 0 | |
| Median (range) time | months | 5 (1-21) | 6 {1-21) | 3 (3-15) | 1 |
| end chemotherapy to first vaccination | |||||
| FcγRIIIa genotype | 158FF | 11 | 8 | 2 | 1 |
| 158FV | 8 | 5 | 3 | 0 | |
| 158VV | 2 | 1 | 1 | 0 |
| . | . | All patients . | FL . | MCL . | LL . |
|---|---|---|---|---|---|
| n | 21 | 14 | 6 | 1 | |
| Median (range) age | years | 54 (29-70) | 51 (29-70) | 56 (44-64) | 37 |
| Stage | IIA* | 1* | 1* | 0 | 0 |
| IIIA | 6 | 5 | 1 | 0 | |
| IIIB | 2 | 2 | 0 | 0 | |
| IVA | 10 | 5 | 5 | 1 | |
| IVB | 2 | 1 | 1 | 0 | |
| Risk score: n | IPI 1/5: 10 | FLIPI 1/5: 4 | MIPI 5-6: 3 | IPI 2/5: 1 | |
| IPI 2-3/5: 9 | FLIPI 2/5: 7 | MIPI 6-7: 3 | |||
| IPI 4-5/5: 2 | FLIPI 3-4/5: 3 | ||||
| Last systemic therapy | CHOP | 2 | 2 | 0 | 0 |
| R-CHOP | 6 | 6 | 0 | 0 | |
| F | 1 | 0 | 1 | 0 | |
| FC | 2 | 1 | 0 | 1 | |
| FC-R | 2 | 2 | 0 | 0 | |
| Auto-SCT | 5 | 0 | 5 | 0 | |
| R mono | 2 | 2 | 0 | 0 | |
| Chloramb | 1 | 1 | 0 | 0 | |
| Remission status | First CR | 11 | 6 | 5 | 0 |
| First PR | 3 | 1 | 1 | 1 | |
| Second CR | 2 | 2 | 0 | 0 | |
| Second PR | 4 | 4 | 0 | 0 | |
| Fifth PR | 1 | 1 | 0 | 0 | |
| Median (range) time | months | 5 (1-21) | 6 {1-21) | 3 (3-15) | 1 |
| end chemotherapy to first vaccination | |||||
| FcγRIIIa genotype | 158FF | 11 | 8 | 2 | 1 |
| 158FV | 8 | 5 | 3 | 0 | |
| 158VV | 2 | 1 | 1 | 0 |
IPI indicates International Prognostic Index; FLIPI, Follicular Lymphoma International Prognostic Index; MIPI, Mantle Cell Lymphoma International Prognostic Index; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CHOP, CHOP and rituximab; F, fludarabine; FC, fludarabine and cyclophosphamide; FC-R, FC and rituximab; Auto-SCT, high-dose chemotherapy and autologous stem cell transplantation; R mono, rituximab monotherapy; and Chloramb, chlorambucil.
One FL patient with stage IIA was deemed unsuitable for radiation therapy because of bulky intra-abdominal disease. She was treated as a stage IIIA patient with chemotherapy and was therefore included into this trial.
Grade 1 or 2 injection site reactions occurred in the majority of patients. The only case of grade 3 toxicity was a generalized erythema. After the sargramostim dose was reduced to 100 μg/day, the patient tolerated all subsequent vaccinations well. All patients completed the planned 6 vaccinations. Eight patients in group A and 9 patients in group B received between 1 and 29 additional maintenance immunizations (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Immune status before vaccination
Six (29%) group A patients had reduced CD4+ and 4 (19%) reduced CD8+ T cells (Figure 1A). Ten of 12 (83%) anti–HBs-negative group A patients failed hepatitis B vaccination.
Immune status and immune responses. (A) Prevaccination lymphocyte subsets in the peripheral blood of untreated lymphoma patients (group A) and patients in remission (group B). Dashed lines indicate normal ranges. R indicates rituximab therapy. (B) Lymphocyte subsets of patients according to detection or absence of cellular (left and middle panels) or humoral immune responses (right panel). n.s. indicates not significant.
Immune status and immune responses. (A) Prevaccination lymphocyte subsets in the peripheral blood of untreated lymphoma patients (group A) and patients in remission (group B). Dashed lines indicate normal ranges. R indicates rituximab therapy. (B) Lymphocyte subsets of patients according to detection or absence of cellular (left and middle panels) or humoral immune responses (right panel). n.s. indicates not significant.
In the remission group, 12 of 19 patients (63%) with complete immune status had reduced CD4+ and one (5%) reduced CD8+ T cells. In association with prior rituximab therapy, 11 patients (58%) had reduced B-cell counts (Figure 1A). Fourteen of 15 (93%) anti–HBs-negative remission patients failed hepatitis B vaccination.
Primary endpoint: induction of immune responses
In group A, 5 of the first 7 patients (n1) developed an immune response, and the trial was continued to the second stage. In the final analysis, 12 of evaluable 20 patients (60%) developed a cellular immune response to idiotype. Nine of 21 patients (43%) developed anti-idiotype IgM or IgG antibodies (5 IgM; 7 IgG). Sixteen patients (76%; 95% confidence interval, 58%-94%) of group A had at least one type of immune response. Both anti-HBs responders also developed anti-idiotype antibodies but remained negative by ELISpot.
In the first stage of group B, all patients had an immune response. In the final analysis, circulating idiotype-reactive cells were induced in 11 (55%) and antibody responses in 10 (50%) of 20 evaluable group B patients (6 IgM, 9 IgG). At least one type of response was detected in 16 patients (76%; 95% confidence interval, 58%-94%).
In both cohorts, the development of cellular or humoral immune responses was not correlated to prevaccination numbers of CD4+ and CD8+ T cells (Figure 1B; and data not shown). Lack of antibody responses was associated in group B with reduced B cells (Figure 1B). Serum immunoglobulin levels were not associated with any type of immune response (data not shown).
Kinetics and qualities of immune responses
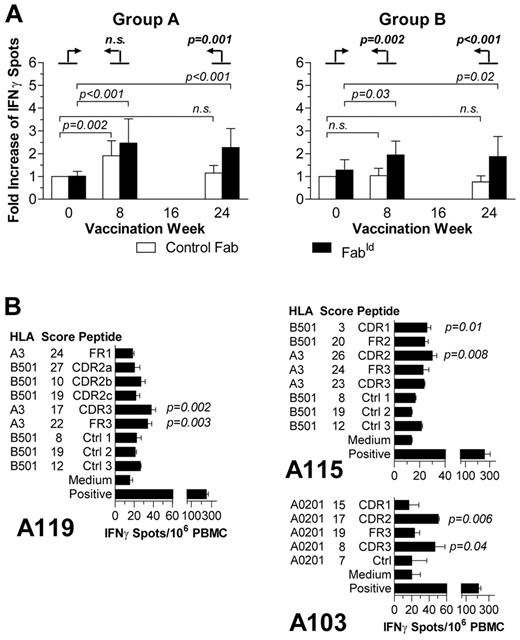
Additional experiments were performed in patients with sufficient available PBMCs to gain more information on the vaccination-induced immune responses. The frequency of idiotype-reactive T cells increased during the initial 6 vaccinations, had a tendency to decline in the following 3-month interval, but could be boosted by maintenance immunization (Figure 2A). AntiFabId antibody titers peaked at the end of the 6 monthly vaccinations and persisted for at least additional 6 months (Figure 2B). In vitro stimulation experiments showed proliferation of PBMCs in response to FabId (Figure 2C). The proliferating cells were composed of 8% to 68% CD4+ and 8% to 62% CD8+ cells (representative example in Figure 2D; raw data in supplemental Table 1). The fraction of cells with a regulatory phenotype within the CD4+ PBMC population declined during the immunization period in all analyzed patients and was significantly decreased at the time point of the primary endpoint after completion of the 6 planned vaccinations (Figure 2E). Cytokine profiling of FabId-stimulated blood cells at the same time point showed increased release of typical Th1 cytokines but not of Th2 cytokines after FabId immunization (Figure 2F).
Kinetics of immune responses. (A) Kinetics of circulating idiotype-reactive cells in responding patients as measured by IFN-γ ELISpot. (B) Kinetics of anti-idiotype IgG titers in responding patients as measured by enzyme-linked immunosorbent assay. (C) PBMC proliferation in groups A (left) and B (right) induced by autologous dendritic cells as determined by flow cytometry measurement of carboxyfluorescein succinimidyl ester loss. (D) Analysis of CD4+ and CD8+ lymphocyte subsets in proliferating cells after FabId stimulation by flow cytometry in a representative patient. (E) Kinetics of the proportion of CD4+CD25+FoxP3+ regulatory T cells in peripheral blood lymphocytes during the vaccination period. (F) Prevaccination and postvaccination release of T helper 1 (IFN-γ and tumor necrosis factor-α) and T helper 2 cytokines (interleukin-4 [IL4] and interleukin-5 [IL5]) by peripheral blood lymphocytes on FabId stimulation.
Kinetics of immune responses. (A) Kinetics of circulating idiotype-reactive cells in responding patients as measured by IFN-γ ELISpot. (B) Kinetics of anti-idiotype IgG titers in responding patients as measured by enzyme-linked immunosorbent assay. (C) PBMC proliferation in groups A (left) and B (right) induced by autologous dendritic cells as determined by flow cytometry measurement of carboxyfluorescein succinimidyl ester loss. (D) Analysis of CD4+ and CD8+ lymphocyte subsets in proliferating cells after FabId stimulation by flow cytometry in a representative patient. (E) Kinetics of the proportion of CD4+CD25+FoxP3+ regulatory T cells in peripheral blood lymphocytes during the vaccination period. (F) Prevaccination and postvaccination release of T helper 1 (IFN-γ and tumor necrosis factor-α) and T helper 2 cytokines (interleukin-4 [IL4] and interleukin-5 [IL5]) by peripheral blood lymphocytes on FabId stimulation.
Target specificity and tumor cell recognition of anti-idiotype immune responses
In group A, the ELISpot assays indicated an increase of T cells with reactivity to the vaccine FabId during the vaccination but also an initial response to control FabId with the same VH family (Figure 3A). Analysis of the induced vaccine-reactive T-cell response against the background of the nonspecific ELISpot counts by means of an objective Poisson regression analysis10 confirmed that the vaccination-induced T-cell reactivity was indeed nonspecific after 2 vaccinations (at week 8) but developed into an antigen-specific response to the patient's idiotype after 6 vaccinations in group A. In group B, the induced cellular immune response was specific throughout the immunization period.
Specificity of cellular anti-idiotype immune response. (A) Comparison of the normalized relative frequency of IFN-γ-releasing cells induced by FabId from all ELISpot responders at various time points against the actual frequency of control Fab-reactive cells. Prevaccination reactivity to control Fab was defined as 1. Error bars represent 95% confidence intervals. The significances of relative differences of FabId-induced ELISpots to week 0 against the background change of control-Fab-induced differences are calculated by a Poisson regression model10 and indicated in boldface type. (B) Epitope mapping analysis of postvaccination peripheral blood mononuclear cells by ELISpot with synthetic HLA-restricted peptides identified by the SYFPEITHI algorithm in the patient's lymphoma idiotype IgH chain.
Specificity of cellular anti-idiotype immune response. (A) Comparison of the normalized relative frequency of IFN-γ-releasing cells induced by FabId from all ELISpot responders at various time points against the actual frequency of control Fab-reactive cells. Prevaccination reactivity to control Fab was defined as 1. Error bars represent 95% confidence intervals. The significances of relative differences of FabId-induced ELISpots to week 0 against the background change of control-Fab-induced differences are calculated by a Poisson regression model10 and indicated in boldface type. (B) Epitope mapping analysis of postvaccination peripheral blood mononuclear cells by ELISpot with synthetic HLA-restricted peptides identified by the SYFPEITHI algorithm in the patient's lymphoma idiotype IgH chain.
Functional epitope mapping experiments demonstrated T-cell activation by peptides derived from complementarity-determining regions (Figure 3B). Framework epitopes did not elicit IFN-γ release, except a framework peptide of patient A119 (AIFRDNAKN) that contained 2 mutations to the germline sequence (TISRDNAKN).
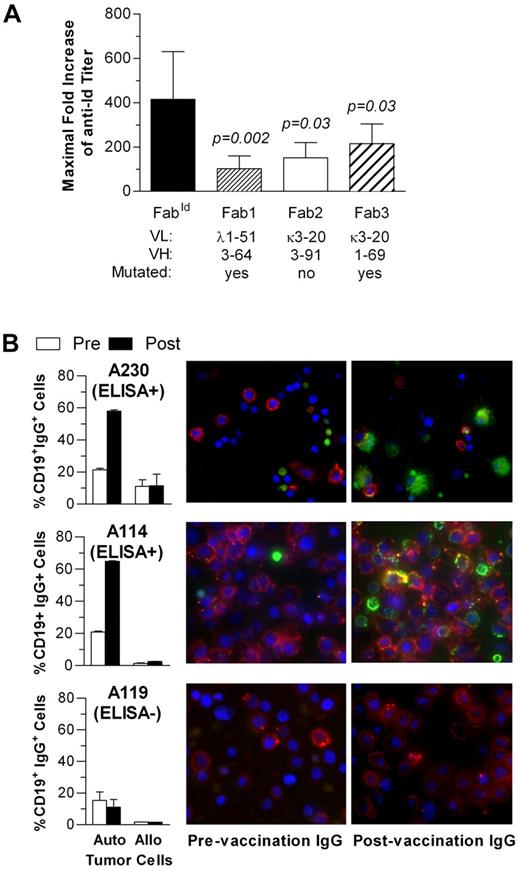
Humoral responses had also predominant specificity for the autologous idiotype as evident from testing for cross-reactivity to a fixed panel of 3 control Fabs (Figure 4A). Binding of vaccination-induced antiFabId antibodies to viable tumor cells was demonstrated for IgG purified from prevaccination and postvaccination sera by automated digital fluorescence microscopy (Figure 4B).
Specificity of humoral anti-idiotype immune response. (A) Maximal anti-FabId IgG titer increase after idiotype vaccination in all enzyme-linked immunosorbent assay responders compared with a fixed panel of 3 control Fab. (B) Binding of FabId vaccination-induced antibodies to autologous and allogeneic tumor cells assessed by automated digital fluorescence microscopy. Bar graphs represent the percentage of tumor cells with surface-bound IgG. Photographs show autologous tumor cells incubated with prevaccination or postvaccination serum IgG. Red represents anti-CD19; green, anti-IgG; and blue, nuclear counterstain with DAPI.
Specificity of humoral anti-idiotype immune response. (A) Maximal anti-FabId IgG titer increase after idiotype vaccination in all enzyme-linked immunosorbent assay responders compared with a fixed panel of 3 control Fab. (B) Binding of FabId vaccination-induced antibodies to autologous and allogeneic tumor cells assessed by automated digital fluorescence microscopy. Bar graphs represent the percentage of tumor cells with surface-bound IgG. Photographs show autologous tumor cells incubated with prevaccination or postvaccination serum IgG. Red represents anti-CD19; green, anti-IgG; and blue, nuclear counterstain with DAPI.
Clinical outcome and immune responses in patients without prior therapy (group A)
After a median follow-up of 54 months (range, 20-78 months), the median progression-free and treatment-free survival after the first vaccination were 18 and 30 months, respectively, and 47 and 48 months after diagnosis (Figure 5A). Fifteen patients (71%) required cytoreductive therapy between 6 and 45 months after vaccination start. Two patients with mantle cell lymphoma died 45 and 46 months after their first vaccination, and a patient with marginal zone lymphoma after 33 months. Six patients (29%), 4 with FL and one with nodal marginal zone lymphoma, remain free of therapy 38 to 64 months after vaccination start and have an excellent quality of life.
Clinical outcome in upfront vaccination (group A). (A) Kaplan-Meier curves of progression-free survival (PFS) and treatment-free survival (TFS) after vaccination start (left panel) and lymphoma diagnosis (right panel). (B) Evolution of total lymphoma burden at 24 weeks after the first vaccination in relationship to cellular immune responses and lymphoma entity. R indicates patients who eventually achieved an objective partial remission; F, follicular lymphoma; M, mantle cell lymphoma; and m, marginal zone lymphoma. (C) Computed tomography scans of patient A119 with a near-complete disappearance of a disfiguring submandibular lymphoma manifestation.
Clinical outcome in upfront vaccination (group A). (A) Kaplan-Meier curves of progression-free survival (PFS) and treatment-free survival (TFS) after vaccination start (left panel) and lymphoma diagnosis (right panel). (B) Evolution of total lymphoma burden at 24 weeks after the first vaccination in relationship to cellular immune responses and lymphoma entity. R indicates patients who eventually achieved an objective partial remission; F, follicular lymphoma; M, mantle cell lymphoma; and m, marginal zone lymphoma. (C) Computed tomography scans of patient A119 with a near-complete disappearance of a disfiguring submandibular lymphoma manifestation.
At the prespecified time point for the analysis of immune responses (24 weeks), the tumor burden appeared to be minimally to moderately reduced in some patients, especially in patients with cellular immune responses (Figure 5B). Tumor regressions continued and also began to occur beyond this time point. In 6 of 15 (40%) FL patients, including 2 patients with progressive disease at the time of their first vaccination, these regressions reached the criteria for objective partial remissions between 6 and 29 months after the start of idiotype vaccination (Figure 5C; patients marked with “R” in Figure 5B). Four patients are in continuing remission at 57 to 64 months; 2 responding lymphoma eventually progressed at 34 and 39 months, respectively.
Cellular immune responses, particularly in FL patients, were associated with freedom from progression (Figure 6A). Because there were no events before the time point to assess the primary endpoint at 24 weeks, equivalent results were observed in a landmark outcome analysis at this time point. Humoral immune responses and the FcγRIIIa genotype were not correlated with outcome. All objective responders developed a cellular immune response (P = .04, Fisher exact test). Anti-idiotype antibodies were induced in 3 of the 6 objectively responding patients.
Progression-free survival and anti-idiotype immune responses. Progression-free survival in patients with upfront idiotype vaccination (group A) and idiotype vaccination as remission consolidation (group B) according to the development of cellular (left) or humoral immune responses (right). HR indicates hazard ratio.
Progression-free survival and anti-idiotype immune responses. Progression-free survival in patients with upfront idiotype vaccination (group A) and idiotype vaccination as remission consolidation (group B) according to the development of cellular (left) or humoral immune responses (right). HR indicates hazard ratio.
Immune responses and outcome in remission consolidation
At a median follow-up of 46 months (range, 10-69 months) after the first vaccination, the estimated median progression-free and treatment-free survival of group B are 30 and 53 months, respectively. Ten patients in this group, including 9 of 14 patients (64%) with FL, remain in remission. Ten patients have actuarially required additional cytoreductive therapy 8 to 53 months after vaccination start and 15 to 70 months after the last chemotherapy, respectively. Two patients with mantle cell lymphoma have died resulting from lymphoma relapse 27 and 52 months after vaccination start. The patient with lymphocytic lymphoma, carrying the prognostically poor del11q22, died 41 months after the first vaccination. Progression-free survival in FL patients was correlated with humoral but not cellular immune responses (Figure 6). There was no correlation of the FcγRIIIa genotype alone or in combination with humoral immune responses and outcome.
Discussion
This trial demonstrates a high immune response rate to intradermal administration of the recombinant FabId vaccine in indolent B-cell lymphoma patients. Both patient cohorts enrolled in this trial had a high incidence of numerical and functional immunodeficiency as indicated by high failure rates of simultaneous hepatitis B vaccination. Despite this impairment, the primary endpoint of a 30% anti-idiotype immune response rate was surpassed for both indications studied.
The immune response rate in the remission consolidation cohort appears to be similar to published idiotype vaccination studies in FL.9,11,13,14,16,29 Most of these reported trials have used idiotype vaccines conjugated to the immunologic carrier keyhole limpet hemocyanin.8,9,11-13 The satisfactory immunogenicity of our unconjugated FabId vaccine may be attributable to a combination of several factors, including the combination with the adjuvant MF59 and extended conditioning of the injection site with sargramostim. We think that the intradermal injection route, which has been recognized to be highly efficacious,30 represents perhaps the single most important aspect for achieving immune responses in immunologically impaired persons. Finally, the FabId vaccine antigen lacks the Fc region of immunoglobulin. The Fc regions are known to harbor Tregitopes (ie, human leukocyte antigen [HLA] class II-presented epitopes that are recognized by regulatory T cells) and induce immunosuppressive activity.31 The existence of Tregitopes may provide a structural basis for immunomodulatory activity of polyclonal immunoglobulin. Therefore, Tregitopes could dampen immune responses to an entire lymphoma-derived idiotype immunoglobulin, and their absence from a vaccine, as is the case in our FabId format, may result in a superior immune response rate. To support this hypothesis, we are currently conducting in vitro stimulation experiments comparing entire monoclonal immunoglobulin with their corresponding recombinant FabId.
In the most commonly studied indication of remission consolidation, the induction of anti-idiotype antibodies has consistently been associated with superior progression-free survival, and possibly with better overall survival.12-14 The clinical outcome of our group B matches these results and suggests similar biologic activity for the FabId vaccine. Importantly, we demonstrate that the vaccination-induced antiFabId antibodies indeed target autologous tumor cells. Furthermore, the absence of the prognostically favorable antibody response in patients with low counts of circulating B cell may explain the failure of an randomized trial testing idiotype vaccination shortly after rituximab monotherapy.15 An association of B-cell depletion through prior administration of rituximab and subsequent failure to develop humoral immune responses strongly suggests that idiotype vaccination should precede a B cell–depleting antibody therapy if active and passive immunotherapy components were to be integrated in a combined regimen.
Most importantly, this report describes the first trial of idiotype vaccination monotherapy in untreated lymphoma patients who carry a considerable tumor burden. Regression of measurable tumor manifestations after idiotype vaccination has been described before8-10,32 but has not been analyzed systematically in a prospective trial.
In prospective studies of watch and wait management of FL, the median time to requirement of cytoreductive therapy has been 23 to 31 months.4 In our FL patients, the median time to therapy was 48 months after diagnosis. These values, however, cannot be compared because of considerable delays between diagnosis and vaccination in our trial. These delays, mostly attributable to the referral of previously diagnosed patients from outside institutions, were not correlated to immune response rate or outcome (data not shown). On the other hand, the presence of additional risk factors beyond advanced stage indicates that our trial did not selectively include a patient population with minimal risk of tumor progression. Nevertheless, the intervals from diagnosis to vaccination may represent a selection bias that calls for a prospective randomized trial.
The occurrence of durable remissions in FL patients may therefore represent a better indicator for potential clinical efficacy of FabId vaccination. The spontaneous remission rate of FL was 4.6% in the only prospective trial reported in the literature.4 We exerted meticulous scrutiny in excluding patients who showed any evidence for spontaneous tumor regression, and indeed withheld therapy in 2 such persons. The occurrence of durable objective remissions in otherwise untreated FL patients, which occurred even in progressing lymphomas and were associated with measurable immune responses in every case, suggest clinical benefit.
In contrast to patients vaccinated in clinical remission after conventional cytoreductive therapy, where long-term suppression of residual lymphoma cells was consistently associated with the induction of anti-idiotype antibodies,12-14 superior progression-free survival was associated in our trial with cellular rather than humoral immune responses. Control or even reduction of large tumor burdens may therefore depend on the induction of tumor-specific cellular immune activity.
Of note, both patients who developed anti-HBs responses failed to develop cellular anti-idiotype responses. Together with the independence of immune responses from the numerical immune status, these results indicate that the potential favorable effect of FabId vaccination is not merely attributable to a superior general state of the immune system.
Cellular idiotype-specific immune responses have been identified previously as indispensable in several animal vaccination models.33-36 In these models, idiotype-specific T cells recognize preferentially unique epitopes from complementarity-determining region or generated through somatic hypermutation, whereas the natural T-cell receptor repertoire is considered tolerant toward germline-encoded immunoglobulin sequences.37 We have recently demonstrated that T regulatory cells (Tregs) appear to play an important role in the suppression of effector T cells with specificity for framework region epitopes.38
In accordance with these animals studies, the Poisson regression analyses of the ELISpot results in our consecutive trials,10 and our in vitro experiments with idiotype-transfected surrogate target cells19 suggest that vaccination-induced T-cell activity is directed predominantly against individual rather than shared idiotype peptides. This conclusion was confirmed by the determination of the precise specificity of the observed T-cell responses in epitope mapping experiments: Together with a case in our previous trial10 and the identification of an individual HLA class II-restricted antigen by others,39 these experiments consistently show that anti-idiotype cellular immunity targets predominantly complementarity-determining region-derived and/or hypermutated idiotype peptides. Cytotoxic T cells with specificity for framework-derived peptides are known to circulate in B-lymphoma patients,40 but these T cells apparently fail to respond to idiotype vaccination in vivo.
In contrast to the question of specificity and regulation of idiotype-directed T-cell immunity, the cellular effector mechanisms that may lead to lymphoma regression are more difficult to identify. Dependent on the particular model and immunization strategy, efficient antilymphoma immunity in mouse studies depended either on both CD4+ and CD8+ effector cells,33,35 or on CD4+ T cells alone as the single dominant effector cell population.34,41 In the CD4+ T cell–dependent models, idiotype vaccination was efficacious even when the effector cells did not directly bind to idiotype-derived peptides on the lymphoma cells. These animal studies suggest that idiotype-specific cellular effector mechanisms may range from classic, perforin- and granzyme-mediated cytotoxicity to humoral mechanisms that do not require direct contacts between T cells and tumor cells. Likewise, the crucial cellular effector mechanisms in humans might vary from patient to patient. T cells with classic cytotoxicity toward lymphoma cells can be stimulated by idiotype vaccination in vivo and in vitro.9,19 Nevertheless, we chose quantitative measurement of IFN-γ secretion as our most important surrogate endpoint because a broad range of studies indicate that IFN-γ is a central and pleiotropic mediator of efficient antitumor immunity.42 Important effects of IFN-γ include the suppression of Treg activity,43 inhibition of angiogenesis,44 and the polarization of T helper cells toward a Th1 milieu.42 Furthermore, IFN-γ appears to play a special role in the immunosurveillance against naturally occurring lymphomas in mice.45
We have recently presented bioinformatic evidence for selection of a given FL idiotype for relatively low immunogenicity by the affected patient's HLA type.17 Although these findings may be taken as evidence for potential spontaneous antilymphoma T-cell activity against HLA class I–presented idiotype epitopes, our current data suggest that active idiotype immunization may convert an apparently inefficient immune recognition of intermediately immunogenic individual idiotypes into a clinically efficacious T-cell response. In this context, it may be a potentially important observation that the fraction of circulating Tregs, the most important cell population to maintain peripheral tolerance,46 decreased in all analyzed patients. In contrast, a vaccination-induced increase of Tregs was strongly associated with failure of idiotype vaccination to induce an effector T-cell response in myeloma patients.47 The cytokine milieu of the cellular immune response may represent another potentially important mechanism for the vaccination effects: We observed conversion of a T helper 2–dominated T-cell milieu into predominance of Th1 cells, but not the opposite phenomenon. Because Th2-biased, idiotype-specific T helper cells can even induce B-cell lymphomagenesis in a transgenic mouse model,48 the induction of idiotype-specific Th2 cells should be avoided in clinical idiotype vaccination, and a Th1-dominated immune response appears to be desirable with respect to both safety and efficacy.
In conclusion, our results demonstrate the induction of specific immunity and associated durable clinical remissions in FL patients receiving idiotype vaccination as first-line therapy. Concomitant immunologic studies identify crucial candidate effector mechanisms and potentially important surrogate markers for the in vivo induced immune responses. This vaccination strategy has the potential to fulfill the requirements for a biologic, well-tolerated intervention to delay or prevent the necessity for cytoreductive therapy in asymptomatic indolent lymphoma patients.
Presented in part as an oral presentation at the 50th annual meeting of the American Society of Hematology, San Francisco, CA, December 2008.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Heide Dierbach for expert technical assistance, and Drs Ola Linden and Thomas Relander, Lund; Bernhard Woermann, Braunschweig; Martin Dreyling, Munich; and Christoph von Schilling, Freising, for referring patients. We are indebted to Marie Follo, PhD, for expert assistance with digital microscopy.
This study was supported in part by the Deutsche Krebshilfe (grant 70-2698-Ve I) (H.V.).
Authorship
Contribution: H.V. and G.I. designed the study; K.H.-M., F.S., A.K.-H., G.D., and H.V. recruited patients and collected clinical data; C.B.-L. and M.A.N. performed immunologic analyses; M.A.N., K.H.-M., G.I., A.K.-H., and H.V. analyzed the data and take responsibility for the accuracy of the data analysis; H.V. and M.A.N. wrote the paper; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hendrik Veelken, Department of Hematology, Leiden University Medical Center, PO Box 9600, C2-R-140, 2300 RC Leiden, The Netherlands; e-mail: j.h.veelken@lumc.nl.


![Figure 2. Kinetics of immune responses. (A) Kinetics of circulating idiotype-reactive cells in responding patients as measured by IFN-γ ELISpot. (B) Kinetics of anti-idiotype IgG titers in responding patients as measured by enzyme-linked immunosorbent assay. (C) PBMC proliferation in groups A (left) and B (right) induced by autologous dendritic cells as determined by flow cytometry measurement of carboxyfluorescein succinimidyl ester loss. (D) Analysis of CD4+ and CD8+ lymphocyte subsets in proliferating cells after FabId stimulation by flow cytometry in a representative patient. (E) Kinetics of the proportion of CD4+CD25+FoxP3+ regulatory T cells in peripheral blood lymphocytes during the vaccination period. (F) Prevaccination and postvaccination release of T helper 1 (IFN-γ and tumor necrosis factor-α) and T helper 2 cytokines (interleukin-4 [IL4] and interleukin-5 [IL5]) by peripheral blood lymphocytes on FabId stimulation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/5/10.1182_blood-2010-06-292342/4/m_zh89991064700002.jpeg?Expires=1765931897&Signature=n2R~v7ymeefs0zaBEe12f2uHQJ~fPgDLutAQ8xld5WvNtDaMmIo3j3SiO4Kt8BJuZQ14WBbhyfPy1Facd60vUYIppFO-v~ihwPwRQdFfhYlzmec~kLfo1cZq2PqLiQ7fH8L7XDjWfyC5sZzXCnZwBVkWrDnha8F3vmd76BqitiHh5l3OSIjHWnXei1k~b7jXy3Je7xsRY3iSFdXvOsByo6atFnBRoaHEgcFf7ltyiJqpiWB8ah0O1AYRaKTq348SiWtvAmeYXeJtougchtjOI072khGhpxCLlC9u5-MS1u1J6A-IVuajUyD5mkcQZ3JkBgIhYLpcBq8CLpEesZi1lA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)