Abstract
Hepcidin is a major regulator of iron homeostasis, and its expression in liver is regulated by iron, inflammation, and erythropoietic activity with mechanisms that involve bone morphogenetic proteins (BMPs) binding their receptors and coreceptors. Here we show that exogenous heparin strongly inhibited hepcidin expression in hepatic HepG2 cells at pharmacologic concentrations, with a mechanism that probably involves bone morphogenetic protein 6 sequestering and the blocking of SMAD signaling. Treatment of mice with pharmacologic doses of heparin inhibited liver hepcidin mRNA expression and SMAD phosphorylation, reduced spleen iron concentration, and increased serum iron. Moreover, we observed a strong reduction of serum hepcidin in 5 patients treated with heparin to prevent deep vein thrombosis, which was accompanied by an increase of serum iron and a reduction of C-reactive protein levels. The data show an unrecognized role for heparin in regulating iron homeostasis and indicate novel approaches to the treatment of iron-restricted iron deficiency anemia.
Introduction
Iron maldistribution is implicated in various diseases, including hemochromatosis, iron-loading anemias, anemia of inflammation, and neurodegenerative disorders.1 Body iron absorption and distribution are controlled by hepcidin, a small peptide secreted by hepatocytes that is expressed as a prepropeptide of 84 amino acids and is processed to the mature hormone of 25 residues by furin.2,3 Hepcidin binds and induces the degradation of ferroportin, the only known cellular iron exporter, and thus it regulates iron absorption from duodenum and iron release from reticuloendothelial macrophages.4 Inappropriately low levels of hepcidin are associated with the various forms of genetic hemochromatosis,5 whereas high levels of hepcidin are found in inflammatory conditions, where they contribute to anemia.6 Liver hepcidin expression is regulated by iron, erythropoietic activity, and inflammation,6 with mechanisms that strongly rely on bone morphogenetic protein (BMP) signaling pathways.7 Hemojuvelin, which is a main hepcidin regulator, acts as a BMP coreceptor,8 and the modulation of its level by proteases tightly regulates hepcidin expression.9,10
Hepcidin expression in hepatic cells is strongly stimulated by BMP2, BMP4, BMP6, and BMP911 and is reduced by inhibitors of the BMP/SMAD signaling pathway, such as noggin or dorsomorphin.12 BMP6 is considered the physiologic BMP regulator of hepcidin because its expression is iron dependent and because BMP6 knockout mice demonstrate massive liver iron overload.13,14 Hepcidin also is stimulated by the inflammatory cytokine interleukin-6 (IL-6) in a pathway that involves signal transducer and activator of transcription 3 (STAT3),15-17 but it is dependent on the presence of SMAD4.18
Methods to control hepcidin expression can be based on the modulation of BMP's activity. These proteins belong to the transforming growth factor-β family and exhibit a wide range of biologic effects on various cell types.19 The recombinant BMP2, BMP4, and BMP7 induce osteoblastic differentiation of myoblasts or osteoblastic cells in vitro assays20,21 with mechanisms that are modulated by various factors, including the antagonists noggin and chordin22 and the heparan sulfate proteoglycans (HSPGs) present on the surface and in the extracellular matrix of various cell types,23 including hepatocytes.24 The consequences of the binding of BMPs to HSPGs vary, depending on whether HSPGs are cell-associated or in a free form. Moreover, HSPGs can bind also BMP antagonists and receptors.25-27 Accordingly, the removal of cell-associated HSPGs by chlorate or heparinase treatments reduced20 or increased28 BMP signaling.
Heparin is a glycosaminoglycan analog to HSPGs,29 and it is known to bind BMPs, which were originally purified from heparin columns.30 In cellular systems, the addition of heparin inhibited the signaling by BMP7 and by BMP6,20,31 whereas it potentiated the biologic activity of BMP2 and BMP431-33 or inhibited BMP2 binding to BMP receptors.34 Altogether, it is proven that heparin has strong and variable effects on BMP osteogenic activity. Heparin and its derivatives were shown to inhibit also a wide range of cytokines and growth factors, implying their potential employment in the treatment of various important pathologies, including tumor and viral diseases.29,35,36 Here, we analyzed the effects of exogenous heparin on BMP signaling and hepcidin expression in hepatic cell lines. Moreover, we analyzed hepcidin expression in mice treated with pharmacologic doses of heparin and in patients who were undergoing treatment to prevent deep vein thrombosis.
Methods
Cell culture
HepG2 cells were cultured in minimal essential medium (PAA Laboratories GmbH) with 10% fetal bovine serum (PAA Laboratories GmbH), 40 μg/mL gentamicin, and 1mM l-glutamine (PAA Laboratories GmbH). The cells were maintained at 37°C in 5% CO2. For studies of SMAD1/5/8 and STAT3 phosphorylation or activation, 105 cells/well were seeded in 12-well plates, grown for 24 hours and for other 16 hours in 0.5% fetal bovine serum. Then, they were incubated with recombinant BMP2, BMP6, IL-6 (R&D Systems), heparin (ie, unfractionated heparin [UFH]; Calciparina, Italfarmaco), or low-molecular-weight heparin (LMWH; enoxaparin sodium; Clexane; Sanofi Aventis) or pentasaccharide fondaparinux sodium (Arixtra; GlaxoSmithKline).
Immunoblot analysis
Cell extracts were lysed in lysis buffer (200mM Tris-HCl, pH 8; 100mM NaCl; 1mM ethylene diamine tetraacetic acid; 0.5% NP-40; and 10% glycerol) containing a mixture of protease inhibitors (Sigma-Aldrich). For assays examining phosphorylated SMAD and STAT3 expression, 1mM sodium orthovanadate (Sigma-Aldrich) and 1mM sodium fluoride (Sigma-Aldrich) were added to the lysis buffer as phosphatase inhibitors.
Cells lysates were analyzed on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and after transfer, the nitrocellulose filters were incubated for 16 hours with specific antibodies, washed, and further incubated for 1 hour with secondary peroxidase-labeled antibodies (antimouse immunoglobulin G; Dako, or antirabbit immunoglobulin G; Pierce). The primary antibodies used were as follows: rabbit anti-PhosphoSMAD1/5/8 antibody (1:1000; Cell Signaling Technology), rabbit anti-SMAD5 antibody (1:1000; Cell Signaling Technology), anti-PhosphoSTAT3antibody (1:1000; Cell Signaling Technology), and rabbit anti-β-actin antibody (1:1000; Sigma-Aldrich). Bound activity was revealed by advance enhanced chemiluminescence kit (Amersham) and detected with the KODAK Image Station 440CF (Kodak).
RNA extraction and real-time reverse-transcriptase polymerase chain reaction
RNA was purified from cells or mouse livers with the use of the guanidinium thiocyanate-phenol-chloroform method (Trizol) according to the manufacturer's instructions (Ambion). Total RNA (1 μg) was used to synthesize the first strand of cDNA with the ImProm-II Reverse Transcription System (Promega) with the use of oligodT. For real-time polymerase chain reaction (PCR) analysis, specific human or murine assays-on-demand products (20×) and TaqMan Master Mix (2×) from Applied Biosystems were used, according to the manufacturer's instructions, and the reactions were run on ABI PRISM 7700 Sequence Detection System (Applied Biosystems) in a final volume of 20 μL for 40 cycles. The expression levels of hepcidin and Id1 were normalized with levels of HPRT1 mRNA in each sample.
Mice
Seven-week-old female mice (Harlan Laboratories) were cared for in accordance with the European Convention for the Protection of Laboratory Animals, and the study was approved by the Institutional Animal Care and Use Committee of the University of Brescia. The mice were daily injected subcutaneously with saline or heparin. After 3-15 days they were killed, and the livers were homogenized and analyzed for hepcidin mRNA transcript (by reverse transcription [RT]-PCR and by real-time RT-PCR) or for phosphoSMAD1/5/8, total SMAD5, and β-actin as a control. For RT-PCR analysis of hepcidin-1 and HPRT1 we used the following primers: hepcidin-1: forward TTGCGATACCAATGCAGAAGAG and reverse AATTGTTACAGCATTTACAGCAGAAGA; and HPRT1: forward GCTTGCTGGTGAAAAGGACCTCTCGAAG and reverse CCCTGAAGTACTCATTATAGTCAAGGGCAT. The PCRs were run for 25 cycles. Liver and spleen iron concentration were determined spectophotometrically by the use of bathophenantroline, following the procedure described in Roette et al,37 except that the heating at 110°C was omitted. Blood was collected from the tail and serum iron determined spectrophotometrically with a commercial kit (Randox Laboratories, Ltd).
Patients
To preliminarily explore the effects of heparins on hepcidin in humans, we evaluated a time-course of serum hepcidin concentration and of indices of iron status in 5 patients (median age 87 years; range 67-93 years) who were admitted to the Section of Internal Medicine at University Hospital of Verona because of an intercurrent illness (pneumonia, n = 3; decompensated heart failure, n = 1; decompensated heart failure and urinary tract infection, n = 1). In all patients, heparin thromboprophylaxis was indicated according to current guidelines,38 with no contraindication. All patients provided informed consent for the collection of a small adjunctive aliquot of blood (1 mL) for hepcidin measurement during scheduled blood drawn in the morning for monitoring of their disease conditions. Serum hepcidin was measured by surface-enhanced laser desorption and ionization time-of-flight mass spectrometry as previously described.39 The protocol was approved by the local ethical committee.
Statistical analysis
Comparison of values between untreated and treated cells or mice was performed by Student t test for unpaired data. Differences were defined as significant for P values less than .05.
Results
Unfractionated heparin strongly inhibits hepcidin expression in HepG2 cells
Exogenous heparin was previously shown to affect the osteogenic activity of different BMPs in various cell lines26,31,33 ; thus, it was of interest to evaluate how it modifies the activity of BMPs and hepcidin expression in hepatic HepG2 cells. We started with unfractionated heparin (UFH). Treatments for 16 hours with different concentrations of UFH (0.4-400 μg/mL) caused a strong dose-dependent inhibition of hepcidin mRNA, represented in a logarithmic scale in Figure 1A and in linear scale in the inset. Heparin at concentrations > 4 μg/mL reduced hepcidin levels below 4% of the basal (Figure 1A) and inhibited also the phosphorylation of SMAD1/5/8 induced by the BMPs (Figure 1B). Then, we tested other commercial and clinically used heparin preparations. The LMWH enoxaparin was also inhibitory of hepcidin mRNA (Figure 1A) and of pSMAD1/5/8 (not shown), but it was less potent than UFH, and at the lowest concentrations tested (0.4-4.0 μg/mL), it was even stimulatory. The pentasaccharide fondaparinux slightly repressed hepcidin expression and SMAD1/5/8 phosphorylation only at very high concentrations (> 200 μg/mL; Figure 1A-B). Thus, all the heparins tested inhibited hepcidin expression and SMAD phosphorylation with a potency that increased with the length of the saccharidic chain. In time-course experiments we found that a decrease of hepcidin mRNA was evident 30 minutes after addition of 200 μg/mL LMWH and continued linearly for 4 hours (Figure 1C). At 4 hours we obtained maximum repression also with 4 and 40 μg/mL LMWH and with 0.4 and 4 μg/mL UFH (not shown). In other experiments we maintained the cells in 4 μg/mL LMWH for up to 7 days, hepcidin mRNA remained less than 10% of the basal for the whole period (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), and this had no evident effect on cell proliferation and viability (not shown). These data showed that the inhibitory effect of heparin on hepcidin is strong, fast, and may last for a long time.
Effect of heparin on hepcidin expression and BMP signaling in HepG2 cells. (A) The cells were grown for 16 hours in the absence of exogenous BMPs with the indicated concentrations of heparin: UFH, LMWH, and fondaparinux pentasaccharide, and the hepcidin mRNA levels were quantified by real-time RT-PCR in relationship to HPRT1 mRNA. The data are expressed in a logarithmic scale; the inset shows the inhibition plot of UFH in a linear scale. Data are means and SD of 3 independent experiments in triplicate. (B) Western blotting of phosphorylated SMAD1/5/8, total SMAD5, and of β-actin as calibrator of the cell extracts after the incubation with UFH and with fondaparinux. Data are from 2 independent experiments in triplicate. (C) Cells were incubated for the indicated time with 200 μg/mL LMWH, and the level of hepcidin mRNA were quantified by real-time RT-PCR. Data are representative of 4 independent experiments.
Effect of heparin on hepcidin expression and BMP signaling in HepG2 cells. (A) The cells were grown for 16 hours in the absence of exogenous BMPs with the indicated concentrations of heparin: UFH, LMWH, and fondaparinux pentasaccharide, and the hepcidin mRNA levels were quantified by real-time RT-PCR in relationship to HPRT1 mRNA. The data are expressed in a logarithmic scale; the inset shows the inhibition plot of UFH in a linear scale. Data are means and SD of 3 independent experiments in triplicate. (B) Western blotting of phosphorylated SMAD1/5/8, total SMAD5, and of β-actin as calibrator of the cell extracts after the incubation with UFH and with fondaparinux. Data are from 2 independent experiments in triplicate. (C) Cells were incubated for the indicated time with 200 μg/mL LMWH, and the level of hepcidin mRNA were quantified by real-time RT-PCR. Data are representative of 4 independent experiments.
Heparin strongly inhibits BMP6-mediated hepcidin induction
Heparin binds the BMPs; thus, we hypothesized that hepcidin inhibition is caused by heparin sequestering the BMPs. To verify this, we analyzed how the heparins modified BMP6-induced hepcidin expression. BMP6 alone (16 hours, 10 ng/mL) increased hepcidin mRNA levels 5- to 7-fold. The presence of UFH not only suppressed hepcidin induction by BMP6, but at high concentrations it reduced hepcidin level to values less than 5% of the basal, as in the absence of exogenous BMP6 (Figure 2A). LMWH was also inhibitory but was less potent than UFH, and fondaparinux was only marginally inhibitory (Figure 2A). The strong similarity of the inhibition plots of the 3 heparins in the presence and in the absence of added BMP6 supports the hypothesis that they act by suppressing the activity of BMP. This finding is further strengthened by the evidence that UFH inhibited BMP6-induced SMAD1/5/8 phosphorylation in a dose-dependent way, parallel to hepcidin inhibition, whereas fondaparinux had little or no effect on pSMAD1/5/8 and hepcidin (Figure 2B).
Effects of heparin on BMP6-induced expression of hepcidin and signaling. (A) The HepG2 cells were grown for 16 hours in the presence of 10 ng/mL BMP6 and of the indicated heparin types and concentrations; the level of hepcidin was analyzed by real-time RT-PCR in relationship to HPRT1 mRNA and expressed in a logarithmic scale. Data are means and SD of 3 independent experiments in triplicate. (B) Western blotting of phosphorylated SMAD1/5/8, total SMAD5 and of β-actin as calibrator of the cells extracts before and after the incubation with UFH and with fondaparinux. Data are representative of 3 independent experiments.
Effects of heparin on BMP6-induced expression of hepcidin and signaling. (A) The HepG2 cells were grown for 16 hours in the presence of 10 ng/mL BMP6 and of the indicated heparin types and concentrations; the level of hepcidin was analyzed by real-time RT-PCR in relationship to HPRT1 mRNA and expressed in a logarithmic scale. Data are means and SD of 3 independent experiments in triplicate. (B) Western blotting of phosphorylated SMAD1/5/8, total SMAD5 and of β-actin as calibrator of the cells extracts before and after the incubation with UFH and with fondaparinux. Data are representative of 3 independent experiments.
Heparin inhibits IL-6–mediated hepcidin induction
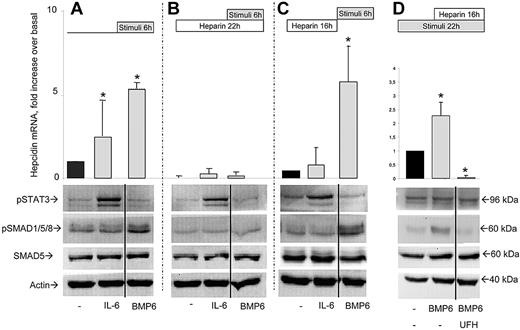
The inflammatory cytokine IL-6 is an activator of hepcidin expression.40 HepG2 cells treatment with IL-6 (4 hours, 50 ng/mL) induced hepcidin mRNA 2.5-fold and increased the phosphorylation of STAT3 but not that of SMAD1/5/8 (Figure 3A). Parallel treatment with BMP6 (10 ng/mL) induced hepcidin (5- to 7-fold) and pSMAD1/5/8 but not pSTAT3, as expected (Figure 3A). To study the effect of heparin, the cells were first incubated with LMWH (16 hours, 200 μg/mL) to suppress hepcidin, then BMP6 or IL-6 was added and the cells were grown for another 6 hours in the presence of heparin and of the stimuli. The presence of heparin blunted both the BMP6 and the IL-6–mediated induction of hepcidin mRNA (Figure 3B). It suppressed pSMAD1/5/8 induction by BMP6, whereas it did not affect the induction of pSTAT3 by IL-6 (Figure 3A-B). Similar effects were obtained with lower concentrations LMWH (4-40 μg /mL, not shown) and with 4 μg/mL of UFH (supplemental Figure 2). Moreover, we also analyzed Id1 expression by real-time RT-PCR, a gene that is tightly regulated by the BMP/SMAD pathway. It was induced 5-fold by BMP6 and was strongly inhibited by UFH even in the presence of BMP6 (supplemental Figure 2). Interestingly, it was induced when BMP6 was added after washing UFH away. Thus, Id1 was modulated by heparin and BMP6 in parallel with hepcidin, as expected. Altogether, the data confirm that heparin inhibits hepcidin expression by reducing the basal and BMP6-induced pSMAD1/5/8 signaling.
Effect of heparin on hepcidin stimulation by BMP6 and IL-6. (A) HepG2 cells were incubated for 6 hours with 50 ng/mL IL-6 or 10 ng/mL BMP6, and then hepcidin mRNA level evaluated by quantitative RT-PCR in relationship to HPRT1 mRNA. (B) Same as panel A, except that the cell were first incubated for 16 hours with 200 μg/mL LMWH, and then added of the stimuli and grown in the presence of heparin for 6 hours. (C) Same as panel B, except that the cells were washed before addiction of the stimulating factors, and grown for 6 hours in the absence of heparin. (D) The cells were grown for 6 hours in the presence or absence of 10 ng/mL BMP6, then added UFH 4 μg /mL and grown for another 16 hours. The schemes of the experiments are represented above the graphs, where stimuli is the incubation with IL-6 or BMP6, and heparin treatment is with 200 μg/mL LMWH (A-C) or with 4 μg/mL UFH (D). Bottom: Western blotting of phosphorylated STAT3 (pSTAT3), of phosphorylated SMAD1/5/8 (pSMAD1/5/8), total SMAD5 and of β-actin as calibrator representative of each panel A, B, C, and D, respectively. The real-time RT-PCR data are means and SD of 2 independent experiments in triplicate. The asterisks indicate significant difference (P < .05) from the nonstimulated control. Western blots are representative of at least 2 independent experiments. The vertical solid lines separate lanes of the same electrophoresis experiment that were spliced out in the figure preparation to eliminate irrelevant or uninformative lanes.
Effect of heparin on hepcidin stimulation by BMP6 and IL-6. (A) HepG2 cells were incubated for 6 hours with 50 ng/mL IL-6 or 10 ng/mL BMP6, and then hepcidin mRNA level evaluated by quantitative RT-PCR in relationship to HPRT1 mRNA. (B) Same as panel A, except that the cell were first incubated for 16 hours with 200 μg/mL LMWH, and then added of the stimuli and grown in the presence of heparin for 6 hours. (C) Same as panel B, except that the cells were washed before addiction of the stimulating factors, and grown for 6 hours in the absence of heparin. (D) The cells were grown for 6 hours in the presence or absence of 10 ng/mL BMP6, then added UFH 4 μg /mL and grown for another 16 hours. The schemes of the experiments are represented above the graphs, where stimuli is the incubation with IL-6 or BMP6, and heparin treatment is with 200 μg/mL LMWH (A-C) or with 4 μg/mL UFH (D). Bottom: Western blotting of phosphorylated STAT3 (pSTAT3), of phosphorylated SMAD1/5/8 (pSMAD1/5/8), total SMAD5 and of β-actin as calibrator representative of each panel A, B, C, and D, respectively. The real-time RT-PCR data are means and SD of 2 independent experiments in triplicate. The asterisks indicate significant difference (P < .05) from the nonstimulated control. Western blots are representative of at least 2 independent experiments. The vertical solid lines separate lanes of the same electrophoresis experiment that were spliced out in the figure preparation to eliminate irrelevant or uninformative lanes.
To verify whether heparin affected soluble or membrane-bound molecules, the cells were first incubated with heparin for 16 hours to suppress hepcidin and pSMAD1/5/8, then washed, BMP6 or IL-6 added, and incubated for 6 hours in heparin-free medium. In this period hepcidin mRNA increased to approximately 40% of the basal in the control cells without stimuli, whereas the addition of BMP6 produced an induction of pSMAD1/5/8 and of hepcidin mRNA comparable with that of the cells that were not treated with heparin (Figure 3A,C). More interestingly, the response to IL-6 remained blunted, with a level of hepcidin mRNA nonsignificantly different from the one of the cells not exposed to IL-6. In these cells the level of pSMAD1/5/8 did not increase, although STAT3 phosphorylation was stimulated as in the presence of heparin (Figure 3B-C). These results support the hypothesis that the suppression of BMP signaling caused by heparin is attributable to the sequestering of BMPs (mainly BMP6) because the response was fully activated by exogenous BMP6. The lack of BMP6 may explain why hepcidin and pSMAD remained repressed and why hepcidin was only poorly induced by IL-6. In other experiments the cells were first induced with BMP6 (6 hours, 10 ng/mL), then UFH (4 μg/mL) was added, and the mixture was incubated for another 16 hours in the presence of BMP6. We observed an inhibition of hepcidin mRNA and of pSMAD1/5/8 that was similar to that in the absence of previous BMP6 stimulation (Figure 3D).
Heparin has a stronger inhibitory activity on BMP6 than on BMP2 signaling
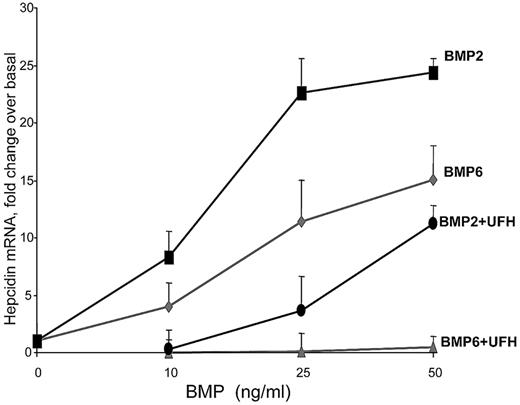
Heparin's effect on BMP osteogenic activity varied with the experimental conditions and BMP type.31-33 Thus, we studied the heparin effect on BMP2 and BMP6, which belong to different BMP subgroups19 and strongly induce SMAD signaling and hepcidin in HepG2 cells. In these experiments BMP2 was more potent than BMP6 (Figure 4), but we observed a remarkable batch to batch variability of the potency for either BMPs (not shown). The presence of 4 μg/mL UFH fully blocked the dose-dependent induction of hepcidin by BMP6, as expected. More interestingly, it reduced only to 30%-50% the induction by BMP2 (Figure 4). In other experiments we found that hepcidin stimulation by BMP2 (10 ng/mL) was only marginally inhibited by increasing concentrations of LMWH and was reduced only ∼10-fold by incubation with UFH (supplemental Figure 3). We concluded that heparin has a much more potent inhibitory effect on BMP6 than BMP2 in HepG2 cells.
Heparin and BMP2 or BMP6. HepG2 cells were grown for 16 hours in the indicated concentrations of BMP2 or BMP6 in the presence or the absence of UFH 4 μg/mL, and then hepcidin mRNA was evaluated.
Heparin and BMP2 or BMP6. HepG2 cells were grown for 16 hours in the indicated concentrations of BMP2 or BMP6 in the presence or the absence of UFH 4 μg/mL, and then hepcidin mRNA was evaluated.
Heparin inhibits hepatic hepcidin expression in vivo
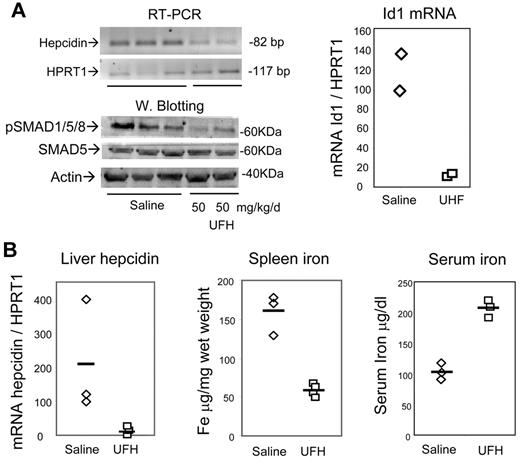
Next we asked whether heparin also has the capacity to modulate the expression of hepcidin in vivo. In initial experiments the mice were subcutaneously injected daily with 50 mg/kg/d with UFH for 7 days, and the liver was analyzed. RT-PCR analysis showed a decrease of hepcidin1 mRNA, and also of Id1 mRNA, and blotting demonstrated a reduction of pSMAD1/5/8 in liver homogenates (Figure 5A). Thus, heparin reduced liver hepcidin expression and SMAD signaling in vivo at concentrations greater than the pharmacologic ones, without causing evident local hemorrhages. Similar results were obtained by treating the mice for 5 days with pharmacologic concentration of UFH 5 mg/kg/d (not shown). In other experiments the mice were treated for 15 days with lower UFH doses (2 mg/kg/d), and then liver mRNA was quantified by real time RT-PCR calibrated on the housekeeping HPRT1 mRNA. The level of hepcidin transcript was rather variable in the 3 control saline-treated animals, but it strongly decreased in the heparin-treated mice (Figure 5B). Heparin treatment caused also an increase in serum iron concentration and a large reduction of spleen iron concentration (Figure 5B).
In vivo effect of heparin in mice. (A) Mice were daily treated subcutaneously for 7 days with 50 mg/kg/d UFH and the level of hepatic hepcidin mRNA evaluated by RT-PCR, HRPT1 was used for calibration, the corresponding real-time RT-PCR values were 2% and 2.7% of the control, for the mice treated with 50 mg/kg/d UFH. Bottom: Western blot of the liver homogenates for evaluation of phosphorylated SMAD1/5/8 and of total SMAD5. β-actin was used as load control. The real-time RT-PCR evaluation of Id1 mRNA in the treated mice is shown on the right. (B) Mice were treated subcutaneously for 15 days with the pharmacologic concentration of 2 mg/kg/d UFH or with saline, and liver hepcidin mRNA was evaluated by real-time RT-PCR of in relationship to HPRT1 mRNA. The animals were analyzed for spleen iron and for serum iron concentration. The horizontal bars represent the mean values.
In vivo effect of heparin in mice. (A) Mice were daily treated subcutaneously for 7 days with 50 mg/kg/d UFH and the level of hepatic hepcidin mRNA evaluated by RT-PCR, HRPT1 was used for calibration, the corresponding real-time RT-PCR values were 2% and 2.7% of the control, for the mice treated with 50 mg/kg/d UFH. Bottom: Western blot of the liver homogenates for evaluation of phosphorylated SMAD1/5/8 and of total SMAD5. β-actin was used as load control. The real-time RT-PCR evaluation of Id1 mRNA in the treated mice is shown on the right. (B) Mice were treated subcutaneously for 15 days with the pharmacologic concentration of 2 mg/kg/d UFH or with saline, and liver hepcidin mRNA was evaluated by real-time RT-PCR of in relationship to HPRT1 mRNA. The animals were analyzed for spleen iron and for serum iron concentration. The horizontal bars represent the mean values.
Heparin reduces circulating hepcidin levels in treated patients
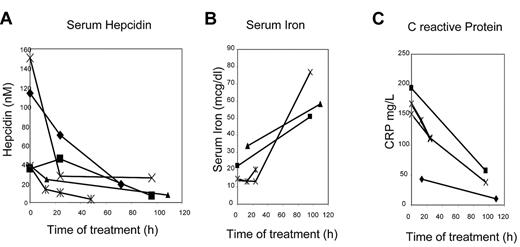
After the evidence that pharmacologic concentrations of heparin reduced the level of hepcidin in mice, we analyzed hematologic parameters and serum hepcidin level of 5 hospitalized patients who had to be treated with heparin to prevent deep vein thrombosis. All the patients had normal hematocrit values, signs of inflammation with high levels of C-reactive protein and of serum hepcidin and low serum iron and transferrin saturation. They were treated for 4-5 days with the usual pharmacologic doses of LMWH. We observed a strong decrease of serum hepcidin, that is, 15%-20% of the initial level, in all 5 patients after 2-5 days of treatment (Figure 6A). Serum iron (Figure 6B) and transferrin saturation (not shown) increased in all the patients analyzed. Also C-reactive protein decreased drastically in all the subjects (Figure 6C). The data suggest an increase in systemic iron availability during the treatment in all the patients analyzed.
Serum hepcidin and iron in patients under heparin treatment. (A) Time-course of serum hepcidin levels in 5 subjects during heparin therapy. (B) Time-course of serum iron concentration in 4 subjects. (C) Time-course of circulating C-reactive protein concentration in 4 subjects. The data on C-reactive protein and serum iron were not available for 1 subject.
Serum hepcidin and iron in patients under heparin treatment. (A) Time-course of serum hepcidin levels in 5 subjects during heparin therapy. (B) Time-course of serum iron concentration in 4 subjects. (C) Time-course of circulating C-reactive protein concentration in 4 subjects. The data on C-reactive protein and serum iron were not available for 1 subject.
Discussion
We demonstrated that heparin inhibits hepcidin expression in hepatoma HepG2 cells in a dose-dependent manner. The effect is evident after 30 minutes, is complete after 4 hours (Figure 1C), and hepcidin mRNA can be maintained at less than 10% of basal level for a week or longer in the presence of heparin (supplemental Figure 1). We used 3 different commercial heparin types of clinical use: the unfractionated heparin (UFH, 12-15 kDa) had an effect approximately 10-fold more potent than LMWH (4.5 kDa), and the smaller pentasaccharide fondaparinux (1.7 kDa) had little inhibitory activity (Figure 1, 2A-B). This size-dependent potency of heparin is not surprising, and it has been already observed on the biologic activity of growth factors, including fibroblast growth factors,41 vascular endothelial growth factors, and HIV-Tat.42 Noticeably, all these growth factors are inhibited by their direct interaction with heparin and its sequestration in the extracellular environment. By analogy, we postulate that heparin binds BMPs to generate complexes that are unable to stimulate SMAD signaling and hepcidin expression. This finding is consistent with all our data, including the evidence that BMP signaling and hepcidin expression were fully restored by the addition of BMP6 to cells pretreated with heparin (Figure 3C). This result demonstrates that heparin does not desensitize cells and does not act on membrane-associated BMP receptors, as indicated to occur in murine osteoblastic NC3T3 cells.34
In addition, the kinetics of inhibition (Figure 1C) and of recovery of hepcidin expression (a few hours) are compatible with heparin sequestering the endogenous BMPs, known to be expressed by HepG2 cells7 and to have autocrine activity on hepcidin expression in hepatic cells.43 Interestingly, heparin did not affect the STAT3 signaling induced by IL-6 but still suppressed its effect on hepcidin regulation (Figure 3). This finding is in full agreement with previous observations that the STAT3 signaling induced by IL-6 stimulates hepcidin expression only in the presence of a functional SMAD pathway.15,18
Heparin is a strongly acidic molecule that binds any positively charged molecule, but it exerts a biologic activity only on molecules with high-affinity binding, as it occurs with antithrombin. Thus, it was of interest to find that heparin had no effect on IL-6 signaling and that it had a different effect on the 2 BMPs we tested. Heparin inhibition was more potent on the signaling and hepcidin induction by BMP6 than that by BMP2 (Figure 4), and this goes along with previous findings that BMP6 has a greater affinity binding to heparin (Kd = 6.3nM)44 than BMP2 (Kd = 15-20nM).45 The binding to BMP2 may be responsible of osteoporosis, a known side effect of prolonged heparin treatments described 40 years ago,46 whereas the binding to BMP6 seems to affect systemic iron homeostasis. We found that low concentrations of LMWH stimulated rather than inhibit hepcidin expression in HepG2 cells (Figure 1,2). These variable effects were already observed for the BMP osteogenic activity19,25,28,32,47 and are probably attributable to competition with endogenous heparan sulfates and/or binding to BMP antagonists. Further work will clarify the role of cellular HSPGs in recruiting BMPs to hepatic cells and the possible competition with exogenous heparin.
Heparins and glycosaminoglycans bind a variety of factors in a specific manner that depends on fragment size and sequence. Thus, it would be of interest to define the size and structure of the heparin fragments that have the greatest inhibitory activity. We expect that such activity is linked to affinity binding to BMP6, the putative physiologic regulator of hepcidin. It was shown that such affinity was reduced in the heparins that have been desulfated in position 2O and 6O, indicating that both sulfate types are involved in the binding.44 Moreover, BMP6 did not bind octasaccharides obtained by fragmentation of heparin, indicating that it has affinity for longer fragments44 in agreement with the higher potency of UFH.
We show that daily heparin treatments cause a significant down-regulation of liver hepcidin in mice, evident after 5 and 15 days treatment (Figure 5). This response occurred with high (50 mg/kg/d) and with low pharmacologic doses of UFH (2 mg/kg/d). For example the strong reduction of liver hepcidin mRNA shown in Figure 5B was obtained with treatments of UFH that correspond to 400 U/kg/d, which are in the range of ones used in clinical settings. These doses were sufficient to increase serum iron and to mobilize iron from splenic macrophages. Thus, heparin-induced reduction of hepcidin levels has the expected effects on systemic iron status. The inhibition of hepcidin is not attributable to heparin anticoagulant activity, however, we found a strong decrease of liver C-reactive protein mRNA after the treatments with 5 mg/kg/d (approximately 50%, not shown), consistent with its known anti-inflammatory activity,48 which might indirectly affect hepcidin expression. These initial findings stimulated the analysis of serum hepcidin levels in a small group of hospitalized patients who had to be treated with low doses of heparin to prevent deep vein thrombosis. We noted effects in the same direction of those observed in cellular and animal models, with a major decrease of serum hepcidin evident as soon as at day 1 or 2 and continuing for the short period of observation of 2-5 days (Figure 6A). Serum iron (Figure 6B) and transferrin saturation increased (not shown). The change in serum iron was so evident that we were surprised that it had not been reported before. Indeed, we found that Braunsteiner et al49 in 1959 already described the doubling of serum iron levels after short treatments with heparin. We observed also a reduction of C-reactive protein level (Figure 6C), as already reported to occur in patients.48 Serum ferritin marginally increased in 3 of the 4 subjects (not shown), and it did not seem to respond to the inflammatory status but rather to the iron status of the subjects. This initial study has inherent limitations, but it indicates unrecognized effects of heparin on iron homeostasis that may be beneficial. The inhibition of liver hepcidin and the increases in serum iron may be attributed both to the anti-inflammatory activity of heparin,48 which would reduce IL-6 expression, and to the antihepcidin activity we describe in the cells, which would reduce BMP6. They act in the same direction, but in different ways: the anti-inflammatory activity is attributed to heparin interfering with leukocytes extravasation,50 whereas the antihepcidin activity seems to occur at a molecular level by heparin sequestering the BMPs. Thus, the fast decrease in hepcidin levels and the trend of iron status improvement may be attributable to the specific antihepcidin activity, to a reduction of inflammation (but we did not measure IL-6), or to a combination of the 2. Whatever the mechanism, this seems to be the first evidence that heparin has a direct effect on iron homeostasis, indicating novel therapeutic approaches for anemia.
The importance of hepcidin-targeted therapeutics has been recently reviewed1 and thought to have applications for various diseases, including chronic kidney diseases.51 The approaches under study include hepcidin agonists based on short peptides of the hepcidin N-terminal region1 ; neutralizing antihepcidin antibodies52 ; inhibitors of BMP type I receptors such as dorsomorphin12 ; soluble hemojuvelin, which acts as an antagonist of BMP signaling7 ; and erythropoiesis stimulating agents such as erythropoietin.53 Heparin is added to this list, and it has the unique advantage of being a drug that has been used in clinical setting for more than 70 years. Its toxicity (mainly thrombocytopenia) and its costs are extremely low compared with other drugs used for the treatment of inflammatory anemia, such as erythropoietin. Moreover, heparin can be modified experimentally to improve its antihepcidin activity.
In conclusion, more accurate studies on a larger number of animals and for longer time are necessary to verify if and how heparin treats the anemia caused by hepcidin overexpression, although some relevant data can be obtained from patients who have to be treated with heparin. Moreover, the evidence that heparin has a strong anti-hepcidin activity in vitro and in vivo stimulates the study and development of modified sulfated polysaccharides with low anti-coagulant and high anti hepcidin activity. This should be feasible, because heparins with low anticoagulant activity were shown to have strong anti-inflammatory activity54 and novel biotechnologic K5 polysaccharide derivatives are promising molecules.55 In addition, the effect of heparin suggests that HSPGs are deeply involved in the BMP signaling pathways that regulate hepcidin expression in hepatic cells. The role of these endogenous molecules on hepcidin regulation is still unexplored, and so is their activity on the various heparin binding molecules involved in the liver BMP signaling.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are particularly grateful to Prof Marco Rusnati for reading the manuscript and helpful discussions and to Dr Laura Silvestri for helpful suggestions.
The work was partially supported by Euroiron1 grant 200-037296 and by Murst-Cofin-2006 to PA.
Authorship
Contribution: M.P. designed the research and analyzed data; D.G. and P.A. wrote the paper; N.C. and A.N. contributed vital new analysis; and D.F., F.M., and S.L. performed research and analyzed data.
Conflict-of-interest disclosure: A patent application regarding the use of heparin for hepcidin inhibition has been submitted by the University of Brescia.
Correspondence: Paolo Arosio, Dipartimento Materno Infantile e Tecnologie Biomediche, Facoltà di Medicina e Chirurgia, Università di Brescia, Viale Europa 11, 25123 Brescia, Italy; e-mail: arosio@med.unibs.it.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal