Abstract
Immunosuppression is a known risk factor for B-cell non-Hodgkin lymphoma (NHL), yet mechanisms of tumor-associated immunosuppression remain to be fully characterized. We examined the immunophenotype of 40 NHL patients and 27 age-matched healthy volunteers to better understand systemic immune suppression. NHL peripheral blood mononuclear cells had significantly decreased interferon-γ production and proliferation. This suppression was not the result of regulatory T cells, interleukin-6 or interleukin-10, as these factors were not different between NHL and healthy volunteers (controls). We were able to restore T-cell proliferation by removing NHL monocytes, suggesting that these monocytes are suppressive. This suppression was mediated in part through arginine metabolism as exogenous arginine supplementation partially overcame monocytes' suppression of T-cell proliferation in vitro and NHL patients had elevated arginase I in their plasma. NHL monocytes had impaired STAT1 phosphorylation and interferon-α production to CpG stimulation and a dendritic cell differentiation deficiency. Further studies demonstrated that monocytes from NHL patients had decreased HLA-DR and Tumor necrosis factor-α receptor II (CD120b) expression compared with controls (CD14+HLA-DRlow/−CD120blow). Patients with increased ratios of CD14+HLA-DRlow/− monocytes had more aggressive disease and suppressed immune functions. In summary, we report that CD14+HLA-DRlow/− monocytes are a major and multifactorial contributor to systemic immunosuppression in NHL.
Introduction
Systemic immune suppression is often seen in cancer patients and is thought to contribute to patient morbidity via tumor-mediated immune evasion. Indeed, patients with compromised immune systems, such as those with HIV infection, or on immunosuppressive medications are at increased risk of developing non-Hodgkin lymphoma (NHL).1,2 Polymorphisms in host germline immune genes also have been associated with risk of developing NHL3 as well as survival in NHL patients.4 Conversely, a small percentage of patients with indolent NHL have spontaneous regression of their disease without any treatment,5 possibly linked to a host antitumor immune response. The presence of host immune cells in the tumor microenvironment has also been correlated with treatment outcome and survival.6-9 Of note, reduction in absolute count of circulating lymphocytes has been identified as a poor prognostic factor for overall survival in newly diagnosed NHL10,11 as well as a predictor of poor treatment response.9,12,13 Although evidence for the role of immune suppression in tumor establishment and pathogenesis is unquestionable, the mechanisms and the cellular phenotype of systemic immune suppression in NHL patients remain to be fully characterized.
In this study, we investigated the qualitative and quantitative systemic immune suppression in B-cell NHL. We found circulating mononuclear cells had suppressed interferon-γ (IFN-γ) recall response and proliferative capacity. These suppressed functions were mediated by circulating monocytes and partly mediated via arginine metabolism. These monocytes also had other impaired adaptive immune response including a decreased capacity to generate mature dendritic cells. In addition, the innate immune response of these monocytes to CpG stimulation was impaired as measured by intracellular STAT1 phosphorylation and interferon-alpha (IFN-α) production. These multifactorial suppressive functions of monocytes in NHL were correlated with decreased HLA-DR expressions, and the presence of CD14+HLA-DRlow/− monocytes was also associated with more aggressive disease. Taken together, we have identified a phenotype of suppressive monocytes in NHL (CD14+HLA-DRlow/−) as a significant source of host immune deficit.
Methods
Study subjects
This study was conducted by the Lymphoma SPORE and approved by the Mayo Clinic Institutional Review Board. All patients provided signed informed consent in accordance with the Declaration of Helsinki to provide a blood sample and to review the medical record for research purposes. Patients were eligible if they had new untreated or relapsed B-cell NHL and no treatment for at least 8 weeks before sample collection. Samples from newly diagnosed patients were taken before any treatments. Patients with coexisting medical illness likely to impact their immune status were excluded from the study. Samples were analyzed within 24 hours of collection.
ELISpot
Peripheral blood mononuclear cells (PBMCs) were incubated in X-Vivo15 (Lonza Walkersville) with 1% human AB serum with either influenza vaccine FLuVirin (5 μL per 3 × 106 cells; Evans Vaccine) or tetanus toxoid (10 μg per 3 × 106 cells; Sigma-Aldrich) for 72 hours. Cells were washed and plated at 105 cells/well in 96-well multiscreen IP plate (Millipore) precoated with anti-IFN-γ, anti-interleukin-4 (IL-4), or anti-IL-17 and incubated for 24 hours (all antibodies from eBioscience). After washing the wells, IFN-γ, IL-4, and IL-17 spots were detected with conjugated horseradish peroxidase antibodies and avidin. The ELISpot images of the wells were scanned and analyzed with AID ELISpot Reader (AID ELISpot).
Allogeneic mixed lymphocyte reaction
Pan T cells (105 total per well pooled from 3 healthy controls) were seeded in 96-well round-bottom plate (Corning Life Sciences) in RPMI 1640 (Invitrogen) with 10% fetal bovine serum (Mediatech) and 1× P/S (Invitrogen) with various ratios of monocytes. Pan T cells incubated with 2 μL of anti-CD3/CD28 beads (Dynabeads, Invitrogen Dynal) were used as positive control. Cells were cultured for 72 hours with tritiated thymidine added 18 hours before collection.
Polyclonal autologous T-cell proliferation assay
PBMCs and monocyte-depleted (by anti-CD14 immunomagnetic beads) PBMCs were seeded in 96-well round-bottom plates at 105 cells per well in RPMI 1640 with 10% fetal bovine serum and 1× P/S. Anti-CD3/CD28 beads (2 μL per well, Dynabeads, Invitrogen Dynal) were added to stimulate polyclonal expansion. Cells were cultured for 72 hours with tritiated thymidine added 18 hours before collection.
To assess the effect of arginine on autologous T-cell proliferation, the above assay was performed with PBMCs from NHL in RPMI 1640 media without arginine, histidine, or tryptophan (Invitrogen) with 5% fetal bovine serum, 1× P/S, and 1mM L-arginine (Quality Biological). An additional 5mM of L-arginine was added to media as indicated in some culture conditions.
Intracellular protein kinase assay
Whole blood was incubated either without stimulation or stimulated with IFN-γ (R&D Systems), IL-6 (R&D Systems), or CpG (InvivoGen). Surface staining with anti-CD14 fluorescein isothiocyanate (BD Biosciences) and anti-HLA-DR APC (BD Biosciences) was performed at the same time. Immediately after stimulation, red blood cells were lysed and PBMCs were fixed with BD PhosFlow Lyse/Fix buffer and permeabilized with Perm buffer II solution per manufacturer's protocol (BD Biosciences). Cells were stained for intracellular expression of either anti-pSTAT1(Y701) (BD Biosciences) or anti-pSTAT3(S747) (BD Biosciences). Data were acquired on BD FACSCalibur flow cytometer and analyzed by FlowJo Version 7.5.5 software.
Cell isolation and ex vivo culture of CD14+ monocytes
PBMCs were isolated by density gradient centrifugation (Lymphoprep, MP Biomedicals LLC). Monocytes and T cells were isolated from PBMCs by incubation with anti-CD14 or pan T-cell immunomagnetic beads, respectively (Miltenyi Biotec) per manufacturer's protocol and selection with AutoMACS (Miltenyi Biotec). CD14+ monocytes were cultured, and the generated mature dendritic cells were analyzed as previously described.14 Results from a small subset of control cultures have been reported previously.15 For sorting CD14+ cells into HLA-DRhi/lo fractions, phycoerythrin-labeled antihuman CD14 (eBioscience) and fluorescein isothiocyanate-labeled antihuman HLA-DR (BD Biosciences PharMingen) were used to stain cells. Cells were sorted based on expression of CD14+ and HLA-DR hi/lo parameters using either FACSVantage or FACSAria flow sort machines (BD Biosciences).
Gene expression analysis by real-time PCR
Sorted CD14+HLA-DRhi and CD14+HLA-DRlo cells were pelleted and resuspended in RNALater Solution (Ambion). RNA was purified from cells using the Qiagen RNeasy Plus kit (QIAGEN). cDNA was made with the Transcriptor First Strand cDNA kit (Roche Diagnostics). Primers were designed with the Roche Universal Probe Library system. Primers for ARG1 (NM_000045.2) are as follows: forward, caaggtggcagaagtcaagaa; reverse, gcttccaattgccaaactgtg; GAPDH (NM_002046.3): forward, agccacatcgctcagacac; reverse, gcccaatacgaccaaatcc. Polymerase chain reaction (PCR) reactions were performed per instructions from the Roche Light Cycler System. Relative transcript levels were determined by assessing the crossing point for each gene and then normalized to the crossing point of GAPDH for each experiment.
Immunophenotyping of peripheral blood
Leukocytes were analyzed by direct antibody staining of whole blood and analyzed by flow cytometry. The protocol for whole blood staining was described previously.15,16 To assess absolute cell count, 50 μL of whole blood was added and stained in Trucount tubes according to the manufacturer's directions (BD Biosciences). Data were acquired on a BD FACSCalibur flow cytometer (BD Biosciences) and analyzed with Cell Quest Version 5.2.1 and Multiset Version 2.2.1 (BD Biosciences) software. The antibodies used for these studies are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
ELISA and cytokine analyses
To measure IFN-α production, PBMCs were incubated for 19 hours in RPMI media with either CpG or polyriboinosinic acid/polyribocytidylic acid (poly I:C). Supernatant was collected and assayed for IFN-α by enzyme linked immunosorbent assays (ELISA) per the manufacturer's protocol (PBL InterferonSource). Plasma concentration of vascular endothelial growth factor (VEGF, R&D Systems), IL-6 (eBioscience), IL-10 (Cell Sciences), and arginase I (Cell Sciences) were measured by ELISA according to the manufacturer's instructions.
Statistical analyses
Values between groups of data were tested for statistical significance using the 2-tailed Student t test. Correlation analysis was performed using Pearson correlation with the coefficient of correlation (r2) calculated using Prism Version 5.0 software (GraphPad Software). Kaplan-Meier survival curve was plotted using Prism Version 5.0 software. Significant P value was set at less than .05.
Results
Monocytes suppress recall responses and lymphocyte proliferation in NHL patients
Human blood samples were collected from 40 patients with NHL and 27 age-matched healthy volunteers (controls). There were 4 newly diagnosed and 36 patients with relapsed or refractory disease. The 4 newly diagnosed patients had received no therapy; and in the relapsed patients, the median time from last treatment to time of sampling was 16.5 months. The NHL disease types were 18 diffuse large B-cell lymphoma (DLBCL), 14 follicular lymphoma (12 grade 1 or 2; 1 grade 3), and others including 4 mantle cell lymphoma, 2 small lymphocytic lymphoma, 1 mucosa-associated lymphoid tissue lymphoma, and 1 lymphoplasmacytoid lymphoma. For the 4 mantle cell lymphoma patients, 2 patients were more than 6 years from time of diagnosis, 1 was more than 2 years from diagnosis, and 1 was 1 year from diagnosis and 6 months from treatment. Because of the clinical indolent course, the mantle cell lymphoma patients were grouped with indolent disease where indicated.
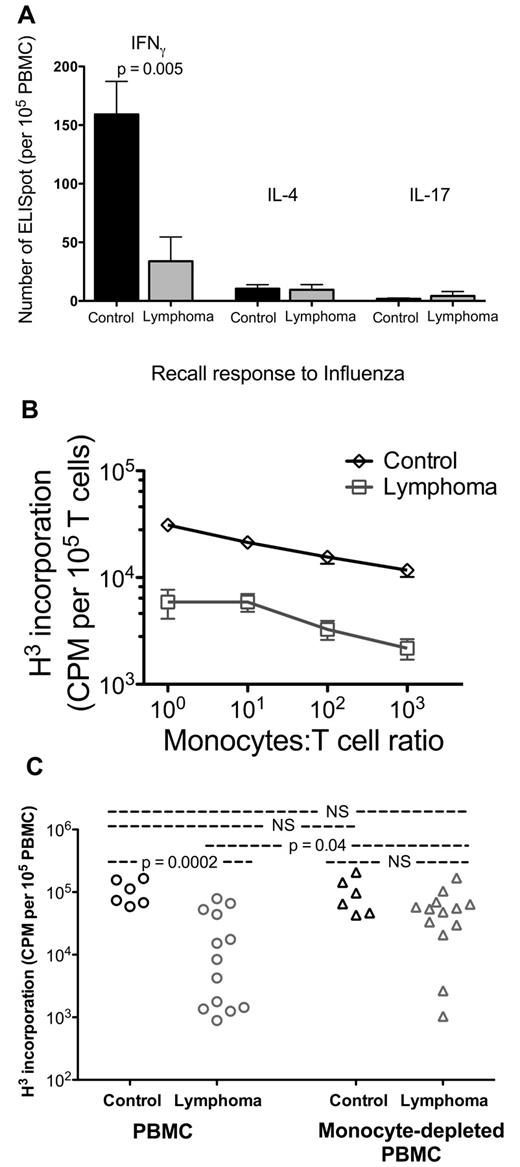
To assess host immune capacity in NHL patients, we measured the recall response of PBMCs to influenza. PBMCs from NHL have significantly decreased Th1 recall response compared with that of age-matched controls as measured by IFN-γ production with ELISpot assay (control 159 ± 28, n = 6; lymphoma 34 ± 21, n = 6; P = .005; Figure 1A). By comparison, NHL PBMC Th2 response (IL-4 production) and Th17 response (IL-17 production) were not different (Figure 1A). However, the response was approximately 10-fold lower for Th2 response and 100-fold lower for Th17 response, limiting our ability to detect differences. Similar results were seen with recall responses to tetanus toxoid (supplemental Figure 1).
PBMCs from lymphoma patients have decreased functions mediated by monocytes. (A) PBMCs from controls (n = 6) and lymphoma patients (n = 6) were incubated with influenza vaccine, and their cytokine expressions were measured by ELISpot assay for IFN-γ, IL-4, and IL-17 production. Each column represents mean plus or minus SD of 6 samples. (B) Monocytes from controls (n = 8) and lymphoma patients (n = 8) were cocultured for 3 days with negatively selected T cells pooled from 3 allogeneic healthy donors. Proliferation was assessed by H3 incorporation over the last 18 hours. (C) PBMCs and PBMCs with monocytes removed were cultured for 3 days with anti-CD3/CD28 beads. Proliferation was assessed by H3 incorporation over the last 18 hours (controls, n = 6; lymphoma, n = 13.) Statistically significant P value is shown between groups. NS indicates that P value was not significant.
PBMCs from lymphoma patients have decreased functions mediated by monocytes. (A) PBMCs from controls (n = 6) and lymphoma patients (n = 6) were incubated with influenza vaccine, and their cytokine expressions were measured by ELISpot assay for IFN-γ, IL-4, and IL-17 production. Each column represents mean plus or minus SD of 6 samples. (B) Monocytes from controls (n = 8) and lymphoma patients (n = 8) were cocultured for 3 days with negatively selected T cells pooled from 3 allogeneic healthy donors. Proliferation was assessed by H3 incorporation over the last 18 hours. (C) PBMCs and PBMCs with monocytes removed were cultured for 3 days with anti-CD3/CD28 beads. Proliferation was assessed by H3 incorporation over the last 18 hours (controls, n = 6; lymphoma, n = 13.) Statistically significant P value is shown between groups. NS indicates that P value was not significant.
The loss of recall response could be a response to poor antigen presentation or poor T-cell response capacity. To examine the contribution of monocytes as antigen-presenting cells, we cocultured monocytes isolated from NHL PBMCs or normal healthy donors with allogeneic pan-T cells pooled from unrelated healthy donors. Pooled allogeneic T cells proliferated less when coincubated with monocytes from NHL than with normal healthy donors (n = 8 for both, P < .01 at all cell ratios tested; Figure 1B).
Having identified that allogeneic healthy T cells were less proliferative in the presence of monocytes from NHL patients, we hypothesized that the effect of monocyte-mediated T-cell suppression existed independent of the antigen-mimicking effect of an allogeneic environment. We stimulated PBMC proliferation in vitro by polyclonal expansion using anti-CD3/CD28 beads. PBMCs from NHL had significantly decreased proliferation (almost 10-fold) compared with control (control, n = 6; lymphoma, n = 13; P = .0005; Figure 1C). However, when we removed the monocytes from PBMCs by anti-CD14 immunomagnetic beads and repeated the stimulation, proliferation of the remaining PBMCs was restored to the level of controls (Figure 1C). Together, these data demonstrate that PBMCs in NHL have decreased recall response and proliferative capacity. These suppressed functions appear to be mediated, at least in part, by autologous monocytes, and removal of the monocytes can restore lymphocyte function.
Monocytes from NHL patients have impaired response to stimuli
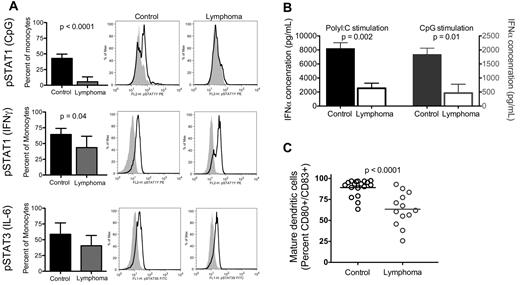
Monocytes are key responders to immune-stimulating cytokines and Toll-like receptors, are a major source of IFN-α,17 and can rapidly and efficiently differentiate into dendritic cells. We conducted in vitro experiments to examine the responsiveness of monocytes to cytokines and Toll-like receptor agonists. Stat1 or Stat3 phosphorylation is one of the early changes in the signal transduction pathway of monocyte activation and differentiation. To examine alterations in the signal transduction pathway of NHL monocytes with minimal in vitro manipulation, peripheral blood from NHL or healthy volunteers were incubated with designated stimulant within a few hours of venipuncture, fixed, and analyzed for intracellular protein kinase phosphorylation by flow cytometry. Monocytes from NHL patients stimulated with CpG had no identifiable change in STAT1 phosphorylation compared with unstimulated monocytes and had significantly decreased phosphorylation compared with healthy volunteers (percentage of monocytes with STAT1 phosphorylation: control, 42.9% ± 4.0%, n = 3; lymphoma, 5.5% ± 2.5%, n = 10; P < .0001; Figure 2A top row). Similarly, monocytes from NHL patients stimulated with IFN-γ had decreased STAT1 phosphorylation compared with control (control, 64.4% ± 4.3%, n = 5; lymphoma, 43.6% ± 6.8%, n = 7; P = .04; Figure 2A middle row). However IL-6 mediated STAT3 phosphorylation was not different in NHL monocytes (control, 58.7% ± 8.0%, n = 5; lymphoma, 40.5% ± 6.2%, n = 7; Figure 2A bottom row).
NHL monocytes have impaired response to stimuli. (A) Whole blood was stimulated as indicated (parentheses on left axis) and immediately processed for analysis by flow cytometry of monocyte intracellular STAT protein phosphorylation. Columns represent mean plus or minus SD (CpG stimulation: controls, n = 3; lymphoma, n = 10; IFN-γ and IL-6 stimulation: control, n = 5; lymphoma, n = 7). Representative histograms for each condition are shown: middle panel, control sample; right panel, lymphoma sample (shaded area represents isotype control; black line, sample protein phosphorylation). (B) PBMCs from control and lymphoma patients were incubated overnight with either poly I:C (left axis, n = 4) or CpG (right axis, n = 4) and assessed for IFN-α concentration in culture supernatant by ELISA. (C) Monocytes from control and lymphoma patients were isolated with anti-CD14 immunomagnetic beads and matured with TNF-α and PGE2. Bars represent mean for each group. Statistically significant P values are shown between groups.
NHL monocytes have impaired response to stimuli. (A) Whole blood was stimulated as indicated (parentheses on left axis) and immediately processed for analysis by flow cytometry of monocyte intracellular STAT protein phosphorylation. Columns represent mean plus or minus SD (CpG stimulation: controls, n = 3; lymphoma, n = 10; IFN-γ and IL-6 stimulation: control, n = 5; lymphoma, n = 7). Representative histograms for each condition are shown: middle panel, control sample; right panel, lymphoma sample (shaded area represents isotype control; black line, sample protein phosphorylation). (B) PBMCs from control and lymphoma patients were incubated overnight with either poly I:C (left axis, n = 4) or CpG (right axis, n = 4) and assessed for IFN-α concentration in culture supernatant by ELISA. (C) Monocytes from control and lymphoma patients were isolated with anti-CD14 immunomagnetic beads and matured with TNF-α and PGE2. Bars represent mean for each group. Statistically significant P values are shown between groups.
Monocytes have been identified as the major source of plasma IFN-α.17 To determine whether the changes we observed in the signal transduction of monocytes resulted in changes of stimulated cytokine release, we measured IFN-α concentrations in PBMCs stimulated with either CpG or poly I:C in vitro. Supernatants from cells from NHL patients had decreased IFN-α concentration compared with control (IFN-α concentration with CpG stimulation, pg/mL, n = 4: control, 1832 ± 231 pg/mL; lymphoma, 464 ± 313 pg/mL, P = .01; poly I:C stimulation, n = 4: control, 8171 ± 855 pg/mL; lymphoma, 2537 ± 729 pg/mL, P = .002; Figure 2B). This was probably not the result of changes in monocyte frequency as we found no change in the frequency of circulating monocytes (supplemental Figure 2) and dendritic cells (data not shown) in NHL patients. In addition, we found a corresponding decrease in IFN-producing monocytes in NHL compared with control by intracellular flow cytometry (data not shown). Taken together, the data indicates decreased production of IFN-α is probably related to changes in the signal transduction responsiveness to cytokines and Toll-like receptors of monocytes from NHL patients.
An additional vital stimulatory response of monocytes to environmental signals is the differentiation of monocytes to dendritic cells. We isolated CD14+ monocytes from NHL patients and healthy donors, cultured them ex vivo, and found that monocytes from NHL are less capable of generating mature dendritic cells compared with control (percentage CD80+CD83+ double-positive: control, 89.2% ± 2.3%, n = 18; lymphoma, 63.4% ± 5.5%, n = 13; P < .0001; Figure 2C). In summary, both innate and adaptive immune stimulatory functions of monocytes are impaired in monocytes from NHL patients.
CD14+HLA-DRlow/− is the suppressive monocyte phenotype in NHL
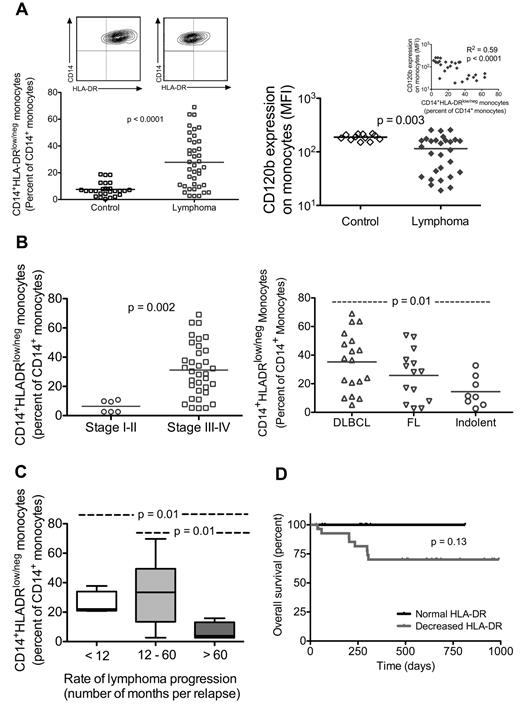
We analyzed an extensive panel of peripheral blood immunophenotypes to identify potential phenotypes of suppressive monocytes in NHL. The percentage of monocytes in peripheral blood is unchanged in NHL compared with control (control, 8.74% ± 0.46%, n = 15; lymphoma, 8.00% ± 0.48%, n = 40; P value not significant). However, CD14+ monocytes in NHL patients were found to have significantly reduced HLA-DR expression compared with control (percentage of CD14+ monocytes that are HLA-DRlow/−: control, 7.54% ± 0.97%, n = 27; lymphoma, 27.8% ± 3.1%, n = 40; P < .0001; representative contour plot shown in Figure 3A top panel).
Lymphoma monocytes have decreased HLA-DR expression, and this phenotype is associated with more aggressive disease. (A) Whole blood was stained for monocyte markers, including CD14 and HLA-DR. Flow cytometric analysis of CD14+ monocyte population that is CD14+HLA-DRlow/− is shown for age-matched controls (n = 27) and lymphoma patients (n = 40). Representative contour plot for each group is shown above (top panel). Flow cytometry analysis of monocyte expression of TNF-αRII (CD120b) is shown in the bottom panel (control, n = 12; lymphoma, n = 24). Decreased TNF-αRII expression is associated with increased percentage of CD14+HLA-DRlow/− monocytes (inset). (B) Within the lymphoma population, those with disseminated disease (stages III or IV disease, n = 34) had a higher percentage of CD14+HLA-DRlow/− monocytes than those with limited disease (stages I or II, n = 6; top panel). Similarly, when lymphoma samples are grouped by disease type (bottom panel), DLBCL (n = 18) had a significantly higher percentage of CD14+HLA-DRlow/− monocytes than the indolent histology group (4 mantle cell, 2 small lymphocytic lymphoma, one mucosa-associated lymphoid tissue, and one lymphoplasmacytoid). (C) The rate of progression for each patient is characterized by the average number of months per relapse, calculated by total number of months from initial diagnosis to time of sampling divided by total number of relapse. Distribution of percentage of CD14+HLA-DRlow/− monocytes is shown for each group as indicated (number of months per relapse: < 12, n = 4; 12-60, n = 28; > 60, n = 4). (D) Overall survival of lymphoma patients is shown. Black line represents patients whose monocytes had normal HLA-DR expression (n = 11; CD14+HLA-DRlow/− < 10% of CD14+ monocytes); and gray line, patients whose monocytes had loss of HLA-DR expression (n = 29; CD14+HLA-DRlow/− monocytes; monocytes where > 10% of CD14+ monocytes had lost HLA-DR expression).
Lymphoma monocytes have decreased HLA-DR expression, and this phenotype is associated with more aggressive disease. (A) Whole blood was stained for monocyte markers, including CD14 and HLA-DR. Flow cytometric analysis of CD14+ monocyte population that is CD14+HLA-DRlow/− is shown for age-matched controls (n = 27) and lymphoma patients (n = 40). Representative contour plot for each group is shown above (top panel). Flow cytometry analysis of monocyte expression of TNF-αRII (CD120b) is shown in the bottom panel (control, n = 12; lymphoma, n = 24). Decreased TNF-αRII expression is associated with increased percentage of CD14+HLA-DRlow/− monocytes (inset). (B) Within the lymphoma population, those with disseminated disease (stages III or IV disease, n = 34) had a higher percentage of CD14+HLA-DRlow/− monocytes than those with limited disease (stages I or II, n = 6; top panel). Similarly, when lymphoma samples are grouped by disease type (bottom panel), DLBCL (n = 18) had a significantly higher percentage of CD14+HLA-DRlow/− monocytes than the indolent histology group (4 mantle cell, 2 small lymphocytic lymphoma, one mucosa-associated lymphoid tissue, and one lymphoplasmacytoid). (C) The rate of progression for each patient is characterized by the average number of months per relapse, calculated by total number of months from initial diagnosis to time of sampling divided by total number of relapse. Distribution of percentage of CD14+HLA-DRlow/− monocytes is shown for each group as indicated (number of months per relapse: < 12, n = 4; 12-60, n = 28; > 60, n = 4). (D) Overall survival of lymphoma patients is shown. Black line represents patients whose monocytes had normal HLA-DR expression (n = 11; CD14+HLA-DRlow/− < 10% of CD14+ monocytes); and gray line, patients whose monocytes had loss of HLA-DR expression (n = 29; CD14+HLA-DRlow/− monocytes; monocytes where > 10% of CD14+ monocytes had lost HLA-DR expression).
Compared with controls, we find no changes in NHL monocyte expression of CD80, CD86, CCL2, TREM1, CD124 (IL-4-receptor-α), CD206, CD163, TLR4, TLR7, or TLR9 (data not shown). We did not find increased expression of B7-H1 on NHL monocytes (percentage of monocytes that is B7-H1+: controls, n = 18, 0.31% ± 0.09%; lymphoma, n = 33, 1.82% ± 0.56%; P = .06). However, one patient did have a small population of B7-H1+ monocytes (14.8% of monocytes). In this patient, increased B7H1 expression was present on HLA-DR− monocytes. Tumor necrosis factor-α receptor II (TNFaRII, CD120b) expression on monocytes of NHL patients was decreased compared with controls (Figure 3A bottom panel). In some patients, the decreased expression is more pronounced on CD14+HLA-DRlow/− monocytes. Decreased TNFaRII on monocytes is associated with increased regulatory T cell (Treg) count, although total circulating Treg count is not increased in NHL (n = 16, R2 = 0.32, P = .02). The presence of CD14+HLA-DRlow/− monocytes correlated with decreased TNFaRII expression (Figure 3A bottom panel inset), suggesting that the immune suppressive phenotype of monocytes in NHL patients is CD14+HLA-DR−/− CD120blow.
Interestingly, patients with limited disease (stages I or II) had similar levels of CD14+HLA-DRlow/− monocytes compared with controls, whereas patients with disseminated disease (stages III or IV) had a significantly increased ratio of CD14+HLA-DRlow/− to CD14+HLA-DR+ within the monocyte population (stages I or II, n = 6, 6.4% ± 1.7%; stages III or IV, n = 34, 31.2% ± 3.1%; P = .002, Figure 3B top panel). When we grouped NHL patients by disease type, all groups had increased ratios of CD14+HLA-DRlow/− monocytes compared with control, with DLBCL having the highest ratio (DLBCL, n = 18, 35.2% ± 4.8%; follicular lymphoma, n = 14, 25.8% ± 4.7%; indolent, n = 8, 14.5% ± 3.7%; Figure 3B bottom panel). The ratio of CD14+HLA-DR−/− monocytes in DLBCL was also higher compared with that of other indolent lymphoma (P = .01).
As our relapsed patients varied greatly in number of relapses and time to relapse before sampling for immunophenotype, we characterized their rate of progression by an average number of months per relapse. This was calculated by dividing the total number of months from initial diagnosis to time of sampling by the total number of relapse(s). Interestingly, those with slow rate of disease progression, as characterized by the average number of months per relapse greater than 60, had less CD14+HLA-DRlow/− monocytes than patients with faster rate of disease progression (average number of months per relapse: < 12 months, n = 4, 25.7% ± 4.1%; 12-60 months, n = 28, 33.8% ± 3.7%; > 60, n = 4, 6.6% ± 3.1%; Figure 3C). In addition, when we examined the overall survival of all NHL patients after sampling, we found that all deaths thus far have been in patients with higher percentages of CD14+ monocytes that have lost HLA-DR expression compared with control (CD14+HLA-DRlow/− > 10% of CD14+ monocytes representing the mean plus 2 SD from the normal values; Figure 3D). Thus, loss of HLA-DR expression on CD14+ monocytes (CD14+HLA-DRlow/−) was associated with more aggressive NHL as characterized by extent of disease involvement, disease type, and rate of disease progression. An increased ratio of CD14+HLA-DRlow/− to CD14+HLA-DR+ monocytes may be a useful measure of prognosis but awaits further investigation. The analysis in Figure 3 was repeated removing the values from the samples of the 4 newly diagnosed patients. No change was seen in the significance in any of the measures.
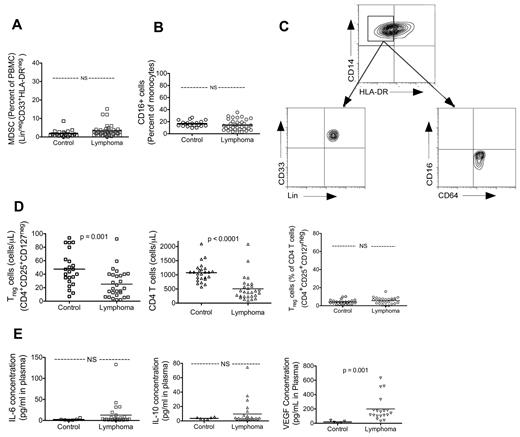
Myeloid-derived suppressor cells (MDSCs)18 and nonclassic monocytes have been reported to suppress immune functions in cancer.19,20 MDSCs have been defined with a strict phenotype, including lineage (including CD14) negative. We examined the population of MDSC (Lin−CD33+HLA-DR− among PBMCs) and found that this cell type was found at low frequency and at similar levels as in controls (control, n = 20, 1.91% ± 0.42%; lymphoma, n = 37, 3.16% ± 0.54%; P value not significant; Figure 4A). In addition, the CD14+HLA-DRlow/− monocyte was a different population of monocytes from MDSCs in that these monocytes expressed lineage markers (Figure 4C). Similarly, we found that the percentage of nonclassic monocytes (CD16+) in NHL were the same as control (control, n = 20, 16.6% ± 1.1%; lymphoma, n = 35, 14.4% ± 1.5%; P value not significant; Figure 4B). Furthermore, CD14+HLA-DRlow/− monocytes are a distinct population from these nonclassic monocytes in that they did not express CD16 (Figure 4C).
Peripheral blood immunosuppressive myeloid phenotype in NHL patients is limited to CD14+HLA-DR−/low. There were no statistically significant differences between controls and lymphoma for MDSCs (A) (MDSCs: control, n = 20; lymphoma, n = 40) or CD16+ monocytes (B) (control, n = 20; lymphoma, n = 40). (C) CD14+HLA-DR− population of monocytes were phenotypically distinct from MDSCs in that they were lineage+ and were not representative of nonclassic monocytes in that they are CD16−. (D) Circulating Tregs were not elevated in lymphoma patients. Absolute count of Tregs and CD4 T cells in peripheral blood was analyzed with TruCount flow cytometry tubes. Percentage of Tregs within CD4 T-cell population is processed and analyzed by standard immunofluorescent flow cytometry. Lymphoma patients had decreased absolute circulating Treg count (top panel: control, n = 23; lymphoma, n = 28). This is reflective of a decrease in absolute CD4 T-cell count in lymphoma (middle panel: control, n = 25; lymphoma, n = 28) as the percentage of Tregs in CD4 T-cell population is not different compared with control (bottom panel: control, n = 23; lymphoma, n = 28). (E) Plasma concentration of IL-6 (top panel: control, n = 6; lymphoma, n = 26) and IL-10 (middle panel: control, n = 6; lymphoma, n = 25) were measured by ELISA. VEGF concentration was elevated in lymphoma patients but not correlated to CD14+HLA-DR− monocytes. Plasma VEGF concentration was measured by ELISA (bottom panel: control, n = 5; lymphoma, n = 19). Lymphoma VEGF concentration did not correlate with the presence of CD14+HLA-DR− monocytes (data not shown). Statistically significant P values are shown between the groups. NS indicates that P value was not significant.
Peripheral blood immunosuppressive myeloid phenotype in NHL patients is limited to CD14+HLA-DR−/low. There were no statistically significant differences between controls and lymphoma for MDSCs (A) (MDSCs: control, n = 20; lymphoma, n = 40) or CD16+ monocytes (B) (control, n = 20; lymphoma, n = 40). (C) CD14+HLA-DR− population of monocytes were phenotypically distinct from MDSCs in that they were lineage+ and were not representative of nonclassic monocytes in that they are CD16−. (D) Circulating Tregs were not elevated in lymphoma patients. Absolute count of Tregs and CD4 T cells in peripheral blood was analyzed with TruCount flow cytometry tubes. Percentage of Tregs within CD4 T-cell population is processed and analyzed by standard immunofluorescent flow cytometry. Lymphoma patients had decreased absolute circulating Treg count (top panel: control, n = 23; lymphoma, n = 28). This is reflective of a decrease in absolute CD4 T-cell count in lymphoma (middle panel: control, n = 25; lymphoma, n = 28) as the percentage of Tregs in CD4 T-cell population is not different compared with control (bottom panel: control, n = 23; lymphoma, n = 28). (E) Plasma concentration of IL-6 (top panel: control, n = 6; lymphoma, n = 26) and IL-10 (middle panel: control, n = 6; lymphoma, n = 25) were measured by ELISA. VEGF concentration was elevated in lymphoma patients but not correlated to CD14+HLA-DR− monocytes. Plasma VEGF concentration was measured by ELISA (bottom panel: control, n = 5; lymphoma, n = 19). Lymphoma VEGF concentration did not correlate with the presence of CD14+HLA-DR− monocytes (data not shown). Statistically significant P values are shown between the groups. NS indicates that P value was not significant.
Systemic immune suppression could be mediated by several other known immune suppressors, such as immunosuppressive cytokines and Tregs. In our analysis of the peripheral blood leukocytes, we found that monocytes were unchanged, lymphocytes decreased, and granulocytes increased (percentage of total blood leukocytes, supplemental Figure 1). The absolute counts of T and B cells were decreased, whereas NK cells were unchanged (supplemental Figure 1). Within the CD4 T cells, we found decreased circulating naive cells (CD4+CD45RA) and no change in the number of memory cells (CD4+CD45RO; supplemental Figure 1). We found that the number of circulating Tregs per microliter of blood (Treg, CD4+CD25+CD127−) in NHL patients was decreased in NHL (Figure 4D; control: n = 23, 47.6 ± 5.3 cells/μL; lymphoma: n = 25, 27.2 ± 4.2 cells/μL; P = .004). This is consistent with the observed lymphopenia as well as the reduction in CD4 T cells (control, n = 25, 1075 ± 66.5 cells/μL; lymphoma, n = 28, 506 ± 81.5 cells/μL; P < .0001; Figure 4D), as the percentage of Tregs in CD4 T-cell population is unchanged (control, n = 23, 4.12% ± 0.52%; lymphoma, n = 25, 5.59% ± 0.69%; P value not significant; Figure 4D). We also did not find significantly elevated plasma levels of IL-6 (Figure 4E), IL-10 (Figure 4E), CCL-2 (data not shown), or transforming growth factor-β (data not shown). Plasma level of VEGF was elevated (control, 18.8 ± 6.9 pg/mL, n = 5; lymphoma, 200 ± 39.4 pg/mL, n = 19, P = .03). However, the increased VEGF level did not correlate with the presence of CD14+HLA-DRlow/− monocytes (data not shown) and may be an independent contributor to systemic immunosuppression.
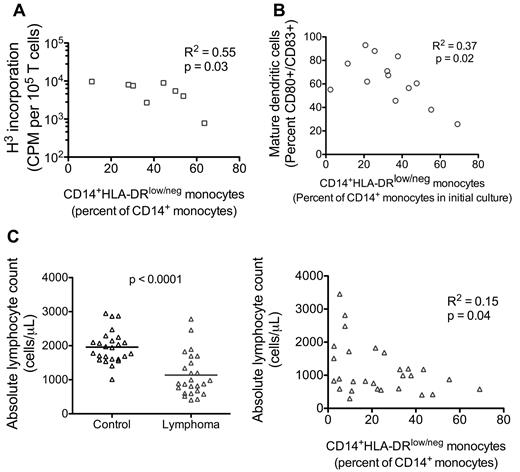
Having identified the predominant suppressive monocyte phenotype (CD14+HLA-DRlow/−) in NHL that was associated with more aggressive clinical disease, we reviewed our in vitro data from patient samples to determine whether the presence of CD14+HLA-DRlow/− monocytes correlated with our observed functional suppression. Increased ratio of CD14+HLA-DRlow/− monocytes correlated with decreased T-cell proliferation in allogeneic mixed lymphocyte reaction (Figure 5A), decreased generation of mature dendritic cells (Figure 5B), and decreased number of circulating lymphocytes (Figure 5C).
CD14+HLA-DRlow/− monocytes correlated with immunosuppressive functions. (A) The percentage of CD14+HLA-DRlow/− monocytes found in peripheral blood of NHL patients is plotted against the in vitro T-cell proliferation response in allogeneic mixed lymphocyte reaction with these NHL monocytes (1:10 monocyte/T-cell ratio; Figure 1B). (B) Similarly, the percentage of CD14+HLA-DRlow/− monocytes in NHL patients was plotted against the ability of these NHL monocytes to differentiate into mature dendritic cell cultures (Figure 2C). (C) The percentage of CD14+HLA-DRlow/− monocytes was plotted against the peripheral blood absolute lymphocyte count as determined using TruCount flow tubes (left panel, n = 25 for control and lymphoma samples). Decreased lymphocyte count in lymphoma patients was correlated to increased percentage of CD14+HLA-DRlow/− monocytes. Statistically significant P value is show between groups. P value for statistically significant correlation.
CD14+HLA-DRlow/− monocytes correlated with immunosuppressive functions. (A) The percentage of CD14+HLA-DRlow/− monocytes found in peripheral blood of NHL patients is plotted against the in vitro T-cell proliferation response in allogeneic mixed lymphocyte reaction with these NHL monocytes (1:10 monocyte/T-cell ratio; Figure 1B). (B) Similarly, the percentage of CD14+HLA-DRlow/− monocytes in NHL patients was plotted against the ability of these NHL monocytes to differentiate into mature dendritic cell cultures (Figure 2C). (C) The percentage of CD14+HLA-DRlow/− monocytes was plotted against the peripheral blood absolute lymphocyte count as determined using TruCount flow tubes (left panel, n = 25 for control and lymphoma samples). Decreased lymphocyte count in lymphoma patients was correlated to increased percentage of CD14+HLA-DRlow/− monocytes. Statistically significant P value is show between groups. P value for statistically significant correlation.
Arginine metabolism may be one of the mechanisms of CD14+HLA-DRlow/− monocyte suppression
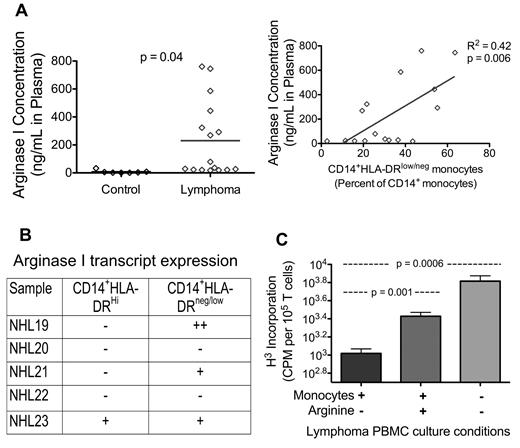
The suppression of T-cell proliferation suggests that the mechanism of immune suppression could be mediated in a cell contact-independent fashion. Serafini et al have suggested that arginine metabolism plays a similar role in immune suppression in a lymphoma model in mice.21 To this end, we measured the plasma level of arginase I in NHL patients and evaluated the ability to correct immune suppression via exogenous arginine addition. Plasma level of arginase I was increased in NHL (control, 7.8 ± 4.4 pg/mL, n = 7; lymphoma, 218.0 ± 64.6 pg/mL, n = 17; P = .05). Furthermore, the increased arginase I level correlated with increased ratio of CD14+HLA-DRlow/− monocytes (Figure 6A). We assessed ARG1 expression levels using quantitative reverse-transcribed PCR in CD14+ populations that had been flow-sorted based on the expression level of DR on CD14+ cells. Cells from 3 of the 5 NHL patients demonstrated marked increase in ARG1 levels, and 2 of the 5 had higher levels of ARG1 in the HLA-DR low fractionated CD14+ populations (Figure 6B). Furthermore, NHL PBMC proliferation was increased with the addition of L-arginine (Figure 6C). Although the degree of increase is not as high as with removal of monocytes, arginine supplementation overcame a significant level of monocyte-mediated suppression.
Arginine metabolism may be one of the mechanisms that CD14+HLA-DRlow/− monocytes suppress T-cell functions. (A) Plasma concentration of arginase I is measured by ELISA (left panel: control, n = 7; lymphoma, n = 16). Increased lymphoma plasma concentration of arginase I is correlated with increased percentage of CD14+HLA-DRlow/− circulating monocytes (right panel). (B) Real-time PCR analysis of arginase I transcript levels in sorted CD14+ monocytes. CD14+ monocytes were stained for HLA-DR and sorted into high-expressing and low-expressing HLA-DR populations. Quantitative reverse-transcribed PCR was performed and normalized to GAPDH (reference gene). Results are reported as follows: − indicates baseline/nondetectable crossing point (Cp); +, positive expression of 2- to 32-fold induction (1-5 Cps lower than baseline Cp); and ++, expression higher than 32-fold increase (> 5 Cp difference) over baseline. (C) Lymphoma PBMC proliferation can be increased with the addition of L-arginine or removal of monocytes (one representative sample, bar represents mean ± SD of 8 replicates). Statistically significant P values are shown between the groups.
Arginine metabolism may be one of the mechanisms that CD14+HLA-DRlow/− monocytes suppress T-cell functions. (A) Plasma concentration of arginase I is measured by ELISA (left panel: control, n = 7; lymphoma, n = 16). Increased lymphoma plasma concentration of arginase I is correlated with increased percentage of CD14+HLA-DRlow/− circulating monocytes (right panel). (B) Real-time PCR analysis of arginase I transcript levels in sorted CD14+ monocytes. CD14+ monocytes were stained for HLA-DR and sorted into high-expressing and low-expressing HLA-DR populations. Quantitative reverse-transcribed PCR was performed and normalized to GAPDH (reference gene). Results are reported as follows: − indicates baseline/nondetectable crossing point (Cp); +, positive expression of 2- to 32-fold induction (1-5 Cps lower than baseline Cp); and ++, expression higher than 32-fold increase (> 5 Cp difference) over baseline. (C) Lymphoma PBMC proliferation can be increased with the addition of L-arginine or removal of monocytes (one representative sample, bar represents mean ± SD of 8 replicates). Statistically significant P values are shown between the groups.
Discussion
Impaired host immunity can affect both pathogenesis of lymphoma and treatment outcome. Typically, the focus of this work has been at the tumor microenvironment level in the form of tumor-infiltrating lymphocytes, tumor-associated leukocytes, or tumor-secreted factors. In this study, we report that immune suppression may be found systemically resulting from an altered monocyte phenotype. This altered phenotype can affect immunity through multiple pathways: innate immunity via altered signal transduction pathways and secreted cytokines, adaptive immune response via impaired dendritic cell differentiation, and direct suppression via interference of T-cell proliferation.
These immunosuppressive monocytes are fundamentally different from the common monocytes (CD14+HLA-DR+) found in healthy controls. They are not detectable on routine automated complete blood counts and white blood cell differentials or by morphology on blood smear. STAT1 phosphorylation is an early step in monocyte differentiation and activation of proinflammatory macrophages in response to type I and II interferons. STAT3 phosphorylation, on the other hand, has been implicated in the arrest of myeloid cell maturation and anti-inflammatory response in macrophages.22 NHL monocytes have decreased STAT1 phosphorylation and normal STAT3 phosphorylation compared with controls, suggesting a predisposed different immune response to the same environmental stimuli (Figure 2). Indeed, these monocytes have decreased production of IFN-α and are less capable of generating mature dendritic cells (Figure 2). Suppression of these 2 functions demonstrates these monocytes' deficits in innate and adaptive immune response. The decreased response to CpG stimulation may have additional clinical implications. CpG, as a TLR9 ligand, has been used for its immunostimulatory capacity as treatment in clinical trials for lymphomas and solid tumors with variable responses.23,24 Our data suggest that patients with poor clinical responses may have a greater frequency of immunosuppressive monocytes incapable of response to CpG stimulation. Identifying patients with a low ratio of CD14+HLA-DRlow/− monocytes may be a method of identifying patients who may potentially respond to CpG treatment.
Extensive immunophenotype analysis of NHL monocytes revealed that the predominant phenotype alteration in these cells is the decreased expression of HLA-DR (Figure 3). Increased ratio of CD14+ monocytes with loss of HLA expression was seen in patients with higher-stage disease, more aggressive pathology, and faster rate of disease progression. Although a trend is seen in decreased survival in patients with increased percentage of CD14+HLA-DRlow/− monocytes, further analysis is needed with larger patient samples over longer periods of time for multivariate analysis with confounding coexisting prognostic factors. Nevertheless, using this limited dataset, we observed that patients with the slowest rate of disease progression had normal ratios of CD14+HLA-DR− monocytes and those with the fastest rate of progression had elevated ratios of CD14+HLA-DR− monocytes. The relationship of CD14+HLA-DRlow/− monocytes with time to progression and overall survival will require evaluation in prospective clinical trials where patients are treated and followed in a similar manner.
A marker of systemic immunity that has been identified as an independent prognostic predictor of poor survival in NHL patients is the decreased absolute lymphocyte count. The underlying mechanism leading to this association may be multifactorial and has not yet been identified.11,25 Our data have provided another cellular immunity alteration that correlates to the reduction of lymphocyte count. Although we do not fully understand the genesis of the 2 phenomena, our data show that the suppressive monocytes have the ability to arrest T-cell proliferation in the presence of antigen stimulation. Arrested T-cell proliferation can lead to apoptosis, providing a potential model for the subsequent loss of lymphocytes.
NHL is not the only disease with changes in monocyte phenotype. Decreased monocyte expression of HLA-DR has been associated with, and predictive of, poor clinical outcome in nonmalignant conditions, such as sepsis,26,27 pancreatitis,28,29 and liver failure.30 Others have also described the presence of immunosuppressive CD14+HLA-DRlow/− monocytes in melanoma,31 hepatocellular carcinoma,32 and pediatric acute leukemia and lymphoma.33 We have reported an increased presence of these cells and associated immunosuppression in prostate cancer34 and glioblastoma.15 Similarities within these diverse cancer types include increased frequency of these cells in disease progression (prostate cancer), loss of CD4 T cells (prostate cancer and glioblastoma multiforme), and inability to differentiate into mature dendritic cells (prostate cancer and glioblastoma multiforme). This volume of data substantiates the importance of this monocyte population in cancer-mediated immune suppression. Additional myeloid suppressors, including Lin−CD33+HLA-DR− (MDSCs)35 and CD14lowCD16+20,36 cells add complexity to this story. Yet the percentage of these CD14+HLA-DRlow/− monocytes in circulation (in some cases, 70% of the total circulating monocytes) suggests a dominant role of these cells for systemic immune suppression.
As we are learning more about the phenotypic heterogeneity of myeloid lineage suppressors, we may also identify heterogeneity in the mechanisms of suppression. CD14+HLA-DRlow/− monocytes have been shown to promote expansion of Tregs32 and suppress NK cells37 as mechanisms of immune suppression in hepatocellular carcinoma. We found decreased circulating Tregs, as a reflection of overall lymphopenia, with no change in the percentage of Tregs among lymphocytes (Figure 4). Although Tregs may have a role in immune suppression in tumor microenvironment, we did not find these cells to be a major component of systemic cellular immunity. Increased B7-H1 expression has been described as a mechanism of monocyte suppression through induction of Treg in T-cell lymphoma.38 In our population of B-cell lymphoma, we did not find an increase in monocyte B7-H1 expression. Anecdotal observation of one patient with a small population of B7-H1+ monocytes showed that these monocytes were HLA-DRlow/−. We found elevated arginase I levels in plasma of NHL patients (Figure 6). Myeloid cells have been shown to suppress T lymphocytes by depletion of L-arginine through increased concentration and activity of arginase I.39 Indeed, the increase in plasma arginase I correlated with increase in CD14+HLA-DRlow/− monocytes, and we found evidence of expression of ARG1 mRNA in monocytes of NHL patients. Furthermore, when we supplemented the cultures with L-arginine, we were able to reverse some of the monocyte-mediated suppression of T-cell proliferation (Figure 6). Therefore, it is probable that arginase I plays a role in the monocyte-mediated inhibition of T-cell proliferation.
IL-6, IL-10, transforming growth factor-β, and VEGF all have been associated with poor prognosis in lymphoma.40-43 In addition, IL-6, IL-10, and VEGF can affect myeloid differentiation, activation, and trafficking.44-47 IL-10 has also been shown to sequester HLA-DR in monocytes.48 However, we did not identify a correlation between these immunosuppressive cytokines and the presence of immunosuppressive monocytes. Thus, if these cytokines do mediate the conversion of HLA-DR+ to HLA-DR− monocytes, it is possible that this may be occurring in the tumor microenvironment.
The relationship between systemic immunity and the tumor microenvironment in lymphoma remains to be characterized. Increased presence of lymphoma-associated macrophages in follicular lymphoma is an independent predictor of poor survival.49 Molecular profiling of follicular lymphoma tumors revealed that gene expression signature consistent with activated T cells in the tumor microenvironment was associated with favorable survival outcome where tumors with gene expression signature consistent with monocyte and dendritic cell activities were associated with poor survival.6 CD14+ monocytes have been identified in the tumor microenvironment of DLBCLs and splenic marginal zone lymphomas.50 The relationship between CD14+HLA-DRlow/− monocytes in circulation and CD14+ monocytes and lymphoma-associated macrophage in tumor remains to be elucidated.
In conclusion, we report here, for the first time, a population of immunosuppressive monocytes in the systemic circulation of patients with B-cell NHL characterized by the CD14+HLA-DRlow/− phenotype. These cells have altered protein kinase phosphorylation response to stimulation and decreased stimulatory functions in innate and adaptive immunity. Their suppression may be mediated through arginine metabolism. These insights may lead to identifying therapeutic strategies to overcome the suppressive properties of these monocytes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Joseph P. Colgan, Dr Luis F. Porrata, Dr David J. Inwards, Dr Stephen M. Ansell, Dr Thomas M. Habermann, Dr Patrick B. Johnston, Dr Ivana N.M. Micallef, Robin R. Klebig, RN, CNP, and the lymphoma group for patient recruitment; Colleen Irlbeck for her administrative assistance with patient recruitment; Mary Maas and Amy Mohr for their assistance with some of the flow cytometry; and Dr Stanimir Vuk-pavlovic for providing laboratory space and guidance.
This work was supported in part by the Gerstner Family Foundation Career Development Grant, the Mayo Clinic Hematology Malignancy Program, the University of Iowa/Mayo Clinic Lymphoma SPORE, and the Henry J Predolin Foundation.
National Institutes of Health
Authorship
Contribution: Y.L. designed and conducted the experiments and prepared the manuscript; M.P.G. assisted with the experiments and manuscript editing; P.A.B. assisted with the experiments; D.A.G. and T.E.W. recruited patients for this study and assisted with manuscript editing; and A.B.D. supervised and reviewed study design and results, and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Allan B. Dietz, Mayo Clinic, Human Cell Therapy Lab, Hilton 2–50, 200 First Street SW, Rochester, MN 55905; e-mail: dietz.allan@mayo.edu.