Abstract
Children with sickle cell disease (SCD) and strokes receive blood transfusion therapy for secondary stroke prevention; despite this, approximately 20% experience second overt strokes. Given this rate of second overt strokes and the clinical significance of silent cerebral infarcts, we tested the hypothesis that silent cerebral infarcts occur among children with SCD being transfused for secondary stroke prevention. A prospective cohort enrolled children with SCD and overt strokes at 7 academic centers. Magnetic resonance imaging and magnetic resonance angiography of the brain were scheduled approximately every 1 to 2 years; studies were reviewed by a panel of neuroradiologists. Eligibility criteria included regularly scheduled blood transfusion therapy. Forty children were included; mean pretransfusion hemoglobin S concentration was 29%. Progressive cerebral infarcts occurred in 45% (18 of 40 children) while receiving chronic blood transfusion therapy; 7 had second overt strokes and 11 had new silent cerebral infarcts. Worsening cerebral vasculopathy was associated with new cerebral infarction (overt or silent; relative risk = 12.7; 95% confidence interval, 2.65-60.5, P = .001). Children with SCD and overt strokes receiving regular blood transfusion therapy experience silent cerebral infarcts at a higher rate than previously recognized. Additional therapies are needed for secondary stroke prevention in children with SCD.
Introduction
Children with sickle cell disease (SCD) have a high rate of overt strokes and silent cerebral infarcts. Overt strokes occur in 11% of patients with homozygous Hemoglobin SS disease (sickle cell anemia) before age 20.1 Standard care for secondary prevention of overt strokes in children with SCD includes regular blood transfusion therapy to suppress synthesis of hemoglobin S (HbS).2 Without transfusion therapy, approximately 67% of these children will have second overt strokes.3 However, approximately 20% of children have a second overt stroke despite receiving regular blood transfusion therapy.2
Silent cerebral infarcts are a relatively newly recognized complication of SCD.4,5 These ischemic lesions seen on magnetic resonance imaging (MRI) studies are, by definition, undetectable by neurologic examination6 and are recognized as areas of increased signal intensity on T2-weighted MRI sequences, greater than 3 mm in one axis, and visible on at least 2 views.7 Children with silent cerebral infarcts are at risk of progression to more silent cerebral infarcts8 and overt strokes.9 Furthermore, an increased volume of cerebral infarcts is associated with a decline in intelligence quotient on neurocognitive testing.10 Moyamoya collateral blood vessels have been described as a risk factor for recurrent overt strokes and transient ischemic attacks (TIAs) in children with SCD receiving regular blood transfusion therapy for secondary stroke prevention.11 The relationship between cerebral vasculopathy and the development of silent cerebral infarcts and overt strokes while receiving secondary prevention of blood transfusion therapy have not been defined.
The incidence of silent cerebral infarcts during secondary stroke prophylaxis with blood transfusion therapy is unknown; however, because silent cerebral infarcts are known to confer additional disease burden,8-10 this information is important for evaluating the efficacy of this secondary prevention strategy. We undertook a prospective cohort study of patients at 7 pediatric SCD centers to accomplish 2 objectives. First, to determine the incidence of silent cerebral infarcts in children with SCD receiving regular blood transfusion therapy for secondary overt stroke prevention, we tested the primary hypothesis that silent cerebral infarcts would be detected with surveillance MRI scans performed every 12 to 24 months. Second, to validate and extend our previous retrospective findings,2 we tested the hypothesis that overt strokes occur in children receiving regular blood transfusion therapy for secondary prevention of strokes and are associated with cerebral vasculopathy.
Methods
A consortium was formed between investigators at (1) Washington University School of Medicine/St Louis Children's Hospital; (2) Medical College of Wisconsin/Children's Hospital of Wisconsin; (3) University of Missouri-Kansas City/Children's Mercy Hospital; (4) Wayne State University School of Medicine/Children's Hospital of Michigan; (5) Northwestern University/Children's Memorial Hospital; (6) Central Middlesex Hospital; and (7) Guy's and St Thomas's Hospital in London, United Kingdom. Consortium members, including 7 hematologists, 3 neurologists, and a pediatric neuroradiologist, met several times over 2 years to develop the consortium guidelines. Human Studies Committee approval was obtained at each site, and subjects or guardians signed informed consent in accordance with the Declaration of Helsinki at the time of enrollment into the study. This was a convenience sample of as many children as each consortium site was able to enroll. The consortium aimed to include all patients with SCD receiving regular blood transfusion therapy after neurologic injury, but analysis for the current study was limited to children with overt strokes.
Subject eligibility
Inclusion criteria for this analysis were diagnosis of SCD (any type) and first overt stroke after January 1, 1996, treated with chronic blood transfusion therapy for secondary stroke prophylaxis. Exclusion criteria were: failure to start chronic blood transfusion therapy within 3 months of the diagnosis of the initial clinical stroke, interval between blood transfusions scheduled for greater than every 6 weeks, presence of another central nervous system condition (eg, neurofibromatosis, traumatic brain injury), or inability to obtain adequate details from medical records.
All consortium members agreed to a clinical protocol that included obtaining MRI and magnetic resonance angiography (MRA) of the brain every 12 to 24 months and providing regular blood transfusion therapy defined as scheduled at least every 6 weeks, to maintain HbS concentrations less than 30% for at least the first 2 years after the initial stroke and less than 50% thereafter for asymptomatic patients. In the event of a recurrent stroke or TIA, the hematologist treating the child individualized the transfusion regimen with the goal to lower HbS concentration. Neurologic examinations were performed primarily by hematologists as part of the participants' standard care. Patients who discontinued chronic blood transfusion therapy or were lost to follow-up were censored at the last transfusion date.
Data collection
In 2006, one investigator (M.L.H.) reviewed all participants' inpatient and outpatient records to capture critical clinical data. Information extracted included clinical events at the time of the first stroke, initial stroke symptoms and treatment, occurrence of second or subsequent strokes, HbS concentration at the time of the second stroke, and other neurologic events, such as TIAs or cerebrovascular surgery. These data were reviewed with the site hematologist for accuracy. Data were recorded onto study data extraction forms and entered into a database. Data entry was double-checked by a second investigator (J.E.M.); discrepancies were resolved by consensus between the 2 persons after reviewing the data extraction sheets and source documents.
MRI studies were obtained in DICOM format on CD-ROM from each site when available. Identifiable medical information was stripped using the Clinical Studies Workstation program.7 Each study was assigned a unique identifier that was linked to the clinical data. The studies were electronically transmitted to the Electronic Radiology Laboratory at Washington University via a Virtual Private Network (Cisco Systems). The images were viewed using the iSite PACS system (Philips Healthcare Informatics) as previously described.7 A single pediatric neuroradiologist (R.C.M.) with expertise in neuroimaging in SCD interpreted MRI studies, without knowledge of the subjects' clinical histories, looking for new or enlarging cerebral infarction. Two neuroradiologists (J.L.L., C.J.M.) independently interpreted each MRA study without knowledge of the subjects' clinical histories. Discrepancies were resolved by a third neuroradiologist's evaluation (R.C.M.). When only reports and not images were available, a neuroradiologist determined whether new cerebral infarcts had occurred (yes or no), as well as whether vasculopathy had worsened (yes or no). If no reports were available for review, or if review of reports was inconclusive, the subject was considered to have no evidence of new infarct and no vasculopathy.
Definitions
First and subsequent overt strokes were defined as new focal neurologic deficits attributable to arterial occlusion lasting greater than 24 hours. If symptoms resolved within 24 hours, an event was considered a stroke if the patient had an imaging study demonstrating acute cerebral ischemia, based on increased signal intensity on T2-weighted MRI and restricted diffusion on diffusion-weighted MRI, in a region of the brain corresponding with the symptoms; there were no patients identified as having recurrent infarctions based on diffusion-weighted imaging without T2-weighted changes. Transient (lasting < 24 hours) weakness or sensory deficits without acute cerebral infarcts on MRI of the brain were considered TIAs. New cerebral infarctions, defined as new 3 mm or greater lesions of increased intensity on T2-weighted MRI sequences visible in at least 2 views, were denoted as present or absent. Enlarging infarctions were defined as an increase in a previously existing area of T2-weighted signal hyperintensity of at least 3 mm in one dimension.7 New or enlarging cerebral infarctions on imaging without colocalizing clinical signs or symptoms were categorized as silent cerebral infarctions. A silent cerebral infarction could be either an enlargement of an existing infarct lesion or a new cerebral infarction, as long as there was no new focal neurologic deficit that was called an overt stroke by the treating hematologists. Time to the first recurrent overt stroke or silent cerebral infarct was noted as the time to the progression of the cerebral infarct after the first stroke. The date for progression of a silent infarction was defined as the date of the MRI on which the new lesion was noted, whereas the date for second overt strokes was defined as the date of symptom onset or recognition. For MRA studies, vessels examined were the internal carotid artery and the first segments of the anterior, middle, and posterior cerebral arteries bilaterally; each vessel was graded as normal, mild stenosis (25%-50%), moderate stenosis (50%-75%), severe stenosis (75%-99%), or occlusion. On the initial MRA, vasculopathy was defined as any presence of moderate or worse stenosis in any vessel segment scored. Worsening vasculopathy was defined as an increase in stenosis from one category to a higher category in any vessel segment, or any new vessel occlusion.
Statistical analysis
The primary and secondary hypotheses were based on preliminary data2 and unpublished experience at a single site. The Kaplan-Meier method was used to estimate event-free duration with respect to second overt strokes and silent cerebral infarcts. These were the only inferential analyses performed for this study, and the level of significance was specified as P less than .05. The rest of the analyses were descriptive of the population, rather than inferential. Categorical data were evaluated with the Fisher exact test or Pearson χ2 when applicable. Statistical analysis was performed with PASW Statistics, Version 17.0 (SPSS Inc).
Results
Subject characteristics
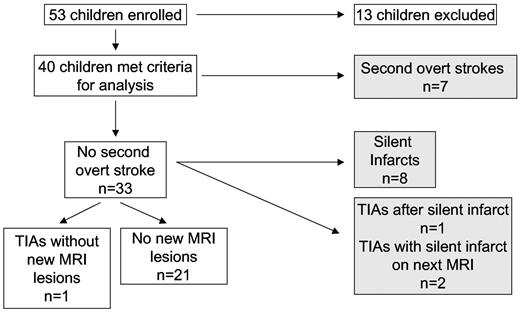
Fifty-three children with SCD were enrolled from the 7 participating centers. Thirteen were excluded from this analysis: 3 children did not have overt ischemic strokes (2 had silent cerebral infarcts, one had a hemorrhagic stroke); 2 were excluded because their initial strokes occurred before January 1, 1996; 4 did not start chronic blood transfusion therapy within 3 months of the diagnosis of the first overt stroke; 1 had neurofibromatosis; and 3 had inadequate clinical details available (Figure 1).
Children with SCD receiving regular blood transfusion therapy for secondary prophylaxis of strokes. Subjects' clinical records were reviewed to determine the occurrence of second overt strokes and TIAs. MRI of the brain was used to determine the occurrence of silent cerebral infarcts. Shaded boxes represent children with second overt strokes or progressive silent cerebral infarctions.
Children with SCD receiving regular blood transfusion therapy for secondary prophylaxis of strokes. Subjects' clinical records were reviewed to determine the occurrence of second overt strokes and TIAs. MRI of the brain was used to determine the occurrence of silent cerebral infarcts. Shaded boxes represent children with second overt strokes or progressive silent cerebral infarctions.
A total of 40 patients met all criteria for analysis; 43% were male. Median age at first stroke was 5.4 years (range, 2.05-15.8 years). Median duration of follow-up from initial stroke was 5.5 years (range, 1.0-9.4 years), with a total of 222 patient-years of follow-up. One patient died during the follow-up period after a haplotype-mismatched allogeneic stem cell transplantation for aplastic anemia, 5.8 years after her stroke. This was presumed to be unrelated to her SCD. Three children were lost to follow-up and were censored at the last follow-up date: 1 voluntarily discontinued blood transfusion therapy 6.9 years after the first overt stroke; 2 started receiving transfusion therapy at nonparticipating institutions at 7.3 and 8.5 years after the first overt stroke.
Of the 40 participants, 37 (93%) had serial MRI studies. Of a total of 135 MRI studies that were reviewed, 109 (81%) were obtained for surveillance after the initiation of the protocol. Central review of images was performed on 82 MRI studies from 25 participants (median, 3 studies/participant; range, 2-7 studies/participant), and institutional reports were reviewed for an additional 12 participants. MRA studies were performed on 35 participants, with central review of images of 46 MRAs from 23 participants (average 2 studies per participant) and institutional reports reviewed for an additional 12 participants. Lack of central MRI and MRA review was the result of either older studies that were not available in DICOM format or the inability to upload the images to the Electronic Radiology Laboratory. Among children with central review of serial MRI images, 40% (10 of 25) had second overt strokes or TIAs; among those without central MRI image review, 13% (2 of 15) had second overt strokes or TIAs. No statistically significant difference (Fisher Exact Test, P = .2) existed between children with and without a central review of the MRI.
Characteristics of regular blood transfusion therapy
Per study protocol, HbS levels were measured before each blood transfusion. HbS levels were analyzed 39 of the 40 participants; one child without a second overt stroke or silent cerebral infarct did not have HbS levels included in the analysis because they were not obtained every month, but sporadic checks of his HbS concentrations were 30% or less. To evaluate the efficacy of chronic blood transfusion therapy at suppressing HbS levels in each child, the mean pretransfusion HbS level was calculated for each participant. The mean of all participants' mean pretransfusion HbS levels was 26.9% (range, 3.94%–48.3%). No significant difference in mean pretransfusion HbS concentration was noted for the patients with overt strokes, silent cerebral infarcts, or no cerebral infarcts. For the group of children who had second overt strokes, the mean of all patients' pretransfusion HbS levels was 25.0% (n = 7); for the group who had silent cerebral infarcts, the mean patients' pretransfusion HbS concentrations was 27.2% (n = 11); and for the group who had no new infarcts on MRI, the mean of all patients' mean pretransfusion HbS concentrations was 27.4% (n = 21) (P = .867).
The mean of all participants' interval between transfusions was 27.9 days (range, 16.5-37.1 days). Furthermore, the mean interval between transfusions was not significantly different between patients with second overt stroke (25.2 days), silent cerebral infarct (27.9 days), and no progression (28.8 days) (P = .13).
Silent cerebral infarcts during regular blood transfusion therapy
New or enlarging cerebral infarcts were identified in 45% (18 of 40) of participants (Figure 1). Seven of 18 participants with new overt or silent cerebral infarcts had second overt strokes as their first endpoint, and the remaining 11 had new silent cerebral infarcts. There was no significant difference in recurrent overt or silent infarctions between males (2 overt and 6 silent, of 17 males) and females (5 overt and 5 silent, of 23 females) (Pearson χ2 2-sided, P = .6).
Among the 11 children whose first events were new silent cerebral infarcts identified on surveillance MRIs, 2 had transient neurologic symptoms before the diagnosis of a silent cerebral infarcts, but without MRIs performed immediately after the neurologic symptoms. Both of these patients were diagnosed by their treating hematologist as having TIAs, not new overt strokes; consequently, we elected to classify these 2 persons as having silent cerebral infarcts with prior TIAs, and not new overt strokes. Regardless of our definition of these 2 cases as having second overt strokes or silent cerebral infarcts, they were both classified among the 18 children with progressive cerebral infarcts despite receiving regular blood transfusion therapy. Eleven children with progressive cerebral infarctions had no new neurologic symptoms noted in their medical record. One of these children had a silent cerebral infarct as well as progressive cerebral vasculopathy, underwent cerebral revascularization surgery, and experienced a new overt stroke postoperatively (HbS 11% at the time of the postoperative stroke; described in “Cerebral revascularization procedures as adjuvant therapy”). Overall, the incidence of silent cerebral infarcts was 4.5 events/100 patient-years (95% confidence interval, 2.2-8.3). The clinical characteristics of children with second overt strokes and silent cerebral infarcts while receiving regular blood transfusion therapy are reported in Table 1.
Clinical characteristics of children with SCD with second overt strokes or progressive silent cerebral infarcts while receiving regular blood transfusion therapy for secondary prevention of strokes
| Patient no. . | Age at first stroke, y . | Type of progression . | Interval between first stroke and second event, y . | HbS at time of second overt stroke, % . | Mean HbS up to second overt stroke, % . | Total no. of overt strokes . | Total no. of MRIs with new or enlarging infarcts . | Clinical comments . | TIAs . | Vasculopathy on initial MRA . | Progression of vasculopathy on follow-up MRA . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5.15 | TIA, then silent | 0.65 | NA | NA | 1 | 2 | MRI after TIA had new infarct—overt stroke? | One TIA reported | Occlusion L MCA/ACA, R ICA, MM | No |
| 2 | 6.31 | Silent | 3.65 | NA | NA | 1 | 1 | None reported | Severe R ICA, L ICA/MCA/ACA | Yes, + MM | |
| 3 | 3.73 | TIAs, then silent | 0.32 | NA | NA | 1 | 1 | MRI after first TIA had new infarct—overt stroke? | TIAs continued after RVS | Moderate R ICA/MCA/ACA, L MCA/ACA/PCA, MM | Yes, severe R MCA/ACA, L MCA/ACA |
| 4 | 3.77 | Overt | 0.75 | 48.1 | * | 4 | 3 | Missed appointment for transfusion week before second stroke | None reported | Severe R ICA/MCA/ACA/PCA, L MCA/ACA/PCA, MM | Yes, occlusion R ICA/ACA, severe L ICA |
| 5 | 4.38 | Overt | 1.98 | 10.2 | 22.9 | 3 | 2 | Yes | Occlusion R ICA, severe R MCA/ACA, L ICA/ACA/PCA, + MM | No change | |
| 6 | 9.16 | Silent | 6.32 | NA | NA | 1 | 1 | None reported | Occlusion R ICA/ACA, severe R MCA, severe L ICA/MCA/ACA, + MM | Yes, occlusion L ICA | |
| 7 | 2.40 | Overt | 0.57 | 16.6 | 17.3 | 2 | † | PFO closed by cardiac catheterization after second stroke | Several times per week | Normal | Yes, bilateral moyamoya |
| 8 | 3.68 | Overt | 0.70 | 28.2 | 30.6 | 2 | 1 | None reported | Moderate R ACA, mild R ICA, MCA | Yes, severe R ICA, MCA, ACA | |
| 9 | 5.80 | Silent, then overt | 3.53 (silent) 5.16 (overt) | 11.0 | 32.4 | 2 | 2 | Overt stroke after RVS that was done because of silent stroke | None reported | Severe R ICA, MCA, L MCA, + MM | Yes, occlusion R ICA, MCA |
| 10 | 8.71 | Overt | 2.89 | 38.0 | 43 | 3 | 1‡ | Had RVS after third stroke | None reported | Mild R ICA, MCA, severe L ICA, MCA, ACA, PCA | Yes, occlusion L ICA, MCA, ACA, + MM |
| 11 | 4.56 | Overt | 0.50 | * | * | 2 | † | None reported | † | † | |
| 12 | 4.89 | Overt | 1.50 | 21.2 | 30.4 | 2 | 1 | Every few months | Severe L ICA, MCA, ACA | Yes, moderate R ACA, mild R ICA, MCA, PCA | |
| 13 | 4.21 | Silent | 0.99 | NA | NA | 1 | 2 | None reported | Normal | Yes, severe R ICA, moderate R MCA, ACA, L ICA | |
| 14 | 3.41 | Silent, then TIAs | 3.41 | NA | NA | 1 | 1 | Silent infarct associated with cerebral AVM | Frequent after AVM resection | Severe R ICA, MCA, ACA, L ICA, MCA, ACA | No change |
| 15 | 3.47 | Silent | 3.96 | NA | NA | 1 | 1 | None reported | Severe R ICA, MCA, ACA, moderate L ICA | No change | |
| 16 | 3.68 | Silent | 7.76 | NA | NA | 1 | 1 | None reported | Normal | Severe R MCA, L MCA | |
| 17 | 5.07 | Silent | 3.89 | NA | NA | 1 | 1 | None reported | Normal | Normal | |
| 18 | 12.22 | Silent | 2.83 | NA | NA | 1 | 1 | None reported | Severe R ACA, L MCA, ACA | No change |
| Patient no. . | Age at first stroke, y . | Type of progression . | Interval between first stroke and second event, y . | HbS at time of second overt stroke, % . | Mean HbS up to second overt stroke, % . | Total no. of overt strokes . | Total no. of MRIs with new or enlarging infarcts . | Clinical comments . | TIAs . | Vasculopathy on initial MRA . | Progression of vasculopathy on follow-up MRA . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5.15 | TIA, then silent | 0.65 | NA | NA | 1 | 2 | MRI after TIA had new infarct—overt stroke? | One TIA reported | Occlusion L MCA/ACA, R ICA, MM | No |
| 2 | 6.31 | Silent | 3.65 | NA | NA | 1 | 1 | None reported | Severe R ICA, L ICA/MCA/ACA | Yes, + MM | |
| 3 | 3.73 | TIAs, then silent | 0.32 | NA | NA | 1 | 1 | MRI after first TIA had new infarct—overt stroke? | TIAs continued after RVS | Moderate R ICA/MCA/ACA, L MCA/ACA/PCA, MM | Yes, severe R MCA/ACA, L MCA/ACA |
| 4 | 3.77 | Overt | 0.75 | 48.1 | * | 4 | 3 | Missed appointment for transfusion week before second stroke | None reported | Severe R ICA/MCA/ACA/PCA, L MCA/ACA/PCA, MM | Yes, occlusion R ICA/ACA, severe L ICA |
| 5 | 4.38 | Overt | 1.98 | 10.2 | 22.9 | 3 | 2 | Yes | Occlusion R ICA, severe R MCA/ACA, L ICA/ACA/PCA, + MM | No change | |
| 6 | 9.16 | Silent | 6.32 | NA | NA | 1 | 1 | None reported | Occlusion R ICA/ACA, severe R MCA, severe L ICA/MCA/ACA, + MM | Yes, occlusion L ICA | |
| 7 | 2.40 | Overt | 0.57 | 16.6 | 17.3 | 2 | † | PFO closed by cardiac catheterization after second stroke | Several times per week | Normal | Yes, bilateral moyamoya |
| 8 | 3.68 | Overt | 0.70 | 28.2 | 30.6 | 2 | 1 | None reported | Moderate R ACA, mild R ICA, MCA | Yes, severe R ICA, MCA, ACA | |
| 9 | 5.80 | Silent, then overt | 3.53 (silent) 5.16 (overt) | 11.0 | 32.4 | 2 | 2 | Overt stroke after RVS that was done because of silent stroke | None reported | Severe R ICA, MCA, L MCA, + MM | Yes, occlusion R ICA, MCA |
| 10 | 8.71 | Overt | 2.89 | 38.0 | 43 | 3 | 1‡ | Had RVS after third stroke | None reported | Mild R ICA, MCA, severe L ICA, MCA, ACA, PCA | Yes, occlusion L ICA, MCA, ACA, + MM |
| 11 | 4.56 | Overt | 0.50 | * | * | 2 | † | None reported | † | † | |
| 12 | 4.89 | Overt | 1.50 | 21.2 | 30.4 | 2 | 1 | Every few months | Severe L ICA, MCA, ACA | Yes, moderate R ACA, mild R ICA, MCA, PCA | |
| 13 | 4.21 | Silent | 0.99 | NA | NA | 1 | 2 | None reported | Normal | Yes, severe R ICA, moderate R MCA, ACA, L ICA | |
| 14 | 3.41 | Silent, then TIAs | 3.41 | NA | NA | 1 | 1 | Silent infarct associated with cerebral AVM | Frequent after AVM resection | Severe R ICA, MCA, ACA, L ICA, MCA, ACA | No change |
| 15 | 3.47 | Silent | 3.96 | NA | NA | 1 | 1 | None reported | Severe R ICA, MCA, ACA, moderate L ICA | No change | |
| 16 | 3.68 | Silent | 7.76 | NA | NA | 1 | 1 | None reported | Normal | Severe R MCA, L MCA | |
| 17 | 5.07 | Silent | 3.89 | NA | NA | 1 | 1 | None reported | Normal | Normal | |
| 18 | 12.22 | Silent | 2.83 | NA | NA | 1 | 1 | None reported | Severe R ACA, L MCA, ACA | No change |
NA indicates HbS not applicable as progressive infarction was clinically silent; RVS, revascularization surgery; L, left; R, right; MCA, middle cerebral artery; ACA, anterior cerebral artery; ICA, internal carotid artery; PCA, posterior cerebral artery; PFO, patent foramen ovale; and AVM, arteriovenous malformation.
HbS data not available.
Serial MRI not available.
Third stroke was clinical with no change in MRI on that date.
No new or enlarging cerebral infarcts were identified in 22 children, including one with recurrent TIAs. Thus, the occurrence of a TIA did not necessarily mean that a patient had a silent cerebral infarct.
Recurrent overt strokes and TIAs despite regular blood transfusion therapy
Of 40 participants, 7 (17.5%) had second overt strokes during the follow-up period, an incidence of 3.2 events/100 patient-years (95% confidence interval, 1.3-6.5). Among these 7 children, the median time between the first and second overt strokes was 0.8 years (range, 0.5-2.9 years). In 4 of 7 patients, second strokes occurred when HbS levels were less than 30% (HbS on hospital admission at the time of the second overt stroke 10%, 17%, 21%, and 28%). In the remaining 3 children with second overt strokes, 2 had HbS concentrations of 38% and 48% at the time of the second stroke, and one did not have HbS measured at the time of the second stroke. For the child with HbS of 38%, greater than 2 years had passed since his first stroke and the goal for HbS concentration was 50%. The child with HbS of 48% had his second overt stroke 0.75 years after the first stroke and had missed a scheduled transfusion appointment the week before the second stroke. Of these 7 children with second overt strokes, 3 subsequently had a third stroke and one had a fourth stroke. HbS was not measured at the time of these events.
Four patients without recurrent overt strokes experienced TIAs (one with a single event, 3 with repeated TIAs) after the initial stroke. One child, described previously as a case report,12 had an enlarging cerebral arteriovenous malformation that was detected on her surveillance MRI with associated cerebral edema, midline shift, and silent cerebral infarction. The lesion was not noted on an earlier surveillance MRI that was completed as part of this protocol. The arteriovenous malformation was resected, and the patient began to have TIAs postoperatively.12 Thus, a total of 25% (10 of 40) of children receiving regular blood transfusion therapy for secondary prevention of overt strokes had neurologic symptoms and radiographic evidence of progressive infarcts, either second overt strokes (n = 7) or TIAs plus silent infarcts (n = 3).
Progression of cerebral vasculopathy during regular blood transfusion therapy
Serial MRA scans were submitted in 88% (35 of 40) of the participants. Mean time between first and last MRA was 3.1 years (range, 0.3-7.8 years). Cerebral vasculopathy was common among this cohort. A total of 63% (25 of 40) had vasculopathy identified on the first MRA. Cerebral vasculopathy worsened, defined as a new vessel segment of stenosis or occlusion with or without new development of moyamoya collateral vessels, in 38% (15 of 40 participants), including in 5 children who had no cerebral vasculopathy identified on their initial MRA.
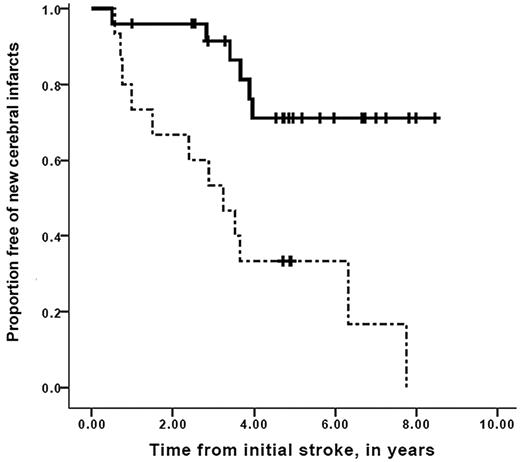
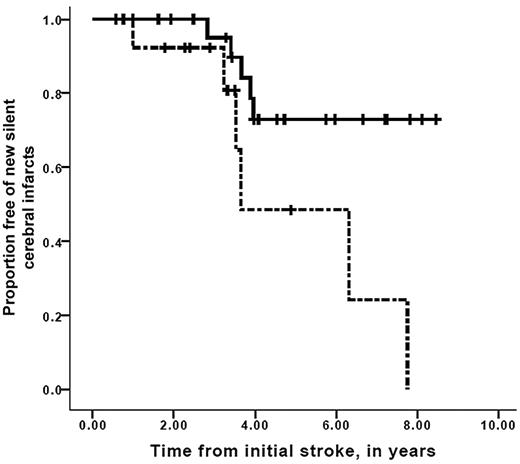
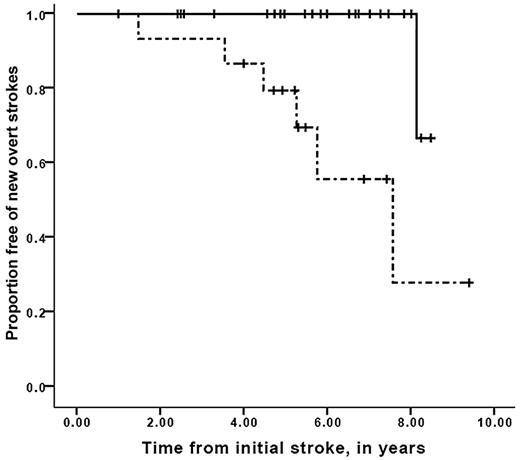
A strong association between worsening vasculopathy by MRA and progressive infarcts on MRI was found, with a relative risk of 12.7 (95% confidence interval, 2.65-60.5, P = .001). Time to recognition of new silent cerebral infarct or overt stroke stratified for progression of cerebral vasculopathy is shown in Figure 2. Median event-free interval for new silent or overt infarction was 3.2 years for the group with progressive vasculopathy, compared with median event-free interval not reached in the group without progressive vasculopathy (Mantel-Cox log-rank, P = .001). Furthermore, the interval between the first overt stroke and the development of new silent cerebral infarct in those persons with progressive cerebral vasculopathy was also decreased compared with those without progressive vasculopathy, with median event-free interval of 3.7 years for those with progressive vasculopathy versus median event-free interval not reached for those without progressive vasculopathy (Mantel-Cox log-rank test, P = .017; Figure 3). Figure 4 demonstrates that the event-free interval of second overt strokes for children with progressive vasculopathy is significantly shorter than for those without progressive vasculopathy, with median event-free interval of 7.6 years for those with progressive vasculopathy versus event-free interval not reached for those without progressive vasculopathy (Mantel-Cox log-rank, P = .003).
Survival free of new overt or silent cerebral infarcts in children with SCD while on transfusion therapy for secondary stroke prophylaxis. Participants with progressive overt and silent cerebral infarction, stratified for the absence (solid line) or the presence (dashed line) of progressive cerebral vasculopathy during chronic blood transfusion therapy. Vertical lines represent censored cases. Median event-free interval for new silent or overt infarction was 3.2 years for the group with progressive vasculopathy, compared with median event-free interval not reached in the group without progressive vasculopathy (Mantel-Cox log-rank, P = .001).
Survival free of new overt or silent cerebral infarcts in children with SCD while on transfusion therapy for secondary stroke prophylaxis. Participants with progressive overt and silent cerebral infarction, stratified for the absence (solid line) or the presence (dashed line) of progressive cerebral vasculopathy during chronic blood transfusion therapy. Vertical lines represent censored cases. Median event-free interval for new silent or overt infarction was 3.2 years for the group with progressive vasculopathy, compared with median event-free interval not reached in the group without progressive vasculopathy (Mantel-Cox log-rank, P = .001).
Survival free of detection of new silent cerebral infarcts in children with SCD while on transfusion therapy for secondary stroke prophylaxis. Participants with progressive silent infarctions only, stratified for the absence (solid line) or the presence (dashed line) of progressive cerebral vasculopathy while receiving chronic blood transfusion therapy. Vertical lines represent censored cases. Median progressive silent infarction only interval was 3.6 years for those with progressive vasculopathy versus median progressive silent infarct–free interval not reached for those without progressive vasculopathy (Mantel-Cox log-rank, P = .017).
Survival free of detection of new silent cerebral infarcts in children with SCD while on transfusion therapy for secondary stroke prophylaxis. Participants with progressive silent infarctions only, stratified for the absence (solid line) or the presence (dashed line) of progressive cerebral vasculopathy while receiving chronic blood transfusion therapy. Vertical lines represent censored cases. Median progressive silent infarction only interval was 3.6 years for those with progressive vasculopathy versus median progressive silent infarct–free interval not reached for those without progressive vasculopathy (Mantel-Cox log-rank, P = .017).
Survival free of second overt strokes in children with SCD while on transfusion therapy for secondary stroke prophylaxis. Participants with second overt strokes, stratified for the absence (solid line) or the presence (dashed line) of progressive cerebral vasculopathy while receiving chronic blood transfusion therapy. Vertical lines represent censored cases. Median second overt stroke–free interval was 7.6 years for those with progressive vasculopathy versus median stroke-free interval not reached for those without progressive vasculopathy (Mantel-Cox log-rank, P = .003).
Survival free of second overt strokes in children with SCD while on transfusion therapy for secondary stroke prophylaxis. Participants with second overt strokes, stratified for the absence (solid line) or the presence (dashed line) of progressive cerebral vasculopathy while receiving chronic blood transfusion therapy. Vertical lines represent censored cases. Median second overt stroke–free interval was 7.6 years for those with progressive vasculopathy versus median stroke-free interval not reached for those without progressive vasculopathy (Mantel-Cox log-rank, P = .003).
Cerebral revascularization procedures as adjuvant therapy
Four children underwent cerebral revascularization procedures while receiving regular blood transfusion therapy resulting from multiple areas of cerebral arterial stenosis or moyamoya changes and either progressive silent or overt strokes or TIAs. One participant who underwent bilateral encephalodurosynangiosis had an overt stroke 5 days after the left-sided revascularization procedure, with new onset of right arm weakness, which resolved within 5 days. HbS concentration was 11% when weakness was noted, and MRI confirmed an acute infarct. The second child who underwent bilateral encephalodurosynangiosis did not have evidence of overt or silent cerebral infarcts in 16 months of follow-up. The third child had right-sided encephalodurosynangiosis and was free of overt or silent cerebral infarcts or TIAs during 4.4 years of follow-up. One child, who had burrhole placement and dural opening as a revascularization procedure for frequent TIAs, did not have any acute complications of the surgery but continued to have intermittent TIAs afterward.
Discussion
To our knowledge, this is the first cohort of children with SCD and strokes followed prospectively with MRI while receiving regular blood transfusion therapy with the goal of identifying silent cerebral infarcts. We demonstrate that both overt strokes and silent cerebral infarcts recur despite regularly scheduled blood transfusion therapy, which averaged approximately every 28 days for secondary prophylaxis of strokes.
The most significant and novel finding in this study was the observation that children receiving blood transfusion therapy for prevention of overt strokes have new silent cerebral infarcts. Previous analyses of children with SCD and progressive cerebral infarction as determined by MRI did not include discussion of regular blood transfusion therapy,8,13,14 so it is not clear from these reports whether progressive infarction occurred during optimal therapy for secondary stroke prevention. The patient population from which our cohort is derived is fundamentally different from the patients described in the Cooperative Study of Sickle Cell Disease, who had a similar rate of progression of silent infarctions,8 because our patients were transfused regularly for secondary stroke prophylaxis and those in the Cooperative Study of Sickle Cell Disease were not.
We postulate that new or enlarging silent cerebral infarcts are associated with increased burden of cognitive deficits in children with a history of overt strokes. Evidence to support the clinical relevance of new or progressive silent cerebral infarcts includes the observation that cognitive deficits in children with SCD and silent cerebral infarctions increase as volume of cerebral infarction increases.10 Silent cerebral infarcts in SCD are associated with poor cognition,10 grade failure or a requirement for special educational services,13 and a risk of new cerebral infarcts.8,9 Specifically, silent cerebral infarcts have been associated with decreased performance in vocabulary, arithmetic, and visual-motor processing tasks on neurocognitive testing compared with children with SCD without silent cerebral infarcts.14 Overall, silent cerebral infarcts in children with SCD are associated with a decrease of 8 points on intelligence quotient testing compared with children with SCD who have normal brain MRI.14 In the setting of preexisting stroke, the impact of progressive silent cerebral infarcts on cognition is not known, but increased volume of cerebral infarction is associated with worsening cognitive performance.10 Given this information, the results of our study suggest that children with SCD and progression of overt or silent cerebral infarctions experience declines in cognitive function. Formal evaluation with cognitive testing is needed to confirm this assertion.
We also demonstrate an association between progressive cerebral vasculopathy and progressive cerebral infarcts, both overt and silent. Our finding of approximately 63% of patients having cerebral vasculopathy at baseline is similar to the 74% reported by Russell et al.15 Dobson et al demonstrated that the presence of moyamoya collaterals on MRA was associated with recurrent overt strokes and TIAs, but they did not describe silent cerebral infarctions in their series.11 Progressive vasculopathy has recently been reported in smaller series by Brousse et al16 and Bader-Meunier et al17 despite regular blood transfusion therapy. However, Bader-Meunier et al did not correlate changes in MRA with clinical outcomes for children with overt strokes while receiving transfusion therapy.17 Brousse et al noted one child with a second overt stroke while receiving blood transfusion therapy, but silent cerebral infarcts were not specifically addressed.16
Previously, Russell et al demonstrated that most patients with overt strokes that receive regular blood transfusion therapy did not have progression of cerebral vasculopathy.15 However, the more recent studies, including ours, that have used MRA instead of angiography, have demonstrated progression of vasculopathy, including new vessel stenoses, occlusions, and moyamoya collateralization.11,16,17 A possible explanation for these differences is that the duration of follow-up was much longer for the studies demonstrating vasculopathy progression: in the Russell et al cohort, angiography was repeated 1 to 2 years after the initial stroke,15 whereas in our study the median time between first and last MRA was 3.1 years (range, 0.3-7.8 years). We postulate that, for some children with SCD and cerebral vasculopathy, transfusion therapy slows progression of vasculopathy but does not prevent progression; thus, the longer follow-up duration of our study revealed the progressive vasculopathy, whereas the duration of follow-up in the Russell et al study15 might have been too short to demonstrate progression in the chronically transfused patients. Taken together, these data indicate that cerebral vasculopathy progresses in many patients despite regular blood transfusion therapy and that progression is associated with cerebral ischemic lesions, both overt strokes and silent cerebral infarcts.
We were not able to differentiate progressive infarctions in large-vessel vascular territories from those in small-vessel vascular territories based on the imaging performed on the children in this study. The primary reason for the inability to distinguish large from small vessel disease is that at baseline most of the participants had moyamoya. In the presence of vascular stenosis and moyamoya, the traditional vascular distribution of border zone regions with low flow is significantly altered from the classic locations.18 For any person, determining specific low flow border zones regions based on static MRI or MRA is not possible.
In this study, the data confirmed that second overt strokes occurred when participants' HbS levels were less than 30%, indicating that regular blood transfusion therapy does not completely prevent recurrent cerebral injury. No significant difference was found between the mean pretransfusion HbS concentrations or the mean transfusion intervals among the children with second overt strokes, silent infarctions, and no new MRI findings. Similar to prior cohorts of children with SCD and strokes who were regularly transfused,2,19 the highest rate of recurrent overt strokes in our cohort occurred during the first 2 years after the initial overt stroke. Based on data from this study and previous studies,2,19 neurologic surveillance with MRI and MRA is important throughout the treatment period but is particularly critical during the first 2 years after the stroke because identification of a new silent infarction may lead to consideration of more intensive or experimental therapies. Knowledge that a student with a stroke has developed additional brain injury is critical for the student's education plan. Such students should receive additional cognitive testing and assessment of their academic achievement because they may require more educational support. Further, the patient, parents and treating physician may elect to participate in experimental therapies, such as matched unrelated donor stem cell transplantation or cerebral revascularization surgery. Lastly, the families and their physicians should be aware that the chosen strategy of blood transfusion therapy does not completely prevent progression of neurologic disease.
Limitations of this study include the small cohort size and short follow-up duration. MRI and MRA scans were not centrally reviewed for all subjects. Uniform imaging protocols and central review for all MRIs and MRAs probably would have identified more, not fewer, children with progressive cerebral infarction because many of the silent infarcts were identified by the study neuroradiologist but missed at the local institution. We scored participants without available MRI or MRA reports as no progressive infarction and no vasculopathy to avoid creating a sampling bias, but this strategy may have underestimated the proportion of children with progressive infarcts or cerebral vasculopathy. Our definition of vasculopathy was not identical to previous studies: we included vascular stenosis as well as moyamoya collateral blood vessels in the definition of vasculopathy because moyamoya is known to progress from vascular stenosis to collateralization.20 In 2 prior studies that evaluated the progression of vasculopathy among this population, Dobson et al included only those children with moyamoya collaterals on MRA,11 whereas Russell et al used conventional cerebral angiography15 to image vasculopathy. In our cohort, despite a different definition of vasculopathy and different imaging modality, we identified a strong association between progressive vasculopathy and new cerebral infarcts in patients being chronically transfused for secondary stroke prevention. However, we are unable to determine whether progression of vasculopathy precedes progression of infarcts because only 2 MRAs per patient were interpreted, whereas a patient may have had multiple MRIs. Progression of infarction may have been noted on an interval MRI for which no MRA interpretation was available.
The results of our study are relevant to children with SCD and overt strokes, but the findings do not translate directly to children with silent cerebral infarction as a primary neurologic event, such as those children enrolled in the ongoing Silent Infarct Transfusion (SIT) Trial,21 because the 2 study populations are completely different. In this analysis, participants had significant burden of disease, as determined by the clinical symptoms of overt strokes, the greater number and size of infarct lesions, and the high prevalence of MRA-defined vasculopathy. In the SIT Trial, most of the lesions are small compared with overt strokes, only a small portion of the participants have MRA-defined vasculopathy, and none of the patients has any evidence of an overt neurologic deficit.21 Although some of the participants in this study have experienced the same endpoint that is being studied in the SIT Trial, because of the very different patient populations in the 2 studies, the risks of silent cerebral infarct progression are not equal; therefore, the results of this study have no bearing on the conduct or validity of the SIT Trial.
Using prospective surveillance MRI and MRA protocols, this prospective cohort study demonstrates that blood transfusion therapy is less effective than previously thought in preventing recurrent cerebral infarction or progression of cerebral vasculopathy in children with SCD. Based on these data, we think that all children with SCD and strokes should undergo MRI and MRA of the brain every 1 to 2 years. Progressive overt strokes or silent cerebral infarcts are a potential indication for escalation of therapy, although currently no evidence-based strategy exists. Rigorous prospective clinical trials are needed to evaluate new therapies, such as stem cell transplantation and cerebral revascularization surgery. New approaches to therapy for secondary prevention of cerebral infarction are needed for this high-risk population of children with SCD and overt strokes.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr John J. Strouse and Dr Michael M. Dowling for their critical review of the manuscript.
This work was supported by the Doris Duke Foundation (M.R.D.) and the National Institutes of Health (grant 5T32HD4301003; M.L.H.). Research in the National Health Service (NHS) Trusts benefits from Research & Development funding received from the NHS executive (F.J.K., B.I., J.H., and K.A.A.).
National Institutes of Health
Authorship
Contribution: M.L.H. reviewed all medical records, analyzed data, and contributed to writing the manuscript; R.C.M. participated in protocol design, interpreted neuroradiology studies, and contributed to writing the manuscript; J.L.L. and C.J.M. interpreted neuroradiology studies and contributed to writing the manuscript; M.R.D. participated in protocol design, enrolled patients, reviewed selected medical records, and contributed to data analysis and writing the manuscript; J.A.P., A.A.T., S.A.S., G.M.W., B.I., J.H., F.J.K., and K.A.A. participated in protocol design, enrolled patients, and contributed to writing the manuscript; J.F.C., R.I., and M.N. participated in the protocol design and writing the manuscript; J.E.M. reviewed all data and participated in data analysis and writing the manuscript; and Y.Y. and M.R. participated in data analysis and writing the manuscript.
Conflict-of-interest disclosure: J.H. traveled to the American Society of Hematology Annual Meeting in 2009 using funds provided by Novartis and served on the advisory committee for Sangart. The remaining authors declare no competing financial interests.
Correspondence: Michael R. DeBaun, 2200 Children's Way, Nashville, TN 37232-9900; e-mail: m.debaun@vanderbilt.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal