The myelodysplastic syndromes (MDSs) are hematologically diverse hematopoietic stem cell malignancies primarily affecting older individuals. The incidence of MDS in the United States is estimated at 3.3 per 100 000; however, evidence suggests underreporting of MDS to centralized cancer registries. Contrary to clinical recommendations, registry guidelines from 2001-2010 required the capture of only one malignancy in the myeloid lineage and did not require blood count (BC) or bone marrow (BM) biopsy for MDS confirmation. To address these potential limitations, we constructed 4 claims-based algorithms to assess MDS incidence, applied the algorithms to the 2000-2008 Surveillance Epidemiology and End Results (SEER)–Medicare database, and assessed algorithm validity using SEER-registered MDS cases. Each algorithm required one or more MDS claims and accounted for recommended diagnostic services during the year before the first claim: 1+, 2+, 2 + BC, and 2 + BCBM (ordered by sensitivity). Each had moderate sensitivities (78.05%-92.90%) and high specificities (98.49%-99.84%), with the 2 + BCBM algorithm demonstrating the highest specificity. Based on the 2 + BCBM algorithm, the annual incidence of MDS is 75 per 100 000 persons 65 years or older—much higher than the 20 per 100 000 reported by SEER using the same sample.
Introduction
The myelodysplastic syndromes (MDSs) include a diverse group of clonal hematopoietic stem cell malignancies characterized by BM failure, peripheral blood cytopenias, and reduced survival. In the ninth revision of the International Classification of Diseases (ICD-9-CM), MDS was coded as a disease of the blood and blood-forming organs, but was reclassified as a neoplasm in the tenth revision (ICD-10) and in the corresponding International Classification of Diseases for Oncology, Third Edition (ICD-O-3). ICD-O-3 is the classification system used by population-based cancer registries such as the National Cancer Institute's (NCI) Surveillance, Epidemiology and End Results (SEER) program and the North American Association of Central Cancer Registries (NAACCR). As a result, MDS became a reportable malignancy to population-based registries for the first time in 2001, the year ICD-O-3 was implemented worldwide. However, evidence suggests that MDS incidence has been grossly underestimated.1,–3
Cases of hematologic malignancies may be overlooked by US cancer registry systems as a result of their reliance on inpatient reporting. Only 4% of the MDS incident cases in NAACCR were reported to registries by physicians' offices.3 This is a surprisingly low proportion given that, in contrast to other malignancies, MDS is more commonly diagnosed and managed outside of the hospital setting. Issues with coding and the potential gap in outpatient registration may be examined through population-based analysis of claims data, particularly outpatient claims.
Given the potential for MDS cases to be uncaptured by population-based cancer registries and the generally older age of affected individuals (median age at diagnosis, 71-76 years),4,5 we sought to use Medicare claims to obtain a more accurate estimate of MDS incidence. Claims-based algorithms have been used in prior reports to identify MDS cases and related complications.2,6,7 In a recent report on clinical complications of MDS, a claims-based methodology estimated that approximately 45 000 new cases of MDS were diagnosed among individuals 65 years and older in 2003,2 representing an approximately 5-fold higher incidence compared with previous SEER and NAACCR reports.3,4 However, several methodologic limitations complicate interpretation of these results, including the use of the nonspecific ICD-9-CM code 238.7 to identify MDS cases,8 the potential inclusion of prevalent MDS cases in the estimation of incidence, the lack of methods to estimate claims algorithm diagnostic sensitivity and specificity, and inadequate methodology validation. To overcome these limitations, we developed 4 claims-based algorithms to identify MDS incident cases, assess the algorithms' sensitivity and specificity using registry information, and estimate the trend in MDS incidence among older Medicare beneficiaries between 2001 and 2005, with and without age adjustment.
Methods
Data sources
We conducted a retrospective review of the SEER-Medicare database for 2000-2008. The SEER program is a national, population-based cancer registry sponsored by the NCI with a catchment area roughly equal to 26% of the US population.9 SEER registers information on patient demographics, cancer characteristics, stage at diagnosis, date of diagnosis, first course treatment, vital status, and date and cause of death.9 Of SEER-registered cancer patients who were diagnosed at age 65 years or older, 93% were matched with Medicare enrollment records and claims, as described previously.9
Medicare, administered by the Centers for Medicare and Medicaid Services (CMS), is the primary insurer for approximately 97% of the US population 65 years of age or older. All Medicare beneficiaries receive Part A coverage for hospital inpatient care, skilled nursing care, home health care, and hospice care.9 Approximately 95% of older beneficiaries also subscribe to Medicare Part B for benefits that cover physician services, durable medical equipment, and outpatient care.9 As an alternative to the traditional fee-for-service (FFS) Medicare, the Medicare Advantage program, Part C, is a managed care benefit that enrolls approximately 11%-14% of older Medicare beneficiaries.10,–12 Because of its reimbursement structure, no claims data were available for Part C beneficiaries and therefore they were not included in this study. All study procedures were approved by the University of Florida Institutional Review Board.
Study population
For study inclusion, a beneficiary must have resided in a SEER region between 2001 and 2005, been enrolled in FFS Medicare due to age for 13 months or more, and not participated in Medicare Advantage. The study population included in the current analysis represents a 5% sample of SEER-registered and nonregistered beneficiaries (n = 44 739 and 230 941, respectively) and an oversampling of all beneficiaries registered in SEER with MDS or leukemia (acute myeloid leukemia [AML] and chronic myeloid leukemia, ICD-O-3 histology codes 9800-9989) (n = 23 756). The oversampling allows for more in-depth examinations of beneficiaries diagnosed with hematologic malignancies.
Claims-based algorithms for MDS incidence
To measure disease incidence, claims-based algorithms are commonly applied to CMS administrative data, specifically ICD-9-CM diagnosis and procedure codes, Current Procedural Terminology (CPT) codes, and enrollment information. Before this study, all published algorithms of MDS incidence examined only ICD-9-CM diagnosis codes for 238.7 (other lymphatic and hematopoietic tissues).2,6,7 This code does not encompass all forms of MDS or related modifications (Table 1), and the previously published algorithms were not validated before their application. SEER provided its registries with specific ICD-9-CM diagnosis codes that correspond to MDS ICD-O3 codes before and after October 2006 when the coding system changed. We used these ICD-9-CM codes to identify claims for MDS services.
Coding myelodysplastic syndrome using ICD-O-3 and ICD-9-CM
| MDS . | Abbreviation . | ICD-O-3 . | ICD-9-CM . | |
|---|---|---|---|---|
| Prior to October 2006 . | October 2006 and later . | |||
| Refractory anemia | RA | 9980 | 284.9 | 238.72 |
| Refractory anemia with ringed sideroblasts | RARS | 9982 | 285.0 | 238.72 |
| Refractory anemia with excess blasts* | RAEB | 9983 | 285.0 | 238.73 |
| Refractory anemia with excess blasts in transformation | RAEB-T | 9984 | 285.0 | 238.73 |
| Refractory cytopenia with multilineage dysplasia | RCMD | 9985 | 238.7 | 238.72 |
| Myelodysplastic syndrome with 5q deletion syndrome | MDS 5q | 9986 | 238.7 | 238.74 |
| Therapy-related myelodysplastic syndrome, NOS† | t-MDS | 9987 | 238.7 | 238.72 |
| Myelodysplastic syndrome, NOS | MDS | 9989 | 238.7 | 238.75 |
| MDS . | Abbreviation . | ICD-O-3 . | ICD-9-CM . | |
|---|---|---|---|---|
| Prior to October 2006 . | October 2006 and later . | |||
| Refractory anemia | RA | 9980 | 284.9 | 238.72 |
| Refractory anemia with ringed sideroblasts | RARS | 9982 | 285.0 | 238.72 |
| Refractory anemia with excess blasts* | RAEB | 9983 | 285.0 | 238.73 |
| Refractory anemia with excess blasts in transformation | RAEB-T | 9984 | 285.0 | 238.73 |
| Refractory cytopenia with multilineage dysplasia | RCMD | 9985 | 238.7 | 238.72 |
| Myelodysplastic syndrome with 5q deletion syndrome | MDS 5q | 9986 | 238.7 | 238.74 |
| Therapy-related myelodysplastic syndrome, NOS† | t-MDS | 9987 | 238.7 | 238.72 |
| Myelodysplastic syndrome, NOS | MDS | 9989 | 238.7 | 238.75 |
In October 2009, RAEB with 5%-9% blasts (RAEB-1) was reclassified from 238.73 to 238.72 to improve coverage of erythropoiesis-stimulating agent treatment for patients with MDS. However, RAEB with 10%-19% (RAEB-2) remained under 238.73.20
In October 2009, a new code for antineoplastic chemotherapy–induced anemia (285.3) was introduced, but not incorporated within SEER. NOS is listed where a topographic or morphologic term is not modified, has an adjective that does not appear elsewhere, or is used in a general sense.20
A minimalist claims-based algorithm of MDS incidence requires one or more MDS claims (the 1+ algorithm),2 whereas more specific algorithms may attempt to remove inaccurate diagnoses by requiring additional information, such as requiring a second claim within a specified period of time7 or after a delay in time to confirm the indication.6 Algorithms based only on ICD-9-CM diagnosis codes do not account for clinical services required for MDS diagnosis, such as blood count (BC) and BM biopsy or aspiration.
We compared the sensitivity and specificity of 4 algorithms. The “1+” algorithm requires a single claim with ICD-9-CM diagnosis of MDS. The “2+” algorithm requires a second claim between 1 and 12 months after the first claim or death or hospice entry within 3 months of the first claim. The latter accounts for the censoring of MDS patients who died within 3 months of the first claim. The final 2 algorithms are based on clinical knowledge of diagnostic services required to confirm MDS, specifically BC (CPT 85004, 85007-85009, 850013, 850014, 850018, 850021-85027, 85031, 85032, 85041, 85044-85049, 85060, 85590, 85595, G0306, G0307) and BM biopsy or aspiration (CPT 38220, 38221, 85095, 85097, 85102, G0364 or ICD-8-CM procedure 413.1 and 413.8). The “2 + BC” algorithm further restricts the 2+ algorithm by requiring a BC during the year before the first claim. The “2 + BCBM” algorithm further requires both a BC and a BM during the year before the first claim. During this period, both World Health Organization (WHO) and French-American-British (FAB) Co-operative Group guidelines for MDS diagnosis required a BC; a BM biopsy was recommended but not required. Each of the claims-based algorithms captures only MDS cases that are clinically diagnosed (eg, no postmortem diagnoses or diagnoses made while in hospice) and are not dependent on the use of any particular treatment or supportive care (eg, blood transfusions).
Data analysis
To assess the validity of the claims-based algorithms, SEER registration was used as the “gold standard,” with MDS cases defined by ICD-O-3 histology codes 9980-9989 on or after 13 months of Medicare enrollment, but before January 1, 2006, death or hospice entry (Table 1). Sensitivity was defined as the proportion of SEER-registered MDS patients who were identified as MDS cases by the claims-based algorithm. Specificity was defined as the proportion of individuals not registered in SEER who were not identified as an MDS case by the claims-based algorithm. Patients registered in SEER with leukemia and other cancers were excluded from the validation analysis because SEER guidelines require that only one cancer in the myeloid line be registered.13 To avoid misclassification of prevalent MDS cases as incident MDS cases, all patients with claims for MDS or unspecified anemia (ICD-9-CM 285.9) in the year 2000 or in their first year of Medicare enrollment were excluded from the analysis.
In addition to assessing the validity of the claims-based algorithms, we applied the 4 algorithms to estimate the incidence of MDS among older adults. Each trend represents the number of incident cases per year per 100 000 beneficiaries in SEER regions and was compared with the trends in SEER incidence for the same population of older adults. The study population contains all registered MDS and leukemia cases and only 5% of the cancer and unregistered beneficiaries in SEER regions. Therefore, we applied sampling weights to adjust for the oversampling. Furthermore, we applied a second set of sampling weights created to adjust for differences in the age distribution between 2001 and 2005 using the 2005 age distribution as the standard.
Results
The demographic characteristics of Medicare beneficiaries residing in SEER regions are shown in Table 2 for 4 groups defined by SEER registration: (1) those registered as MDS cases, (2) those registered with ICD-O-3 histology codes 9800-9989 excluding the MDS cases, (3) those registered with other cancers or before 2001, and (4) those not registered for any cancer diagnosis. The MDS sample contained the 52 cases that also had a registered leukemia diagnosis. The leukemia sample contained 509 cases that had an MDS diagnosis (3%), but were excluded from the MDS sample because the diagnosis occurred before the thirteenth month of Medicare enrollment (ie, prevalent cases) or after death or hospice entry. Consistent with previous reports,3,4,14 MDS and leukemia patients were older and more likely to be male and white compared with those not registered with any cancer.
Demographic characteristics and MDS claims among Medicare beneficiaries by SEER registration status, 2000-2005
| Beneficiary characteristics . | MDS (n = 5493) . | Leukemia (n = 18 263) . | Other cancers (n = 44 739) . | Not registered (n = 230 941) . |
|---|---|---|---|---|
| Median age (y) in January 2001 (interquartile range) | 77 (72-82) | 75 (69-81) | 74 (68-80) | 72 (66-79) |
| Sex, n (%) | ||||
| Male | 2965 (54) | 9,692 (53) | 21 612 (48) | 88 748 (38) |
| Female | 2528 (46) | 8571 (47) | 23 127 (52) | 142 193 (62) |
| Race/ethnicity, n (%) | ||||
| White | 4885 (89) | 16 524 (90) | 39 353 (88) | 194 754 (84) |
| Black | 300 (5) | 905 (5) | 2652 (6) | 14 522 (6) |
| Other | 67 (1) | 206 (1) | 780 (2) | 5 275 (2) |
| Asian | 152 (3) | 353 (2) | 1256 (3) | 9 903 (4) |
| Hispanic | 69 (1) | 210 (1) | 543 (1) | 5156 (2) |
| North American Native | 9 (0) | 34 (0) | 95 (0) | 876 (0) |
| Unknown | 11 (0) | 31 (0) | 60 (0) | 455 (0) |
| Claims between 2001 and 2005, n (%) | ||||
| MDS or unspecified anemia claims within first data year* | 1958 (36) | 5152 (28) | 6390 (14) | 30 153 (13) |
| No MDS claims | 251 (5) | 8624 (47) | 37 109 (83) | 197 765 (86) |
| 1 or more MDS claims | 3284 (60) | 4487 (25) | 1240 (3) | 3023 (1) |
| Second MDS claim 1-12 months after first claim, death, or hospice entry within 3 months | 3050 | 3145 | 545 | 1176 |
| Second MDS claim and BC within 12 months before first claim | 3021 | 3088 | 535 | 1138 |
| Second MDS claim and BM biopsy within 12 months before first claim | 2759 | 2439 | 159 | 321 |
| Beneficiary characteristics . | MDS (n = 5493) . | Leukemia (n = 18 263) . | Other cancers (n = 44 739) . | Not registered (n = 230 941) . |
|---|---|---|---|---|
| Median age (y) in January 2001 (interquartile range) | 77 (72-82) | 75 (69-81) | 74 (68-80) | 72 (66-79) |
| Sex, n (%) | ||||
| Male | 2965 (54) | 9,692 (53) | 21 612 (48) | 88 748 (38) |
| Female | 2528 (46) | 8571 (47) | 23 127 (52) | 142 193 (62) |
| Race/ethnicity, n (%) | ||||
| White | 4885 (89) | 16 524 (90) | 39 353 (88) | 194 754 (84) |
| Black | 300 (5) | 905 (5) | 2652 (6) | 14 522 (6) |
| Other | 67 (1) | 206 (1) | 780 (2) | 5 275 (2) |
| Asian | 152 (3) | 353 (2) | 1256 (3) | 9 903 (4) |
| Hispanic | 69 (1) | 210 (1) | 543 (1) | 5156 (2) |
| North American Native | 9 (0) | 34 (0) | 95 (0) | 876 (0) |
| Unknown | 11 (0) | 31 (0) | 60 (0) | 455 (0) |
| Claims between 2001 and 2005, n (%) | ||||
| MDS or unspecified anemia claims within first data year* | 1958 (36) | 5152 (28) | 6390 (14) | 30 153 (13) |
| No MDS claims | 251 (5) | 8624 (47) | 37 109 (83) | 197 765 (86) |
| 1 or more MDS claims | 3284 (60) | 4487 (25) | 1240 (3) | 3023 (1) |
| Second MDS claim 1-12 months after first claim, death, or hospice entry within 3 months | 3050 | 3145 | 545 | 1176 |
| Second MDS claim and BC within 12 months before first claim | 3021 | 3088 | 535 | 1138 |
| Second MDS claim and BM biopsy within 12 months before first claim | 2759 | 2439 | 159 | 321 |
A person with MDS or unspecified anemia claims within the first year of claim data may be a prevalent case and therefore must be removed from the validation study and incidence estimation.
The distribution of MDS claims are also presented for each of the 4 subgroups in Table 2. Across subgroups, 13%-36% of beneficiaries had claims for MDS or unspecified anemia in 2000 or in their first year of Medicare enrollment. These individuals were considered “prevalent” cases and were excluded from the calculations of sensitivity of specificity.
After removing the prevalent cases, 251 (5%) of the remaining 3535 SEER-registered MDS cases lacked claims for MDS between 2001 and 2005. Among these cases, 9 (4%) had no claims between 2001 and 2005, 185 (74%) had a claim for unspecified anemia (ICD-9-CM 285.9) between 2001 and 2005, and an additional 48 (19%) had claims for MDS or unspecified anemia claims between 2006 and 2008, which is outside the study period.
The sensitivity and specificity for correctly classifying SEER-registered MDS cases is presented for each of the 4 claims-based algorithms in Table 3. Sensitivity was highest for the 1+ algorithm (92.9%). Requiring a second claim for MDS within 1-12 months after the first claim or death or hospice entry within 3 months of the first claim, (ie, the 2+ algorithm) reduced the sensitivity to 86.28%. Further requiring a BC claim within the year before the first claim had little effect on sensitivity (85.46%); however, requiring a BM biopsy reduced the sensitivity to 78.05%. As sensitivity decreased, specificity increased, ranging from 98.49% for the 1+ algorithm to 99.84% for the 2 + BCBM algorithm.
Sensitivity and specificity of 4 Medicare claims–based algorithms for classifying SEER-registered MDS cases in 2000-2005
| Algorithms . | Sensitivity (%) . | Specificity (%) . |
|---|---|---|
| 1+ algorithm | 92.90 | 98.49 |
| 2+ algorithm | 86.28 | 99.41 |
| 2 + BC algorithm | 85.46 | 99.43 |
| 2 + BCBM algorithm | 78.05 | 99.84 |
| Algorithms . | Sensitivity (%) . | Specificity (%) . |
|---|---|---|
| 1+ algorithm | 92.90 | 98.49 |
| 2+ algorithm | 86.28 | 99.41 |
| 2 + BC algorithm | 85.46 | 99.43 |
| 2 + BCBM algorithm | 78.05 | 99.84 |
The most conservative algorithm (2 + BCBM) identified 480 MDS cases among patients who were not registered with MDS or leukemia. Because the analytic sample included only 1 of every 20 beneficiaries who were not registered with MDS or leukemia, the results imply that more than 9600 MDS cases (480 × 20) were not captured by SEER.
Between 2001 and 2009, SEER guidelines stipulated that “a myeloid malignancy diagnosed after a previous myeloid malignancy would not count as a subsequent primary,” thereby reducing incidence estimates for all myeloid malignancies including MDS and AML. Because antecedent MDS is the most common risk factor for AML, it is possible that patients presenting to an inpatient facility with AML and an outpatient history of MDS were coded by hospital-based cancer registries as AML only. Therefore, we applied the 2 + BCBM claims algorithm to patients registered with AML, and discovered an additional 2439 MDS cases.
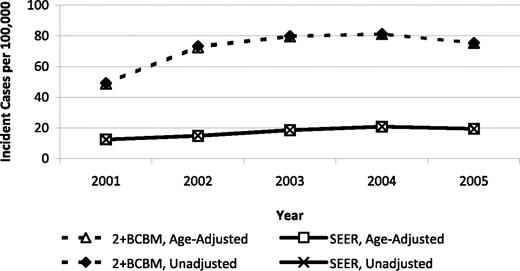
In summary, SEER registered less than one-third of the MDS cases as defined by the 2 + BCBM algorithm, and our validation study results suggest that this is a conservative estimate. Figure 1 illustrates the trend over time in MDS incidence rates estimated using the 2 + BCBM algorithm and the SEER registry with and without age adjustment. For the year 2005, MDS incidence was estimated to be 75 per 100 000 persons 65 years of age or older based on 2 + BCBM. In the same year, MDS incidence was reported by SEER to be 20 per 100 000.
Temporal trends in MDS incidence rates based on a conservative claims-based algorithm and on SEER registry evidence, 2001-2005.
Temporal trends in MDS incidence rates based on a conservative claims-based algorithm and on SEER registry evidence, 2001-2005.
Discussion
Given the potential for MDS cases to be uncaptured by population-based cancer registries, we sought to develop and validate a Medicare claims–based algorithm for the identification of MDS to estimate incidence in individuals 65 years of age and older.3,4 The results of this validation study demonstrate that claims-based algorithms of MDS incidence are moderately sensitive and highly specific. Historically, an approach of applying a 1+ algorithm has been applied2 ; however, the reduced specificity of this approach may overestimate incidence and obscure patterns identified by more definitive algorithms. Our work validates and supports the use of the 2 + BCBM claims algorithm in identifying MDS cases. Although this algorithm showed lower sensitivity compared with other algorithms, it had the highest specificity, which is of critical importance if this tool is to be used as the basis for extrapolations and investigations of MDS treatment patterns.
Based on population registry data between 2001 and 2004, the average annual age-adjusted incidence rate for MDS in the US was 3.3 per 100 000 persons, and the prevalence of MDS was estimated to be 55 000 persons in the United States.15,16 The findings of the present study indicate that the registry-based data underestimated MDS incidence. Among individuals 65 years and older, MDS incidence captured by the most conservative claims-based algorithm (2 + BCBM) was nearly 4-fold higher than the incidence estimated by population-based cancer registries.3,4
The implications of these findings expand beyond Medicare and suggest that specific changes should be considered in the cancer registry system. When analyzing the uncaptured MDS cases, we found that many of these cases were linked to individuals already registered in SEER for another cancer. In cases when MDS is diagnosed subsequent to a solid tumor, SEER guidelines state that the MDS case should be abstracted as a separate primary. Our findings highlight an opportunity for cancer registries to focus on capturing MDS as a potential subsequent primary to a solid tumor diagnosis. In the context of MDS and other myeloid malignancies, SEER updated its guidelines in January 2010 to permit coding of multiple myeloid malignancies as multiple primaries.13 However, with only 4% of MDS cases reported from outpatient clinics to cancer registries,3 the greatest limitation is registry reliance on inpatient surveillance for MDS incidence.1,3,14 Because BM confirmation is required for MDS diagnosis,17 greater outreach to free-standing pathology laboratories would assist in the capture of MDS cases diagnosed in the office setting. Nevertheless, the evidence of bias against comorbid registration and outpatient diagnosis suggests that MDS cases successfully captured in SEER may favor inclusion of intermediate-risk MDS cases and exclude high-risk cases (eg, those rapidly transforming into AML) and low-risk cases (ie, those requiring no inpatient care).
Unlike the 2 + BCBM algorithm, SEER includes cases without BM confirmation of MDS based on evidence that these underdiagnosed patients have received active and prolonged treatment for MDS. Even in confirmed cases, SEER accepts BC as a form of microscopic confirmation. The population of MDS patients without BM confirmation of disease—those captured by the less-specific 2 + BC algorithm—may be of significant interest given that they use MDS resources. To assess the importance of diagnostic data obtained from BM, further study is required to determine whether the absence of BM pathology findings influences clinical outcomes, because a lack of information on MDS pathology and karyotype may prevent accurate prognostication, thereby mitigating informed treatment choices and potentially resulting in inferior outcomes. Moreover, in the absence of BM confirmation, it is difficult to interpret outcome data from SEER-registered MDS patients, because these cases may represent an inaccurate diagnosis and therefore may be managed inappropriately.
A primary limitation of claims-based algorithms is their reliance on ICD-9 codes. The ICD-9 codes generated by the treating physician at the time of billing are assumed to be the physician's impression of the diagnosis rather than a confirmed pathologic diagnosis. A small proportion of the SEER-registered cases did not have claims with MDS codes, and MDS patients may not consent to undergo BM biopsy. The imprecise coding of MDS as unspecified anemia and the use of supportive care without confirmatory BM biopsy suggest that claims-based algorithms may miss MDS cases (ie, they have poor sensitivity). In addition, no claims algorithm can measure incidence in the first 12 months of Medicare enrollment because of the lack of data before enrollment to exclude MDS prevalence. Claims-based algorithms are also limited to insured populations and exclude persons enrolled in hospice or managed care organizations due to the absence of claims (eg, Medicare Advantage).
In the present study, a large number of MDS cases were identified using the claims-based algorithms that were not captured within SEER. This finding likely reflects the outpatient diagnosis and care of MDS patients outside of the sites of institutional cancer registration from certified cancer registrars. For both the registry and claims-based methods, the incidence of MDS may be underreported because of underdiagnosis. For example, given the chronic nature of disease, a possible MDS patient may be asymptomatic with diagnostic MDS morphology or symptomatic with incompletely diagnosed unspecified anemia. These cases would not be captured by a centralized registry or claims-based system until the physician performed blood work and BM examination. Moreover, cytologic recognition of diagnostic features of MDS can be challenging.18 Considering that approximately 70% of MDS patients succumb to complications associated with cytopenias,19 a large number of MDS patients may be underdiagnosed and/or underreported because of life-threatening infections, bleeding, or anemia. Medicare claims analysis complements registry evidence on MDS, but does not address issues relating to underdiagnosis.
In summary, we evaluated 4 claims-based algorithms for sensitivity and specificity using the SEER-Medicare database, which includes registered MDS patients as gold-standard comparators. Our findings validate and support the use of the 2 + BCBM claims algorithm in identifying MDS cases. Moreover, the use of this conservative and highly specific algorithm identified a large number of uncaptured MDS cases. Our results call for the commitment of more public resources so that centralized cancer registries may improve MDS case ascertainment. This will ensure that trends in MDS incidence may more accurately describe the general population for the allocation of health care resources.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the staff in B.M.C.'s laboratory at H. Lee Moffitt Cancer Center & Research Institute for their contributions to the research and creation of this paper: Andrea Collado for database management, Riddhi Patel for research assistance, and Carol Templeton for copy editing.
Funding for this research was provided by a National Institutes of Health grant: “Developing Information Infrastructure Focused on Cancer Comparative Effectiveness Research” (RC2-CA148332 to the principal investigator, Dr David Fenstermacher, H. Lee Moffitt Cancer Center & Research Institute); by a research grant from Celgene: “Pilot Study of SEER-Medicare Database to Define Incidence of MDS” (to C.R.C.); and by a National Cancer Institute (National Institutes of Health, Bethesda, MD) Career Development Award (K25-CA122176 to B.M.C.).
National Institutes of Health
Authorship
Contribution: C.R.C. and B.M.C. designed the concept, analyzed the data, and wrote and approved the manuscript; and D.E.R. and A.F.L. edited and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christopher R. Cogle, MD, Division of Hematology and Oncology, Department of Medicine, College of Medicine, University of Florida, Box 100278, Gainesville, FL 32610-0278; e-mail: c@ufl.edu.