Members of the TGF-β family act on many, if not all, cell types within the body, producing diverse and complex cellular outcomes. Activation of the endothelial cell-restricted TGF-β type I receptor ALK1 results from the binding of several different ligands of the TGF-β family, including bone morphogenetic protein (BMP) 9, BMP10, and TGF-β. Mounting genetic, pharmacologic, and histopathologic evidence supports a critical role for ALK1 signaling in regulation of both developmental and pathologic blood vessel formation. However, the precise function of TGF-β family signaling in endothelial cells is difficult to predict and appears highly context dependent because of the multitude of ligands and receptors influencing the final outcome. Pharmacologic inhibitors of ALK1 have recently been developed and will allow for more accurate studies of ALK1 function in vivo, as well as for assessment of ALK1 as a target for suppression of angiogenesis during tumor development. Herein, we will summarize the current view of ALK1 regulation of endothelial cell phenotype in vitro and in vivo as well as provide an outlook for the ongoing clinical trials of ALK1 inhibitors in malignant disease.
Introduction
The introduction of antiangiogenic therapies into oncologic practice has been highly anticipated because of the success of extensive preclinical testing. However, the initial clinical experience with this new class of anticancer drugs has been sobering, with a measurable therapeutic benefit of a few months observed and very little effect on overall patient survival. To fully realize the potential of therapies inhibiting neoangiogenesis, it is likely that drugs impinging on multiple regulatory pathways must be combined, and thus we must learn more about the biology of various signaling factors affecting endothelial cell (EC) growth and function. Activin receptor-like kinase 1 (ALK1) is an EC-restricted receptor of the large TGF-β family.1 Herein, we will review our current understanding of ALK1 signaling and the potential of ALK1 to serve as a drug target for antiangiogenic therapy for cancer.
Signaling by ALK1 in ECs
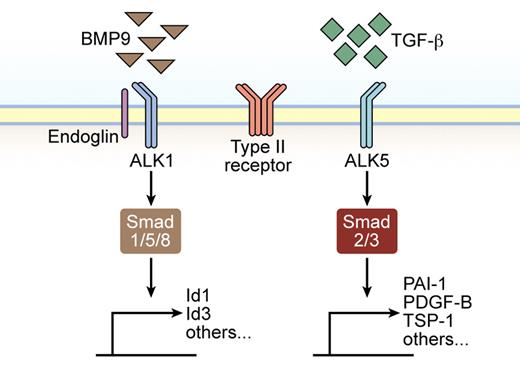
The large family of TGF-β extracellular ligands consists of > 30 cytokines that exert influence on several cellular compartments, notably epithelial cells, fibroblasts, immune cells, and endothelial and perivascular cells. TGF-β, the prototypical member of the family, elicits a diverse set of cellular responses, such as growth arrest, immune suppression, differentiation, apoptosis, and specification of developmental cell fate during embryogenesis and pathogenesis, in species ranging from flies and worms to mammals.2,3 On secretion and subsequent activation, the mature TGF-β ligand initiates signaling by inducing specific serine/threonine kinase type I and type II receptor heterotetrameric complexes.4 Ligand binding results in signal propagation inside the cell by phosphorylation of specific effector proteins, so-called Smads, which translocate to the nucleus and activate transcription of target genes.2,5 In ECs, TGF-β has been shown to signal via both the ubiquitously expressed type I receptor ALK5 and through the predominantly EC restricted receptor ALK1 (Figure 1). Depending on which type I receptor is recruited, different Smad signaling cascades are activated; ALK1 activation induces phosphorylation of Smad1/5/8, whereas ALK5 leads to Smad 2/3 activation.6,,–9 Consequent to engagement of either Smad pathway, the receptor-activated Smads further form a heteromeric complex with a common and related partner molecule, Smad4, which translocates the complexes into the nucleus, where cell type-specific transcriptional modulators collaborate to activate or repress transcription of specific target genes in the angiogenic response.10,11 In addition to the canonical signaling through Smad activation, TGF-β stimulation may lead to Smad-independent regulation of cellular outcomes, such as apoptosis and cell-cycle progression, through the direct modulation of prototypical signaling mediators, including MAP kinases and p21.12
Illustration of TGF-β family signaling in ECs. TGF-β activates both ALK1 and ALK5 type I receptor expressed by ECs, whereas BMP9 only binds ALK1. The affinity of BMP9 for ALK1 is greater than that of TGF-β, making it likely that ALK1 will predominantly bind BMP9 when both ligands are available. In addition, endoglin acts as a coreceptor modulating signaling through ALK1. Smad 1, 5, and 8 are preferentially phosphorylated and activated by ALK1, whereas Smad 2 and 3 act downstream of ALK5. Subsequently, Smads are translocated to the nucleus, where they regulate specific gene expression.
Illustration of TGF-β family signaling in ECs. TGF-β activates both ALK1 and ALK5 type I receptor expressed by ECs, whereas BMP9 only binds ALK1. The affinity of BMP9 for ALK1 is greater than that of TGF-β, making it likely that ALK1 will predominantly bind BMP9 when both ligands are available. In addition, endoglin acts as a coreceptor modulating signaling through ALK1. Smad 1, 5, and 8 are preferentially phosphorylated and activated by ALK1, whereas Smad 2 and 3 act downstream of ALK5. Subsequently, Smads are translocated to the nucleus, where they regulate specific gene expression.
Furthermore, a third type of TGF-β receptor, the type III receptors, is represented by betaglycan and endoglin. Endoglin, primarily a vascular marker, is an auxiliary receptor for TGF-β signaling required for angiogenesis during development and increasingly expressed during EC activation, inflammation, and tumor angiogenesis (Figure 1).13,–15 There is no enzymatic kinase activity associated with endoglin, but possibly through presenting various ligands to the receptors, endoglin modulates efficient TGF-β/ALK1 signaling but not TGF-β/ALK5 signaling (see “Related signaling pathways” and Lebrin et al16 ).
Cellular effects of ALK1 signaling in ECs
Effects of TGF-β on ECs in vitro
Several lines of evidence suggest that TGF-β regulates a fine balance between ALK1 and ALK5 signaling in the endothelium.17,18 However, many reports on the action of ALK1 signaling in ECs have revealed paradoxical results, highlighting the pleiotropic effects of TGF-β. For example, the ALK1 signaling pathway, on stimulation with TGF-β, leads to EC proliferation and migration (Figure 2A). Accordingly, ectopic expression of the ALK1 downstream target gene Id1 induces migration and EC tube formation,19 whereas specific disturbance of Id1 expression inhibits ALK1-induced EC migration.17 Further attempts to dissect the biologic effect of ALK1 activation have shown that infection of ECs with a constitutively active form of ALK1 inhibits proliferation, migration, and cell adhesion, suggesting that rather than an activator, ALK1 mediates the maturation phase of angiogenesis.20 Possible reasons for these opposing results may rely on the context-specific nature of TGF-β signaling, dosage, cell type, culture conditions, as well as the artificial experimental settings in vitro. In sharp contrast, ALK5 signaling induces inhibition of EC migration and proliferation in response to TGF-β (Figure 2A), indicating that TGF-β stimulation of ALK1 and ALK5 signaling gives rise to opposing outcomes.17 Similar antagonistic results have been obtained with human chondrocytes.21
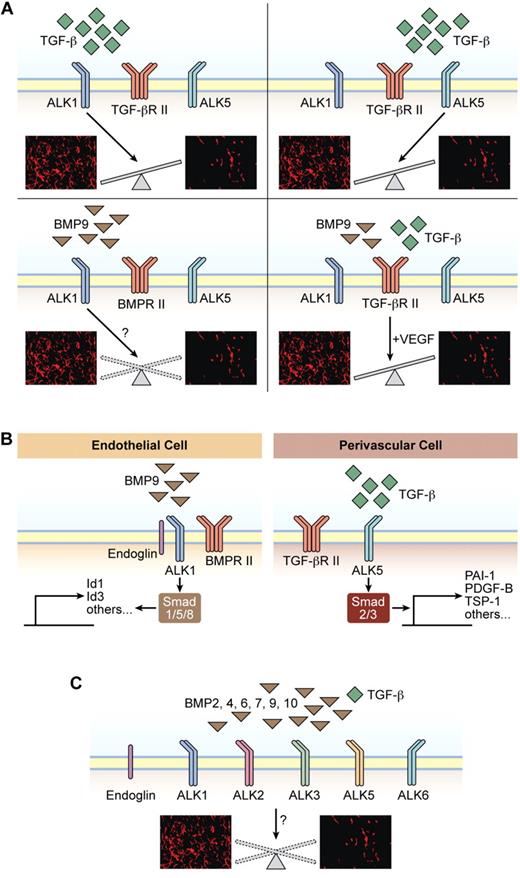
The outcome of signaling by TGF-β family members in ECs is highly context dependent. The net effect of stimulation or inhibition of EC migration, proliferation, and survival is illustrated by images from immunostaining of tumor sections for the vascular marker CD31 (red; images are only representations and are not derived from actual experimental settings). (A) TGF-β signaling through ALK1 predominantly acts to stimulate EC growth and migration. TGF-β signaling through ALK5 predominantly acts to inhibit EC growth and migration. BMP9 stimulation of ALK1 has been reported to be either inhibitory or stimulatory in terms of EC growth and migration. Stimulation of ECs with both TGF-β and BMP9, thus engaging both ALK1 and ALK5 simultaneously, primes ECs to an angiogenic stimulus by prototypical angiogenic inducers such as VEGF. (B) Representation of paracrine interaction between ALK1 expressed by ECs and ALK5 expressed by mural cells. (C) Illustration of the large complexity of BMP ligands and type I receptors that collectively regulate EC functionality in a context-dependent manner.
The outcome of signaling by TGF-β family members in ECs is highly context dependent. The net effect of stimulation or inhibition of EC migration, proliferation, and survival is illustrated by images from immunostaining of tumor sections for the vascular marker CD31 (red; images are only representations and are not derived from actual experimental settings). (A) TGF-β signaling through ALK1 predominantly acts to stimulate EC growth and migration. TGF-β signaling through ALK5 predominantly acts to inhibit EC growth and migration. BMP9 stimulation of ALK1 has been reported to be either inhibitory or stimulatory in terms of EC growth and migration. Stimulation of ECs with both TGF-β and BMP9, thus engaging both ALK1 and ALK5 simultaneously, primes ECs to an angiogenic stimulus by prototypical angiogenic inducers such as VEGF. (B) Representation of paracrine interaction between ALK1 expressed by ECs and ALK5 expressed by mural cells. (C) Illustration of the large complexity of BMP ligands and type I receptors that collectively regulate EC functionality in a context-dependent manner.
Effects of BMP9 on ECs in vitro
Similar to TGF-β, the recently described high-affinity ligand for ALK1, bone morphogenetic protein (BMP) 9,22 has also been reported to have disparate effects on ECs. On the one hand, BMP9 exhibits antiangiogenic effects, inhibiting fibroblast growth factor (FGF)–induced angiogenesis in ex vivo models (Figure 2A).22,23 On the other, Suzuki et al24 have shown induction of proliferation by BMP9 in multiple types of ECs in vitro and a proangiogenic effect of BMP9 on Matrigel plug vascularization and tumor angiogenesis in a xenograft model of pancreatic cancer (Figure 2A).
In an attempt to further clarify the role of ALK1 signaling in ECs, we recently described a synergistic effect of TGF-β together with BMP9, where the combined action of both ligands improved the EC response to angiogenic stimuli (Figure 2A).25 Although either ligand alone impairs EC functionality in this context, the presence of both ligands primes ECs to an enhanced response to prototypical angiogenic cues, such as VEGF and FGF-2, both in vitro in different cell lines and in vascular in-growth into subcutaneously injected Matrigel plugs in vivo.
From a molecular point of view, double stimulation of ECs with TGF-β and BMP9 induces a substantial up-regulation of ALK5 target gene products, such as PAI-1 and PDGF-B, supporting a previous report on induction of Smad2 phosphorylation on BMP9 stimulation.26 In agreement with these findings, ALK1 signal inhibition proved to interfere not only with its own target genes but also with ALK5 downstream effectors in a tumor model of multistep tumorigenesis.25 In this context, it is interesting to note that the relative, rather than the absolute, levels of ALK1 and ALK5 signaling may be crucial for proper regulation of gene expression.27
Interdependency of ALK1/ALK5 signaling
Transcriptional profiling of ECs expressing constitutively active forms of ALK1 or ALK5 demonstrates substantial differences in the molecular output obtained from either signaling pathway.28 Moreover, the nonoverlapping expression patterns of murine ALK1 and ALK5 in vivo29 suggest divergent roles in vascular development for each of the 2 type I receptors expressed by ECs. Further complexity to this signaling system is added by the observation that although TGF-β/ALK1 signaling appears to directly antagonize TGF-β/ALK5 signaling, in some circumstances, the presence of ALK5 is an absolute requirement for efficient TGF-β/ALK1 signaling, as demonstrated by studies in which the authors used ECs deficient for ALK5.30
However, the dependency of ALK1 on ALK5 signaling has been questioned and addressed by several groups. For example, Shao et al31 demonstrated that ALK5 suppression does not interfere with BMP9/ALK1-induced phosphorylation of Smad1/5/8 in bovine aortic ECs. In addition, in agreement with the ALK5-independent action of ALK1 is the notion that ALK5 is present in ECs in vivo only at low levels and instead is postulated to be mainly expressed by perivascular cells, thereby regulating angiogenesis through a paracrine mode of action (Figure 2B).29,32
Consistent with these findings, specific deletion of ALK5 in the endothelium does not result in vascular malformations in mice,32 nor does ALK5 genetic or pharmacologic inhibition in zebrafish.32 Nonetheless, embryos from knockin mice expressing ALK5 with a L45 loop mutation (a mutation that interferes with downstream Smad2 phosphorylation while preserving the capability of ALK5 to mediate non-Smad signaling and lateral signaling to ALK130 ) partially rescued the earliest vascular defects observed in ALK5 knockout mice.33
Collectively, these reports suggest that ALK5 signaling is indeed relevant for endothelial homeostasis, but the codependency on ALK1 signaling remains unclear, as does the issue whether this function is EC autonomous or involves crosstalk between ECs and mural cells. In addition to being critical to ensure endothelial architecture, ALK1 appears to also modulate smooth muscle cell differentiation and recruitment, as observed by Oh et al,18 through the analysis of ALK1 knockout mice. Further studies to address ALK1 vs ALK5 signaling in ECs and in perivascular cells are thus highly warranted to clarify and conciliate the seemingly divergent conclusions from previous studies.
Related signaling pathways
Both BMP9 and BMP10 have been identified as functional activators of ALK1 in ECs, inducing comparable cellular effects. Nevertheless, BMP10 binds to ALK1 with lower affinity than BMP9 and is mainly expressed in the murine heart.22 Moreover, BMP10 ablation leads to embryonic lethality because of impaired cardiac growth and function,34 suggesting that BMP10 action on ALK1 may be cardiac tissue specific. Whether BMP10 mediates vascular effects similar to BMP9 direct remains to be elucidated.
ALK1 shares similar properties in terms of BMP-dependent activation of Smad1/5/8 signaling with the related BMP type I receptors ALK2, ALK3, and ALK6. Ligand specificity has not been exhaustively elucidated, and many ligands, including BMP2, BMP4, BMP6, BMP7, BMP9, and BMP10, show a diverse range of effects on ECs, making it difficult to ascertain the net effect of signaling in any given situation (Figure 2C).35,,,,,–41 Although recently described as the physiologic ligand for ALK1, BMP9 can also bind ALK2 in non-ECs, such as myoblasts and breast tumor cells.23 Accordingly, BMP9/ALK2 signaling has been highlighted to promote ovarian cancer cell proliferation.42 In addition, ALK1 and ALK2 are both important for BMP9-induced osteogenic signaling in mesenchymal stem cells.43 Thus, the interplay and/or compensatory crosstalk between ALK1 and ALK2, which is of critical importance in a therapeutic context, should be the subject of further studies.
Of note, ALK2 has been demonstrated to up-regulate ALK1 in ECs in response to high-density lipoproteins, after which ALK1 in turn promotes survival by inducing expression of VEGF-A.35 ALK1 and ALK2 are also coregulated in aortic ECs by high levels of glucose.36 Moreover, ALK2 stimulates ectopic endothelial-to-mesenchymal transition as a result of activating mutations in the familial syndrome fibrodysplasia ossificans progressiva or after BMP4 stimulation.37 In agreement with this finding, ECs conditionally deficient for ALK2 fail to undergo endothelial-to-mesenchymal transition during the formation of the endocardial cushion during embryogenesis.44 In contrast, BMP7 counteracts endothelial-to-mesenchymal transition induced by TGF-β.38,45
Taken together, the various BMP ligands and type I receptors exert a variety of effects on ECs, not all of which are easily consolidated with the fact that the receptors activate the same set of Smad mediators. In this context, it is interesting to note studies by Nohe et al46,47 in which they suggest that preformed BMP receptor complexes mainly induce Smad-dependent signaling, whereas ligand-induced oligomerization of type I and type II receptors predominantly activates non-Smad signaling. In addition, specific binding of the downstream effector kinase Limk1 to the BMP type II receptor, but not to TGF-β or Activin type II receptors, have been reported, indicating that the choice of type II receptor also can influence the signaling outcome of BMP stimulation.48,49 Clearly, signaling through non-Smad effectors, and recruitment of distinct type II receptors, should be further examined as the explanations for the diverse effects.12
Endoglin, the accessory receptor for TGF-β signaling, shares considerable phenotypic similarities with ALK1 when mutated in humans or when manipulated in mice.50 Despite the lack of enzymatic activity, endoglin is known to be able to directly bind BMP9, TGFβ1, and TGFβ3 and modulate ALK1 signaling23,51,52 but not ALK5 signaling.16 However, despite the fact that endoglin is better described and strongly connected to regulation of ALK1 signaling, it is important to mention that it interacts directly not only with other ligands (Activin A, BMP2, and BMP7) but also with several different type I and type II receptors involved in BMP and TGF-β signaling.53 Thus, the promiscuity of endoglin adds yet another level of complexity to TGF-β family signaling in general and to ALK1 signaling in particular.
Physiologic role of ALK1 in the vasculature
Genetic studies of ALK1 function in vivo
ALK1 expression overlaps with sites of vasculogenesis and angiogenesis in early mouse development.54 Nevertheless, it has also been described to be present in other cell types, such as hepatic stellate cells55 and chondrocytes.21 Rodent ALK1 expression has been appreciated in the brain, lung, kidney, and spleen,56,57 being predominantly expressed in developing arterial endothelium, whereas it is almost inexistent in capillaries.29 As the mouse develops, ALK1 expression in adult blood vessels is suppressed but induced once again during neoangiogenesis in wound healing or tumors.29
The importance of TGF-β signaling in angiogenesis and vascular remodeling has been emphasized during the last decades by numerous gene-targeting studies in mice that have shown loss of TGF-β signaling components leading, in most cases, to embryonic lethality because of cardiovascular defects.58 As a case in point, Acvrl1 (ALK1) knockout mice die at midgestation around embryonic day 11.5 because of severe vascular abnormalities, including excessive capillary fusion, hyperdilation of vessels, and deficiency of differentiation and recruitment of vascular smooth muscle cells.18 The zebrafish ALK1 mutant, violet beauregarde, also presents with vascular malformations with blood flow constrained to a limited number of hyperproliferative cranial vessels,59 suggesting that ALK1 possesses an important and evolutionarily preserved function in the vasculature.
Aside from the breakthrough in dissecting the importance of ALKI on embryonic vascular development, the ALK1-lacZ reporter mice have proven very useful in adulthood to show that ALK1 is fundamental for placental development60 because ALK1 deficiency results in impairment of umbilical and placental blood vessel formation. The critical relevance of TGF-β signaling in vascular development has been further acknowledged by the identification of mutations in TGF-β receptor genes, ACVRL1 and ENG (endoglin), in familial vascular pathologies.61 Germline mutations in ACVRL1 or ENG are underlying the development of the human syndrome of hereditary hemorrhagic telangiectasia (HHT), which is characterized by cutaneous telangiectases, increasingly severe nosebleeds, and gastrointestinal hemorrhage.62,63 In addition, major arteriovenous malformations occur in lung, liver, spleen, or brain and may cause severe morbidity and mortality. Although ALK1 or endoglin-null mice are embryonic lethal as a result of severe vascular malformations,18,64 mice lacking one copy of the gene for either ALK1 or endoglin recapitulate the HHT phenotype with age,65,66 making them useful models for the study of this vascular pathology.
EC-specific ALK1 knockout in the mouse through deletion of the ALK1 gene from an Acvrl12loxP allele with the EC-specific L1-Cre line results in postnatal lethality at P5, and mice exhibiting hemorrhaging in the brain, lung, and gastrointestinal tract,67 mirroring to some extent the full ALK1 knockout phenotype. To evaluate the contribution of ALK1 to vascular homeostasis in adult mice, Park et al32 globally deleted the ALK1 gene by tamoxifen treatment of 2 months old R26+/CreERAcvrl12loxP/loxP mice. One single tamoxifen administration resulted in severe internal hemorrhaging and lethality, suggesting ALK1 relevance for vascular homeostasis in small intestine, lung and uterine vessels.
Other relevant mouse models have been generated for loss-of-function studies of the ALK1 downstream target genes, Id1 and Id3. Although transcription of Id1/Id3 is regulated by many BMP ligands and type I receptors through Smad 1/5, their robust induction by constitutively active ALK1 and by BMP9, as well as the requirement of ALK1 for Id1/Id3 to stimulate EC migration,17 makes it tempting to speculate on a functional relationship between the effects of ALK1 on ECs and Id1/Id3. Id1/Id3 double-knockout mice show abnormal angiogenesis, exhibiting enlarged dilated vessels. Moreover, Id1+/−; Id3−/− mice fail to support tumor growth and metastasis because of poor vascularization.68 Furthermore, selective knockdown of Id1 in BM-derived endothelial progenitor cells suppresses angiogenesis and consequently impairs metastatic spread of breast tumors to the lung.69
ALK1 signaling in lymphangiogenesis
In addition to the well-established expression of ALK1 in blood ECs, ALK1 was recently also reported to be readily expressed by lymphatic ECs.70 Indeed, stimulation of lymphatic ECs with BMP9 induces downstream target genes, such as Smad6, and inhibition of ALK1 signaling by the use of an ALK1-Fc fusion protein diminishes neonatal lymphangiogenesis. Interestingly, lymph vessel development is coordinately and synergistically regulated by ALK1 and VEGF receptor-3 signaling, reminiscent of the crosstalk observed between ALK1/ALK5 and VEGF-receptor signaling in blood vessel angiogenesis.25,71
Targeting ALK1 in vivo
Drugs incorporating inhibitory action against ALK1
Despite the large literature on the diverse effects of signaling emanating from ALK1 in ECs, it is difficult to predict the net outcome of acute inhibition of ALK1 in a tumor setting. Moreover, the complex ligand/receptor binding specificities within the TGF-β family add further uncertainty to the forecast of therapeutic efficacy and side effect profile of different classes of ALK1 inhibitors currently under development. Small molecules incorporating broad inhibitory action against BMP type I receptor kinase activity, including ALK1, such as dorsomorphin and the structural analog LDN-193189, have recently been developed and shown to block both Smad-dependent and -independent signaling induced by BMPs.72,73
Although such drugs may be very useful and potent in inhibiting BMP type I receptor signaling in a range of diseases and familial syndromes,74 it still remains to determine whether they affect tumor angiogenesis induced by BMPs. In fact, the blockade of zebrafish intersomitic vessel formation observed after treatment with dorsomorphin was determined to be because of off-target effects on VEGF signaling.75 Nevertheless, these recent efforts show that small molecule inhibitors of BMP signaling can be readily identified, and although dorsomorphin appears as a poor candidate for clinical development because of its additional targeting of the tumor suppressor AMP kinase, further development of small molecule compounds with a narrower inhibition profile of BMP type I receptors should be pursued.
The use of biologic compounds, such as antibodies or soluble extracellular domains (traps), may seemingly allow for more specific inhibition of ALK1 activity. However, caution is warranted because the extensive crosstalk between different signaling pathways and sharing of receptor complexes may result in undesired off-target effects. Thus, an antibody against the extracellular domain of ALK1 would conceivably block the binding and downstream signaling of all BMPs/TGF-βs that activate ALK1 while preserving the capability of those ligands to bind to related receptors, such as ALK5 or ALK2, on ECs or other cell types. Conversely, an ALK1-Fc fusion protein may neutralize all ligands that bind directly to ALK1 and at the same time block signaling by those ligands through other receptors, thereby incorporating a wider inhibition profile. To date, the development of 3 different biologic ALK1 inhibitors for use in vivo has been reported from pharmaceutical companies. First, researchers at Pfizer are currently conducting phase 1 trials of PF-3446962, a fully human monoclonal antibody against ALK1.76 Second, Niessen et al70 from Genentech reported on the use of an ALK1-Fc fusion protein (amino acids 23-119 of mouse ALK1) to study the dependence of lymphatic vessel formation on ALK1 signaling in mouse. Third, ACE-041 is a human ALK1-Fc fusion protein currently in clinical development by Acceleron Pharma (mouse counterpart RAP-041, amino acids 22-117 of mouse ALK1).77 Interestingly, ACE-041 only binds and neutralizes BMP9 and BMP10, but not TGF-β.25,71
PF-3446962
Preclinical tumor studies in which the authors used PF-3446962 were recently reported.78 Blocking of ALK1 was demonstrated to attenuate VEGF-induced EC proliferation and tube formation in vitro. Furthermore, treatment with the ALK1-neutralizing antibody delays tumor growth in vivo when different mouse models are used, including MDA-MB-231 breast carcinoma and M24met/R melanoma. Notably, ALK1 inhibition acts in concert with VEGF-blocking agents to further reduce angiogenesis and growth of tumors. Of particular interest is the observation that ALK1 signaling may in part be responsible for compensatory signaling in tumors refractory to VEGF inhibition, possibly by modulating the crosstalk between ECs and pericytes.78 Finally, administration of PF-3446962 attenuates blood flow in tumors, a parameter that may be useful as a surrogate marker of clinical efficacy.
ACE-041/RAP-041
We have recently reported on the effects of RAP-041 in the RIP1-Tag2 genetically engineered mouse model of pancreatic neuroendocrine tumorigenesis.25 Tumors from RIP1-Tag2 mice exhibit expression of ALK1 exclusively on ECs, and the expression is strongly correlated with other vascular markers during the course of tumor development. Treatment of RIP1-Tag2 mice with 1-12 mg/kg RAP-041 twice weekly results in a dose-dependent delay in tumor growth, and the greatest dose effectively blocks further tumor growth, albeit without producing regression. Histologic analysis established that the growth-inhibitory effect of RAP-041 was mainly the result of antiangiogenic effects. The specificity of the drug treatment was confirmed by demonstrating a near-identical phenotype in RIP1-Tag2 mice deficient for one copy of the Acvrl1 gene and by revealing decreased expression of ALK1 downstream target genes in tumors from mice treated with the ALK1-Fc fusion protein. In parallel studies, RAP-041 exhibited growth-inhibitory properties in orthotopically implanted MCF-7 breast carcinomas, although potential antivascular effects were not assessed.71 Taken together, the limited preclinical data currently available point to an antiangiogenic and growth inhibitory effect of attenuated ALK1 signaling in mouse models of cancer, and thus justifies continued clinical development of drugs blocking ALK1.
Clinical relevance of ALK1 in tumor biology
Expression of ALK1 in human tumors
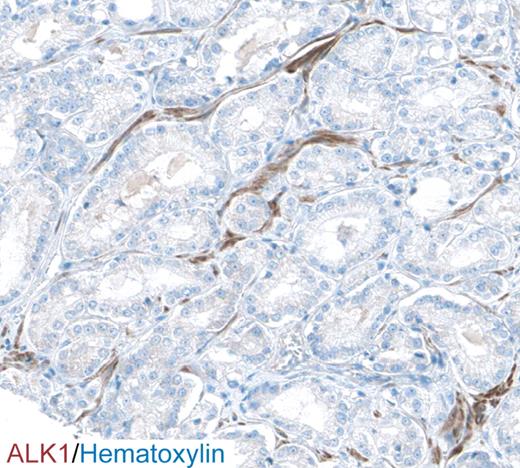
Little is known about the pattern and extent of ALK1 expression in human normal and malignant tissues, mainly because of a lack of suitable reagents for immunohistochemical analysis. The authors of a preliminary study reported a weak but widespread pattern of expression in the vasculature of normal tissues, including positive staining in lymphatic tissues, lung, intestines and pancreas.79 In a recent study by the same organization, ALK1 was found to be widely present on tumor blood vessels, most notably in lymphomas and cancers of the prostate, skin, thyroid, kidney, ovary, lung, pancreas, and liver.78 In the effort to map the abundance and localization of all human proteins, the public Human Proteome Resource program80 has developed and used an antibody against ALK1 for immunostaining of both normal and neoplastic tissues. In this study, ALK1 exhibited abundant expression in the vasculature and stroma by a high proportion of prostate carcinomas (Figure 3). Intriguingly, expression of TGF-β is correlated to vascular density and survival in prostate cancer, making this tumor type of particular interest for further clinical studies using ALK1 inhibitors.81 Further studies investigating the prognostic significance of ALK1 expression in a wide variety of cancers are, however, still lacking and should be a high priority for future efforts.
Immunostaining for ALK1 (brown) of a section from human prostate cancer reveals a vascular staining pattern. Cell nuclei are counterstained with hematoxylin (blue). Image taken from the Human Protein Atlas (http://www.proteinatlas.org).
Immunostaining for ALK1 (brown) of a section from human prostate cancer reveals a vascular staining pattern. Cell nuclei are counterstained with hematoxylin (blue). Image taken from the Human Protein Atlas (http://www.proteinatlas.org).
Possible side effects from ALK1 inhibition
Antiangiogenic therapies, mainly in the form of inhibitors of VEGF signaling, have been in routine clinical use for > 5 years for various malignancies. Side effects from inhibiting angiogenesis generally are milder than for conventional cytotoxic drugs and include bleeding, hypertension, fatigue, and nausea. Specifically, given the causal relationship between impaired ALK1 signaling and HHT-related symptoms, inhibition of ALK1 signaling in the vasculature may induce arteriovenous malformations and hemorrhaging. Indeed, hemorrhaging and signs of arteriovenous malformations of the lung and intestine ultimately resulting in lethality were observed only 9-21 days after inducible cre-mediated deletion of both copies of the ALK1 gene in mice.67 In addition, these mice developed de novo formation of arteriovenous shunts in the subdermis in conjunction with wound healing.67
Loss-of-function mutations in ALK1 are also linked to hereditary pulmonary arterial hypertension,82 raising the risk that treatment with ALK1 inhibitors may influence the hemodynamic status of the pulmonary circulation. However, it should be noted that predictions of side effects from an acute inhibition of signaling using drugs that variously impinge on the ALK1 and related signaling pathways on the basis of the phenotype of global or induced gene knockout studies are not necessarily straightforward. Finally, because ALK1 is reported to be expressed by, and possibly functionally important for, lymphatic ECs, cells of the pituitary gland, hepatic stellate cells, chondrocytes, and pancreatic ductal cells, special care should be taken to record adverse events from the treatment of ALK1 inhibitors related to processes regulated by these particular tissues.21,55,70,83,84
Clinical trials of ALK1 inhibitors
Despite the paucity of target expression and validation studies, 2 different clinical phase 1 trials have been launched. First, researchers from Pfizer are testing treatment with PF-3446962 in patients with advanced solid tumors.76 The primary end point for the study is to recommend a maximum tolerated dose for further phase 2 trials, whereas secondary outcomes include to record preliminary signs of antitumor activity. Importantly, the trial design incorporates both functional readouts for vessel patency by dynamic contrast-enhanced magnetic resonance imaging, as well as a search for surrogate markers of target inhibition by measuring the abundance of ALK1-expressing circulating ECs.85 Second, Acceleron Pharma recently concluded a phase 1 trial for the ALK1-Fc fusion protein ACE-041.77 The study recruited patients with advanced solid tumors or refractory multiple myeloma and will assess the safety and tolerability of ACE-041, as well as any apparent changes in the tumor metabolism, as judged by 18F deoxyglucose positron emission tomography.
Perspective
Members of the TGF-β family have pleiotropic and complex effects on many, if not all, cell types within the body. Specifically, for malignant disease, it is well established that TGF-β acts as a tumor suppressor during the initial stages of tumor development, whereas at advanced stages, TGF-β promotes tumor growth and metastatic spread.86 Thus, it will prove important to consider different aspects of the biology of TGF-β in the development and use of inhibitors of TGF-β actions for the treatment of cancer because blockade of signaling may lead to divergent effects depending on the stage of the disease.
Similarly, in ECs, the net outcome of signaling from TGF-β family receptors is multifarious and determined by which ligands engage ALK1, ALK5, and other BMP type I receptors, as well as endoglin (Figure 2).25 Inhibition of the proangiogenic aspects of ALK1/ALK5 downstream signaling must be accompanied by preserved growth-inhibitory capabilities of TGF-β signaling through ALK5 on ECs and malignant cells. In this aspect, both ACE-041 and PF-3446962 are promising agents because they block signaling exclusively by BMP9/BMP10 and ALK1, respectively, and do not directly interfere with ALK5 signaling on other cell types within the tumor. The prospects of developing ALK1 inhibitors in the context of combinatorial treatment regimens with cytotoxic drugs and/or other antiangiogenic agents, including VEGF inhibitors, appear particularly appealing. Despite the challenges of the tremendous complexity of signaling outcomes from the TGF-β receptors in the various cell types of a tumor, the reward for harnessing the TGF-β system for therapeutic purposes may be large. The current preclinical data lend support to the notion of ALK1 being an important target for antiangiogenic therapy of human cancer.
Acknowledgments
K.P. is the recipient of a Young Investigator Award from the Swedish Cancer Society.
Authorship
Contribution: S.I.C. and K.P. wrote the paper.
Conflict-of-interest disclosure: K.P. is named as an inventor on patents owned by the Ludwig Institute for Cancer Research Ltd and licensed to Acceleron Pharma, which is developing the ALK1 inhibitor ACE-041. S.I.C. declares no competing financial interests. No commercial entities had any influence on the conception of this article.
Correspondence: Kristian Pietras, Department of Medical Biochemistry and Biophysics, Division of Vascular Biology Karolinska Institutet, Scheeles Väg 2, SE-171 77 Stockholm, Sweden; e-mail: Kristian.Pietras@ki.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal