Abstract
Deregulated expression of microRNAs is associated with neoplasia. Here, we show that mature miR-16 levels are abnormally increased in CD34+ cells of patients with polycythemia vera as a consequence of preferential expression of miR-16-2 on chromosome 3 rather than of miR-16-1 on chromosome 13. Forced expression of miRNA-16 in normal CD34+ cells stimulated erythroid cell proliferation and maturation. Conversely, exposure of polycythemia vera CD34+ cells to small interfering RNA against pre-miR-16-2 reduced erythroid colonies and largely prevented formation of erythropoietin-independent colonies; myeloid progenitors remained unaffected. Experiments with knock down of JAK2 indicated that overexpression of miR-16 was independent of JAK/STAT pathway activation. Mice injected with an miR-16 antagomir showed a blunted erythroid response to exogenous erythropoietin, which indicates a role of miR-16 in normal erythropoiesis. These data suggest that deregulation of miR-16-2 contributes to abnormal expansion of erythroid lineage in polycythemia vera. However, the mechanisms for miR-16-2 overexpression remain to be elucidated, because no genetic abnormalities at the miR-16-2 locus were discovered.
Introduction
MicroRNAs (miRNAs) are small noncoding RNAs that intervene in the regulation of cell proliferation, differentiation, and apoptosis by silencing target genes.1 Deregulated expression of miRNAs, because of defective transcriptional control, mutations,2 or epigenetic abnormalities,3 is associated with neoplasia through tumor suppressor gene down-regulation4 or oncogene overexpression.5,6
The myeloproliferative neoplasms polycythemia vera (PV), essential thrombocythemia, and primary myelofibrosis (PMF) originate from deregulated proliferation of hematopoietic stem cells, which leads to overproduction of mature blood cells.7 Virtually all patients with PV and ≃ 60% of those with essential thrombocythemia or PMF harbor a JAK2V617F mutation, which causes activation of the JAK/STAT pathway. Expression of JAK2V617F in murine models recapitulates the myeloproliferative phenotype; however, it remains unclear how a single mutation underlies different clinical entities, which points to additional genetic or postgenetic abnormalities.8
In PV, the differentiation potential of stem cells is skewed toward the erythroid lineage.9 MicroRNAs regulate hematopoietic cell–lineage specification and maturation,10,11 and their role in normal12-15 and pathologic16,17 erythropoiesis is becoming appreciated. Herein, we show that abnormal expression of miRNA-16 contributes to hyperactive erythropoiesis in PV.
Methods
Myeloproliferative neoplasms were diagnosed according to the 2008 World Health Organization criteria.18 CD34+ cells were purified with immunomagnetic beads. A 2-phase liquid culture system was used for erythroid cell generation. Colony assays were performed in methylcellulose. miRNAs were quantified with TaqMan microRNA assays. Cell transfection was performed with Amaxa Nucleofector technology. For in vivo experiments, C57Bl/6J mice were injected with erythropoietin with or without antagomir, euthanized at various time intervals, and analyzed. For a detailed description, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the article).
Results and discussion
While studying the miRNA expression profile in PV CD34+ cells induced to erythroid differentiation, we observed that expression of miR-16 remained steadily high over time, unlike in normal cells, in which a prompt decline after culture initiation was followed by a late increase at day 9-12 coincident with erythroblast generation (Figure 1A).10,12,17 Therefore, we postulated that miR-16 deregulation could contribute to abnormal erythropoiesis in PV. First, we compared miR-16 levels in CD34+ cells from patients with myeloproliferative neoplasms to their normal counterparts. We found that miR-16 was 35-fold (range 1-357-fold) more expressed in PV than in controls (P < .0001), unlike in essential thrombocythemia or PMF (Figure 1B). Thus, PV CD34+ cells overexpressed miR16, the levels of which increased further during erythroid differentiation in vitro (Figure 1A). miR-16 levels in PV T lymphocytes were superimposable with controls, which indicates that miR-16 overexpression was confined to the myeloid clone (supplemental Figure 1). To rule out that miR-16 overexpression simply reflected a stimulated erythropoiesis, we compared the levels measured in PV CD34+ cells with those of subjects with reactive erythrocytosis, hemolytic anemia, or myelodysplastic syndromes; we found that in each of the latter cases, miR-16 levels were normal (Figure 1B). A much less evident, yet statistically significant (P = .016), increase of miR-16 was also detected in PV granulocytes, unlike reactive erythrocytosis granulocytes (supplemental Figure 1). Finally, to ascertain whether increased miR-16 expression in PV cells was linked to JAK/STAT activation, we silenced JAK2 with small interfering RNA (siRNA) in JAK2V617F-mutated HEL and UKE-1 cells. We found that although STAT5 phosphorylation was robustly down-regulated by siRNA, the levels of miR-16 remained unchanged (supplemental Figure 2A), which argues against a functional relationship between activated JAK/STAT and the regulation of miR-16. Furthermore, no correlation was found between JAK2V617F allele burden and miR-16 levels in PV CD34+ cells; conversely, there was a trend between higher miR-16 level and hemoglobin (P = .061).
miR-16-2 is overexpressed in PV CD34+ cells and contributes to abnormal erythropoiesis. (A) Levels of miR-16 were measured during induced erythroid differentiation of control (black line) and PV (red line) CD34+ cells. A 2-phase liquid culture system was used in which erythropoietin (EPO) was added on day 6 of culture (phase 2). Data were normalized to the mean miR-16 level measured on day 0 in the CD34+ cells of each patient group, which comprised 7 PV patients and 5 healthy subjects, and were expressed as percent variation. **P < .01 or greater in PV vs control cells. (B) Levels of mature miR-16 were measured in CD34+ cells from PV (n = 75), essential thrombocythemia (ET; n = 10), or PMF (n = 25) patients by RTQ-PCR and normalized (2−ΔΔCT; expressed as relative quantity [RQ]) to control CD34+ cells (n = 10; upper and lower limits of control subjects are indicated by dashed lines). Also included were subjects with reactive erythrocytosis (RE; n = 3), hemolytic anemia (HA; n = 2), and low-risk myelodysplastic syndromes (MDS; n = 5); because all of these subjects had levels within the normal range, they have been grouped together in the plot. (C) PV CD34+ cells were transfected with siRNA against pre–miR-16-1 or pre–miR-16-2, and levels of mature miR-16 were measured 24 hours later; an aliquot of cells transfected with scramble (Scr) siRNA served as control. **P < .0001. (D) CD34+ cells from healthy subjects were transfected with pre–miR-16 (+) or scramble pre-miR (−) and plated in semisolid medium in the presence of an optimal cytokine cocktail that included EPO to permit the growth of erythroid (CFU-E, BFU-E) or myeloid (CFU–granulocyte macrophage [CFU-GM]) progenitors. Pre–miR-16 transfection rates were assessed by FACS analysis and were in the range of 60% to 70%; cultures were established with unsorted cells. Data shown were generated from 6 controls in 3 independent experiments. *P < .05; **P < .01. (E) The effect of pre–miR-16 transfection on the expression of erythroid differentiation markers was measured in a 2-phase liquid culture system that had been supplemented (“with”) or not (“w/o”) EPO on day 6. These cultures were established with pre–miR-16–transfected, unsorted, normal CD34+ cells (n = 5 subjects); transfection rates assessed by FACS analysis were in the range of 60% to 65%. Flow cytometric analysis for membrane erythroid marker expression was performed 4 days after EPO supplementation. *P < .05; **P < .01. (F) CD34+ cells from PV patients were transfected with siRNA against pre–miR-16-1 or pre–miR-16-2 and the growth of EECs, BFU-E, and CFU-GM was evaluated. **P < .01. (G) PV CD34+ cells were transfected with siRNA against pre–miR-16-1 or pre–miR-16-2, sorted by flow cytometry, and then plated for EEC growth. **P < .01. Representative images of EECs obtained by plating PV CD34+ cells that had been transfected with siRNA against pre–miR-16-1 or pre–miR-16-2 are presented on the right. Microphotographs were obtained with a Nikon Eclipse TS100 contrast-phase microscope, objective Nikon plan 40×/0.65.
miR-16-2 is overexpressed in PV CD34+ cells and contributes to abnormal erythropoiesis. (A) Levels of miR-16 were measured during induced erythroid differentiation of control (black line) and PV (red line) CD34+ cells. A 2-phase liquid culture system was used in which erythropoietin (EPO) was added on day 6 of culture (phase 2). Data were normalized to the mean miR-16 level measured on day 0 in the CD34+ cells of each patient group, which comprised 7 PV patients and 5 healthy subjects, and were expressed as percent variation. **P < .01 or greater in PV vs control cells. (B) Levels of mature miR-16 were measured in CD34+ cells from PV (n = 75), essential thrombocythemia (ET; n = 10), or PMF (n = 25) patients by RTQ-PCR and normalized (2−ΔΔCT; expressed as relative quantity [RQ]) to control CD34+ cells (n = 10; upper and lower limits of control subjects are indicated by dashed lines). Also included were subjects with reactive erythrocytosis (RE; n = 3), hemolytic anemia (HA; n = 2), and low-risk myelodysplastic syndromes (MDS; n = 5); because all of these subjects had levels within the normal range, they have been grouped together in the plot. (C) PV CD34+ cells were transfected with siRNA against pre–miR-16-1 or pre–miR-16-2, and levels of mature miR-16 were measured 24 hours later; an aliquot of cells transfected with scramble (Scr) siRNA served as control. **P < .0001. (D) CD34+ cells from healthy subjects were transfected with pre–miR-16 (+) or scramble pre-miR (−) and plated in semisolid medium in the presence of an optimal cytokine cocktail that included EPO to permit the growth of erythroid (CFU-E, BFU-E) or myeloid (CFU–granulocyte macrophage [CFU-GM]) progenitors. Pre–miR-16 transfection rates were assessed by FACS analysis and were in the range of 60% to 70%; cultures were established with unsorted cells. Data shown were generated from 6 controls in 3 independent experiments. *P < .05; **P < .01. (E) The effect of pre–miR-16 transfection on the expression of erythroid differentiation markers was measured in a 2-phase liquid culture system that had been supplemented (“with”) or not (“w/o”) EPO on day 6. These cultures were established with pre–miR-16–transfected, unsorted, normal CD34+ cells (n = 5 subjects); transfection rates assessed by FACS analysis were in the range of 60% to 65%. Flow cytometric analysis for membrane erythroid marker expression was performed 4 days after EPO supplementation. *P < .05; **P < .01. (F) CD34+ cells from PV patients were transfected with siRNA against pre–miR-16-1 or pre–miR-16-2 and the growth of EECs, BFU-E, and CFU-GM was evaluated. **P < .01. (G) PV CD34+ cells were transfected with siRNA against pre–miR-16-1 or pre–miR-16-2, sorted by flow cytometry, and then plated for EEC growth. **P < .01. Representative images of EECs obtained by plating PV CD34+ cells that had been transfected with siRNA against pre–miR-16-1 or pre–miR-16-2 are presented on the right. Microphotographs were obtained with a Nikon Eclipse TS100 contrast-phase microscope, objective Nikon plan 40×/0.65.
Mature miR-16 derives from miR-16-1 at chromosome 13q14 and miR-16-2 at chromosome 3q25, where miR-16 is in a cluster, respectively, with miR-15a and miR-15b (supplemental Figure 3A-B). However, miR-15a/miR-15b levels in PV CD34+ cells were comparable to controls (supplemental Figure 4), which makes it unlikely that miR-16 overexpression was caused by amplification of the miR-16/miR-15 loci. This was corroborated by miR-16-1/miR-16-2 copy number quantification with quantitative RT-PCR (RTQ-PCR) and SNP 6.0 Affymetrix array (not shown in detail). Sequencing of the pre-miR-16-1 and pre-miR-16-2 regions in 12 PV patients did not uncover any mutations (supplemental Table 1).
To discriminate between miR-16-1- or miR-16-2-derived mature miR-16, we measured the levels of their respective precursors (pre-miRNA) by specific RTQ-PCR and calculated their ratio in PV (n = 19) or control (n = 10) CD34+ cells. The pre–miR-16-1/pre–miR-16-2 ratio was 0.6 ± 0.05 in controls compared with 0.34 ± 0.11 in PV (P = .0013), which suggests a prevalence of miR-16-2–derived mature miR-16 in PV. To corroborate this finding, we measured the level of mature miR-16 in PV CD34+ cells after transfection with siRNA against each pre-miRNA. Mature miR-16 levels decreased dramatically in cells transfected with pre–miR-16-2 siRNA (10 ± 0.02% vs scramble siRNA; P < .0001), unlike pre–miR-16-1 siRNA (83 ± 5% of scramble; Figure 1C); conversely, mature miR-16 levels were similarly down-regulated by each siRNA in control CD34+ cells (supplemental Figure 5). Finally, miR-195, which is 90% identical to miR-16-2, was not increased in PV CD34+ cells, which rules out cross-reactivity (supplemental Figure 6). Overall, these experiments indicated that raised levels of miR-16 in PV CD34+ cells derived preferentially from miR-16-2. miR-16-2 is intronic to SMC4, which encodes for structural maintenance of chromosomes protein 4, a component of condensin complex (supplemental Figure 3A). We found that SMC4 mRNA was overrepresented in PV CD34+ cells and correlated to miR-16 levels (supplemental Figure 7); SCM4 levels showed a trend toward a late increase during erythroid cultures (supplemental Figure 8), which somewhat mirrored the behavior of miR-16 (Figure 1A). These data suggest that miR-16-2 may be cotranscribed with SMC4, as reported for most intronic miRNAs,19 although formal proof is warranted.
Defective gene methylation may cause microRNA overexpression in tumors.20 Although a functionally defined promoter of miR-16-2 has not yet been characterized,21 we evaluated the methylation status of 5 CpG-rich regions ≃ 4 kb upstream of miR-16-2 (supplemental Table 1; supplemental Figure 9). We found a very low extent of methylation in both normal and PV CD34+ cells (not shown), which makes hypomethylation an unlikely mechanism for miR-16-2 overexpression. Thus, the ultimate mechanism underlying miR16-2 overexpression in PV cells remains to be clarified.
To determine whether a high level of miR-16 could facilitate an expansion of erythroid progenitors, we overexpressed miR-16 in normal CD34+ cells. We found that burst-forming unit erythroid (BFU-E) increased from 670 ± 35 to 1330 ± 180 × 104 cells (P = .004) and CFU-erythroid from 105 ± 60 to 174 ± 100 × 104 cells (P = .02) in cultures of scramble-treated and miR-16–overexpressing CD34+ cells, respectively; CFU–granulocyte-macrophage did not change (Figure 1D). In another set of experiments, miR-16 was overexpressed in immature erythroid precursors obtained at day 6 of 2-step liquid cultures. We observed that the frequency of glycophorin A–positive/CD71+ cells increased from 5 ± 3% to 22 ± 9% (P < .001) in erythropoietin-supplemented cultures that had been established with control or miR-16–overexpressing CD34+ cells, respectively, and that of glycophorin A–positive/CD36+ cells increased from 12 ± 3% to 23 ± 9% (P = .031; Figure 1E). Next, we evaluated the effects of knocking down the expression of miR-16 in PV CD34+ cells using siRNAs specific for pre–miR-16-1 or pre–miR-16-2. We found that PV CD34+ cells transfected with siRNA anti–pre–miR-16-2 generated significantly less BFU-E and less erythropoietin-independent erythroid colonies (EECs) than CD34+ cells transfected with anti–pre–miR-16-1 siRNA or control cells (Figure 1F). However, because the transfection efficiency was 60%-67%, in an independent set of experiments, cultures for EECs were established with sorted CD34+ cells after transfection. The pre–miR-16-2 siRNA, unlike the pre–miR-16-1, caused a > 60% reduction of EECs generated from sorted CD34+ cells, and the few remaining colonies showed defective hemoglobinization (Figure 1G). Overall, these results provided evidence that miR-16-2 overexpression contributes to erythroid expansion in PV and to the growth of EECs.
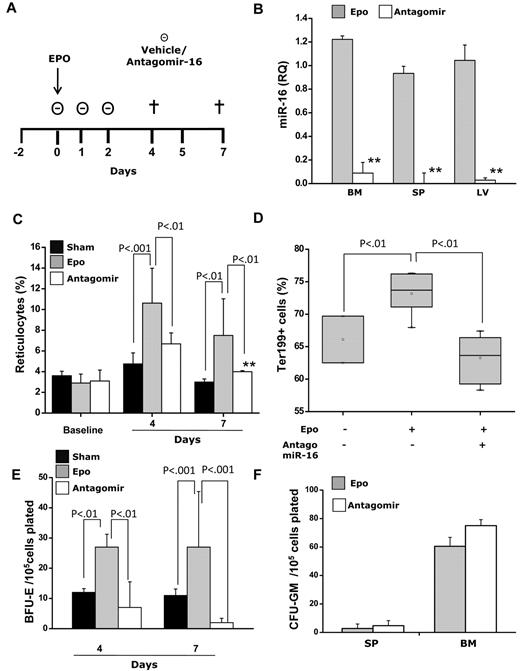
Finally, we investigated the relevance of miR-16 in erythropoiesis in vivo using mice acutely treated with erythropoietin, a useful model for investigating erythropoiesis.22 A group of mice received systemic delivery of phosphorothioate-cholesterol–modified antagomirs, which efficiently knock down miRNAs.23,24 Mice were injected with a single dose of erythropoietin on day 0 (EPO-mice) with or without 3 daily doses of miR-16 antagomir or vehicle (sham mice); they were then euthanized on day 4 or 7 (Figure 2A). Antagomir treatment caused ≥ 90% inhibition of endogenous miR-16 in bone marrow, spleen, and liver extracts (Figure 2B). Erythropoietin injection resulted in a significant increase in reticulocyte count on day 4 from 3.6 ± 0.6% in sham mice to 11.6 ± 2.5% in EPO-mice, which was largely prevented by antagomir-16 treatment (6.1 ± 0.8%; P < .01); similar effects were seen on day 7 (Figure 2C). In addition, the increase in Ter-119+ erythroblasts in the bone marrow of EPO-mice was significantly hampered by antagomir-16: 74.2 ± 5.3% vs 62.0 ± 2.9%, respectively (P < .01; Figure 2D). Finally, the number of BFU-Es obtained from antagomir-16–treated mice was markedly reduced compared with EPO-mice (Figure 2E; P < .01). Myeloid colonies were not modified (Figure 2F). Thus, in line with the observations obtained in vitro, knock down of miR-16 expression in mice resulted in inhibition of erythroid cell generation.
Effects of treatment with miR-16 antagomir on erythropoietin (EPO)-induced erythrocytosis in mice. (A) Normal C57Bl/6J mice were injected with EPO only (600 U/kg; EPO-mice) on day 0 with or without daily intravenous injections of antagomir-16 (55 mg/kg; antagomir mice) on days 0 to 2 or an equivalent volume of vehicle (sham mice). The data presented in the plots derive from 2 independent experiments that involved at least 3 mice per time point in each experiment. (B) Antagomir injection resulted in almost complete suppression of endogenous miR-16 in bone marrow (BM), spleen (SP), and liver (LV) extracts on day 7 (measured by RTQ-PCR). **P < .001. (C) A blood sample was collected from all mice on day −2 (baseline) and on days 4 and 7 after EPO administration to estimate reticulocyte count. The > 3-fold increase of reticulocytes that followed EPO administration in EPO-mice was largely prevented by antagomir-16 treatment. Similarly, the proportion of Ter-119+ cells in the bone marrow of mice at day 7 after EPO injection was significantly higher in EPO-mice than in those receiving antagomir (Antago) or in sham mice (D). The number of BFU-E colonies obtained from bone marrow cells of antagomir-treated mice was significantly lower than that of mice treated with EPO only at both day 4 and day 7 (E), whereas the number of CFU–granulocyte macrophages (CFU-GM) grown from the spleen and the bone marrow at day 4 (not shown) or day 7 was unaffected (F).
Effects of treatment with miR-16 antagomir on erythropoietin (EPO)-induced erythrocytosis in mice. (A) Normal C57Bl/6J mice were injected with EPO only (600 U/kg; EPO-mice) on day 0 with or without daily intravenous injections of antagomir-16 (55 mg/kg; antagomir mice) on days 0 to 2 or an equivalent volume of vehicle (sham mice). The data presented in the plots derive from 2 independent experiments that involved at least 3 mice per time point in each experiment. (B) Antagomir injection resulted in almost complete suppression of endogenous miR-16 in bone marrow (BM), spleen (SP), and liver (LV) extracts on day 7 (measured by RTQ-PCR). **P < .001. (C) A blood sample was collected from all mice on day −2 (baseline) and on days 4 and 7 after EPO administration to estimate reticulocyte count. The > 3-fold increase of reticulocytes that followed EPO administration in EPO-mice was largely prevented by antagomir-16 treatment. Similarly, the proportion of Ter-119+ cells in the bone marrow of mice at day 7 after EPO injection was significantly higher in EPO-mice than in those receiving antagomir (Antago) or in sham mice (D). The number of BFU-E colonies obtained from bone marrow cells of antagomir-treated mice was significantly lower than that of mice treated with EPO only at both day 4 and day 7 (E), whereas the number of CFU–granulocyte macrophages (CFU-GM) grown from the spleen and the bone marrow at day 4 (not shown) or day 7 was unaffected (F).
There is strong evidence that miRNAs are involved in cancer development and progression and could represent targets for therapy. The present data establish a role for miR-16 in normal erythropoiesis and suggest that abnormally increased levels may contribute to PV pathogenesis. Of interest, a role of miR16 in the elevated fetal hemoglobin expression in human trisomy 13 has been reported and has been shown to be mediated by inhibition of Myb.25 We foresee that identification of the mechanism(s) underlying miR-16-2 deregulation, as well as discovery of the ultimate miR-16 targets, in PV cells will contribute to a better understanding of the molecular basis of this disorder.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr J. S. Song, the Simon Center for Systems Biology at Princeton, NJ, for sharing unpublished information about the SMC4 promoter. We gratefully acknowledge Dr Radek Skoda, Basel University, and Dr U. Fiedler, Hamburg University, for providing us with the JAK2 transfected Ba/F3 cell and UKE-1 cell lines, respectively. The JAK2 inhibitor AZD1480 was generously provided by Dr D. Huszar from AstraZeneca, Waltham, MA. We thank Professor A. R. Arcangeli and members from LIGeMA, Florence, Italy, for help with animal experiments.
This work was supported in part by the Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Italy, and the Istituto Toscano Tumori, Florence, Italy (A.M.V.). The study was funded by a grant from AIRC, “Special Program Molecular Clinical Oncology 5 × 1000,” to the AIRC-Gruppo Italiano Malattie Mieloproliferative (AGIMM). P. G. was supported by a grant from Societá Italiana Ematologia Sperinentale (SIES).
A detailed description of the AGIMM project is available at http://www.progettoagimm.it.
Authorship
Contribution: P.G. collected patient samples, performed research, and contributed to data analysis and manuscript writing; L.T. performed research and contributed to data analysis and manuscript writing; C.B. performed research and contributed to manuscript writing; I.I. performed and analyzed single-nucleotide polymorphism arrays; V.P. performed methylation analysis; G.M. analyzed and discussed single-nucleotide polymorphism array data; A.B. contributed patient samples and revised the manuscript; and A.M.V. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the AGIMM Investigators is available as an online supplemental Appendix.
Correspondence: Professor Alessandro M. Vannucchi, MD, Department of Hematology, University of Florence, Viale Morgagni 85, 50134 Florence, Italy; e-mail: amvannucchi@unifi.it.
References
Author notes
P.G. and L.T. contributed equally to this study.

![Figure 1. miR-16-2 is overexpressed in PV CD34+ cells and contributes to abnormal erythropoiesis. (A) Levels of miR-16 were measured during induced erythroid differentiation of control (black line) and PV (red line) CD34+ cells. A 2-phase liquid culture system was used in which erythropoietin (EPO) was added on day 6 of culture (phase 2). Data were normalized to the mean miR-16 level measured on day 0 in the CD34+ cells of each patient group, which comprised 7 PV patients and 5 healthy subjects, and were expressed as percent variation. **P < .01 or greater in PV vs control cells. (B) Levels of mature miR-16 were measured in CD34+ cells from PV (n = 75), essential thrombocythemia (ET; n = 10), or PMF (n = 25) patients by RTQ-PCR and normalized (2−ΔΔCT; expressed as relative quantity [RQ]) to control CD34+ cells (n = 10; upper and lower limits of control subjects are indicated by dashed lines). Also included were subjects with reactive erythrocytosis (RE; n = 3), hemolytic anemia (HA; n = 2), and low-risk myelodysplastic syndromes (MDS; n = 5); because all of these subjects had levels within the normal range, they have been grouped together in the plot. (C) PV CD34+ cells were transfected with siRNA against pre–miR-16-1 or pre–miR-16-2, and levels of mature miR-16 were measured 24 hours later; an aliquot of cells transfected with scramble (Scr) siRNA served as control. **P < .0001. (D) CD34+ cells from healthy subjects were transfected with pre–miR-16 (+) or scramble pre-miR (−) and plated in semisolid medium in the presence of an optimal cytokine cocktail that included EPO to permit the growth of erythroid (CFU-E, BFU-E) or myeloid (CFU–granulocyte macrophage [CFU-GM]) progenitors. Pre–miR-16 transfection rates were assessed by FACS analysis and were in the range of 60% to 70%; cultures were established with unsorted cells. Data shown were generated from 6 controls in 3 independent experiments. *P < .05; **P < .01. (E) The effect of pre–miR-16 transfection on the expression of erythroid differentiation markers was measured in a 2-phase liquid culture system that had been supplemented (“with”) or not (“w/o”) EPO on day 6. These cultures were established with pre–miR-16–transfected, unsorted, normal CD34+ cells (n = 5 subjects); transfection rates assessed by FACS analysis were in the range of 60% to 65%. Flow cytometric analysis for membrane erythroid marker expression was performed 4 days after EPO supplementation. *P < .05; **P < .01. (F) CD34+ cells from PV patients were transfected with siRNA against pre–miR-16-1 or pre–miR-16-2 and the growth of EECs, BFU-E, and CFU-GM was evaluated. **P < .01. (G) PV CD34+ cells were transfected with siRNA against pre–miR-16-1 or pre–miR-16-2, sorted by flow cytometry, and then plated for EEC growth. **P < .01. Representative images of EECs obtained by plating PV CD34+ cells that had been transfected with siRNA against pre–miR-16-1 or pre–miR-16-2 are presented on the right. Microphotographs were obtained with a Nikon Eclipse TS100 contrast-phase microscope, objective Nikon plan 40×/0.65.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/25/10.1182_blood-2010-09-306506/4/m_zh89991173150001.jpeg?Expires=1767773447&Signature=g3AbbRsp2LNaT3uQF8BIH7FXPT8o0SMKvrKvN96VoRoz-LWv1N1BmKB4~VfwY4l5uuZTSURepRr~QLxd1GLeC3mDGiTZDewMTw6r18yNSY00SdHUU71cES14n136CRyDlBYyrPT0~cpmz7a1FqyLU6dV1tCau8KiQ~JOu9OMNRwvTO-1b26MRF7bGBvB-~TykQ7Mrb8GtZtZ-sfrDL2jTBTIxvGg8N4VQ60xfuU-Pc4SNSCPrlvVWrAcUqMlaU22aNZ9gHjNzD0Iljx4KMXfDJ8vTV4AMKz3l-B8lLa59196BcAWR7ZUaDBLnthN2SQzkwoxrDwTlerfmuFXvWrkwA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal