Abstract
The activating mutations in JAK2 (including JAK2V617F) that have been described in patients with myeloproliferative neoplasms (MPNs) are linked directly to MPN pathogenesis. We developed R723, an orally bioavailable small molecule that inhibits JAK2 activity in vitro by 50% at a concentration of 2nM, while having minimal effects on JAK3, TYK2, and JAK1 activity. R723 inhibited cytokine-independent CFU-E growth and constitutive activation of STAT5 in primary hematopoietic cells expressing JAK2V617F. In an anemia mouse model induced by phenylhydrazine, R723 inhibited erythropoiesis. In a leukemia mouse model using Ba/F3 cells expressing JAK2V617F, R723 treatment prolonged survival and decreased tumor burden. In V617F-transgenic mice that closely mimic human primary myelofibrosis, R723 treatment improved survival, hepatosplenomegaly, leukocytosis, and thrombocytosis. R723 preferentially targeted the JAK2-dependent pathway rather than the JAK1- and JAK3-dependent pathways in vivo, and its effects on T and B lymphocytes were mild compared with its effects on myeloid cells. Our preclinical data indicate that R723 has a favorable safety profile and the potential to become an efficacious treatment for patients with JAK2V617F-positive MPNs.
Introduction
Myeloproliferative neoplasms (MPNs) are clonal hematologic diseases characterized by excess production of one or more lineages of mature blood cells, resulting in a predisposition to bleeding and thrombosis, extramedullary hematopoiesis (EMH), and a progression to acute leukemia.1 A somatic activating mutation encoding a valine to phenylalanine substitution at position 617 (V617F) in JAK2 was discovered as a common molecular marker in Philadelphia chromosome–negative MPNs.2-5 The incidence of the JAK2V617F mutation is found in > 90% of patients with polycythemia vera (PV) and affects approximately half of the patients with essential thrombocythemia (ET) and primary myelofibrosis (PMF). Expression of JAK2V617F in vivo in a murine BM transplantation assay resulted in erythrocytosis resembling PV.6-8 Moreover, we and others have reported that JAK2V617F-transgenic (V617F-TG) mice develop 3 kinds of MPNs: PV like, ET like, and PMF like.9-11 These studies support a critical role for JAK2V617F in the pathogenesis of the 3 types of MPNs.
Current therapy for MPNs12-14 is empirically derived and includes phlebotomy,15 hydroxyurea,16 IFN-α,17 anagrelide,16 and thalidomide18 and its analogs.19 Most patients are not candidates for stem-cell transplantation, which is the curative treatment for MPNs,20 given their advanced age at the time of diagnosis, considerably high ratio of transplantation-related mortality, and relatively indolent progression. Identification of specific JAK2 inhibitors appears to be an important step toward the development of targeted therapy for MPNs. Several groups of investigators have begun to develop specific, potent, orally bioavailable JAK2 inhibitors for the treatment of MPNs,21-24 and these compounds are currently undergoing clinical trials.25-27
In the present study, we report the development of R723, a selective small-molecule JAK2 inhibitor. We show that R723 is a potent inhibitor of JAK2V617F in cell-based assays. In 3 mouse models, R723 had significant in vivo activity against JAK2V617F, was well tolerated, and had a minimal impact on T- and B-cell numbers.
Methods
Materials
Cell lines, the JAK2V617F clone, and vectors are described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Identification of JAK2 inhibitors
To identify JAK2 inhibitors, we used a cell-based approach using murine leukemia Ba/F3 cells expressing JAK2V617F with the erythropoietin (EPO) receptor Ba/F3-EPOR-JAK2V617F. Initially, we screened a focused, diversified kinase inhibitor library to identify inhibitors of Ba/F3-EPOR-JAK2V617F cell proliferation. To avoid nonselective compounds targeting other members of the JAK family and molecules with general toxicity, we used 2 additional assays assessing the effects on IL-2–dependent proliferation of human primary T lymphocytes, CTLL-2 mouse T-cell leukemia cells, and JAK1-dependent Ba/F3 cells expressing JAK1V658F. The use of these counterscreen assays also allowed us to exclude the possibility of cell-type- and species-specific artifacts. R723 was obtained as a result of the systemic chemical modification of the initial hits, followed by testing of the resulting compounds in the assays mentioned above.
In vitro proliferation assays
The half-maximal inhibitory concentration (IC50) for the inhibition of proliferation of all the cell lines was determined using CellTiter-Glo Luminescent Cell Viability Assay reagent (Promega). For details, see supplemental Methods.
Cell-based STAT5 phosphorylation assay
Effects of R723 on STAT5 phosphorylation in human primary T cells and cell lines were determined by FACS. For details, see supplemental Methods.
Animals
Eight-week-old female BALB/c mice and 7-week-old female NOD/SCID mice (The Jackson Laboratory) were used in the induced hemolytic anemia model and PK-PD studies and in the Ba/F3-JAK2V617F leukemia study, respectively. Two lines of V617F-TG mice were established and analyzed as described previously.9 In this experiment, we used line 2 mice that showed the spectrum of clinicopathologic features of human PMF.9 Animal studies were performed in the United States in accordance with the Institutional Animal Care and Use Committee of Rigel Pharmaceuticals Inc, and in Japan in accordance with the Miyazaki University Ethics Committee.
Analysis of JAK2- and JAK1/JAK3-dependent STAT5 phosphorylation in primary cells
Wild-type (WT) mice (female BALB/c) were dosed orally with R723 at 50 or 100 mg/kg or vehicle, and blood was collected at 1 and 3 hours after treatment. After stimulation with either 10 ng/mL of GM-CSF for the assessment of JAK2 activity in granulocytes (Gr-1+) or 100 ng/mL of IL-15 for the assessment of JAK1/JAK3 activity in T cells (CD8+), the cells were fixed and permeabilized and then stained with Alexa Fluor 647–conjugated pSTAT5 and either PE-conjugated Gr-1 or peridinin chlorophyll A protein–cyanin 5.5–conjugated CD8. Samples were analyzed by FACS.
PHZ-induced hemolytic anemia model and Ba/F3-JAK2V617F engraftment mouse model
Phenylhydrazine (PHZ; Sigma-Aldrich) was administered at 50 mg/kg/d intraperitoneally for 2 consecutive days to deplete RBCs and to stimulate erythropoiesis. R723 formulated in 0.1% hydroxypropyl methylcellulose was administered by oral gavage starting the day after the final PHZ administration (day 2). R723 dosing continued through day 7 at 50 mg/kg or 75 mg/kg twice daily. Blood was collected on days 3, 6, and 8 to assess RBC number and hematocrit levels. The Ba/F3-JAK2V617F engraftment mouse model is described in supplemental Methods.
Murine MPN model and analysis of mice after R723 treatment
Twelve-week-old V617F-TG mice were treated by oral gavage twice daily with 35 mg/kg R723, 70 mg/kg R723, or vehicle for 16 weeks. In this study, a salt form of R723 was formulated in water to ease the formulation process. As a control for V617F-TG treated with vehicle, we prepared WT mice treated with vehicle. Differential blood counts were assessed by retro-orbital eye bleeds before study initiation, during the study, and at the end of the study. Mice were killed at the study end point. For pathologic examination, tissue samples were fixed in formalin, embedded in paraffin blocks, and sectioned for H&E staining or Gomori silver staining. FACS was performed as described previously.9
V617F-TG BM cell engraftment mouse model
BM cells (2 to 8 × 106) from CD45.2+ V617F-TG mice, together with 2 × 105 CD45.1+ WT BM cells, were injected into 9.5 Gy–irradiated recipient CD45.2+ WT mice. In our preliminary experiments, we injected 2 × 106 CD45.1 BM cells into lethally irradiated (9.5 Gy) CD45.2 recipients. Reconstitution of recipients with donor BM cells was monitored by assessing the frequency of CD45.1+/CD45.2+ cells in the peripheral blood (PB) after transplantation. Twelve weeks after the transplantation, donor-derived (CD45.1) cells had nearly completely replaced (99%) the recipient (CD45.2) cells in the PB. The remaining recipient cells in PB were 1%. Therefore, under the conditions of our cotransplantation experiments, nearly all of the CD45.2+ cells in the PB were derived from V617F-TG. Twenty weeks after BM transplantation, recipient mice were divided into R723 treatment or vehicle control groups. R723 was administered by oral gavage at 70 mg/kg twice daily for 4 weeks, whereas the control group received vehicle only. Complete blood counts and the ratio of CD45.1+ to CD45.2+ cells were monitored by FACS before and after treatment. All mice were killed at study end points and spleens were weighed.
Progenitor cell assays
Colony forming assays were performed as described previously9 (for details, see supplemental Methods). CFU-E colonies were counted on day 3 and other colonies were counted on day 7. To observe the effect of R723 on the proportion of progenitors, V617F-TG mice were dosed orally with R723 at 70 mg/kg or vehicle twice daily for 4 weeks. Lin−Sca-1−cKit+ (LSK), common myeloid progenitor (CMP), granulocyte-macrophage progenitor (GMP), erythromegakaryocyte progenitor (MEP), megakaryocyte progenitor (MKP), early erythroblast, and late erythroblast populations in BM cells were determined by FACS. Antibodies were used as indicated in supplemental Methods.
Statistical analysis
Results are presented as means ± SE. To assess the statistical significance between the 2 groups, the 2-tailed Student t test was used. Statistical analyses of R723-treated groups versus vehicle groups in survival studies were performed with the log-rank test.
Results
R723 is a potent selective JAK2 inhibitor
To identify JAK2 inhibitors, we used a cell-based approach using murine leukemia Ba/F3 cells expressing JAK2V617F with the EPO receptor Ba/F3-EPOR-JAK2V617F. R723 was obtained as a potent and selective inhibitor of Ba/F3-EPOR-JAK2V617F proliferation through a screening of a focused, diversified kinase inhibitor library and through the structure-activity relationship study on the initial hits from the screening. The chemical structure of R723 and the crystal structure of R723 complexed with JAK2 catalytic domain are shown in supplemental Figure 1 (US Patent Application Pub. No. 2009-0258864 A1, October 15, 2009). R723 strongly suppressed proliferation of Ba/F3-EPOR-JAK2V617F and 2 other cell lines dependent solely on JAK2V617F signaling for survival: UKE-1 and SET-2.28 R723 strongly diminished JAK2-dependent STAT5 phosphorylation in these cells (Table 1 and supplemental Figure 2A). R723 potently targeted WT JAK2 activity, as indicated by the inhibition of EPO-driven proliferation of human CD34+-derived erythroid progenitors. We then compared the ability of R723 to inhibit WT JAK2-dependent proliferation of Ba/F3-EPOR cells in the presence of EPO with that of JAK2V617F-dependent (and cytokine-independent) proliferation of Ba/F3-EPOR-JAK2V617F cells. Both cell lines were inhibited equally well by the compound, showing the lack of R723 selectivity between WT and mutant forms of the enzyme in cells (supplemental Figure 2B). This observation agrees with the in vitro kinase inhibition data, in which no differences between the 2 were found (supplemental Table 1).
R723 activity in cell-based assays
| Assay . | Target . | IC50, nM . |
|---|---|---|
| Ba/F3-EPOR-JAK2V617F proliferation | JAK2 V617F | 191 |
| UKE1 proliferation | JAK2 V617F | 168 |
| SET2 proliferation | JAK2 V617F | 139 |
| EPO-dependent human CD34+ progenitor proliferation | JAK2 | 124 |
| Ba/F3-EPOR-JAK2V617F pSTAT5 FACS | JAK2 V617F | 390 |
| SET2 pSTAT5 FACS | JAK2 V617F | 39 |
| IL-2 CTLL2 proliferation | JAK1/JAK3 | 528 |
| IL-2 human primary T-cell proliferation | JAK1/JAK3 | 1260 |
| IL-2 CTLL2 pSTAT5 FACS | JAK1/JAK3 | 2300 |
| IL-2 human primary T-cell pSTAT5 FACS | JAK1/JAK3 | 2700 |
| Ba/F3-JAK1V658F proliferation | JAK1 V658F | 885 |
| CMK proliferation | JAK3 A572V | 2340 |
| A549 proliferation | Multiple | 3680 |
| H1299 proliferation | Multiple | 4100 |
| IgE CHMC tryptase | Syk | 760 |
| Insulin HeLa pAKT in cell western | InsR | 10 800 |
| VEGF HUVEC pVEGFR in cell western | VEGFR | 3250 |
| Assay . | Target . | IC50, nM . |
|---|---|---|
| Ba/F3-EPOR-JAK2V617F proliferation | JAK2 V617F | 191 |
| UKE1 proliferation | JAK2 V617F | 168 |
| SET2 proliferation | JAK2 V617F | 139 |
| EPO-dependent human CD34+ progenitor proliferation | JAK2 | 124 |
| Ba/F3-EPOR-JAK2V617F pSTAT5 FACS | JAK2 V617F | 390 |
| SET2 pSTAT5 FACS | JAK2 V617F | 39 |
| IL-2 CTLL2 proliferation | JAK1/JAK3 | 528 |
| IL-2 human primary T-cell proliferation | JAK1/JAK3 | 1260 |
| IL-2 CTLL2 pSTAT5 FACS | JAK1/JAK3 | 2300 |
| IL-2 human primary T-cell pSTAT5 FACS | JAK1/JAK3 | 2700 |
| Ba/F3-JAK1V658F proliferation | JAK1 V658F | 885 |
| CMK proliferation | JAK3 A572V | 2340 |
| A549 proliferation | Multiple | 3680 |
| H1299 proliferation | Multiple | 4100 |
| IgE CHMC tryptase | Syk | 760 |
| Insulin HeLa pAKT in cell western | InsR | 10 800 |
| VEGF HUVEC pVEGFR in cell western | VEGFR | 3250 |
Characterization of R723 included a variety of cell-based assays probing multiple pathways (summarized in Table 1). The effects of R723 on the key off-target assays, IL-2–dependent proliferation and STAT5 phosphorylation via JAK1/JAK3 in human primary T lymphocytes and CTLL-2 cells, were significantly lower than the effects observed in the JAK2-dependent cell lines (Table 1). We also used a Ba/F3 cell line expressing the V658F mutant of JAK1 kinase (Ba/F3-JAK1V658F cells), which demonstrates IL3-independent but JAK1-dependent growth,29 and the CMK cell line, which is dependent on the constitutively active JAK3A572V mutant, for survival and proliferation assays.30 Both cell lines were weakly affected by R723. We observed moderate inhibition of Syk kinase activity, leading to a suppression of IgE-stimulated tryptase release in human mast cells. This effect, however, became pronounced only at a concentration higher than the one required for efficient inhibition of the JAK2-dependent pathway.
Biochemical selectivity of R723
As expected from the results of the cell-based assays, R723 was shown to be an extremely potent JAK2 inhibitor, with a biochemical IC50 of 2nM (Table 2). It had a nearly identical inhibition profile for both the WT and the V617F mutant of JAK2 in a 2-point in vitro kinase assay (supplemental Table 1). R723 demonstrated good selectivity against all other JAK family kinases when tested at a wide range of concentrations. Selectivity ratios for IC50 varied from 13-fold for JAK3 to a few hundred–fold for 2 other members of the family, JAK1 and Tyk2 (Table 2). The R723 selectivity was further confirmed by 2-point testing against a full panel of more than 200 enzymes. Only 13 other kinases cleared a threshold of 30% inhibition at 100nM, with the majority of them failing to show any discernable inhibition at 20nM, a concentration that is 10-fold higher than the JAK2 IC50 (supplemental Table 1). These results indicate that R723 is a highly potent and selective JAK2 inhibitor in vitro.
R723 selectively inhibits JAK2
| Kinase . | IC50, nM . | Selectivity over JAK2, -fold . |
|---|---|---|
| JAK2 | 2 | NA |
| JAK1 | 740 | 370 |
| JAK3 | 26 | 13 |
| TYK2 | 3950 | > 500 |
| VEGFR2 | 1400 | > 500 |
| Syk | 300 | 150 |
| PKCa | 3960 | > 500 |
| PKCb1 | 7990 | > 500 |
| PKCd | > 10 000 | > 500 |
| PKCe | > 10 000 | > 500 |
| PKCq | > 10 000 | > 500 |
| PLK1 | > 10 000 | > 500 |
| RET | 109 | 55 |
| Kinase . | IC50, nM . | Selectivity over JAK2, -fold . |
|---|---|---|
| JAK2 | 2 | NA |
| JAK1 | 740 | 370 |
| JAK3 | 26 | 13 |
| TYK2 | 3950 | > 500 |
| VEGFR2 | 1400 | > 500 |
| Syk | 300 | 150 |
| PKCa | 3960 | > 500 |
| PKCb1 | 7990 | > 500 |
| PKCd | > 10 000 | > 500 |
| PKCe | > 10 000 | > 500 |
| PKCq | > 10 000 | > 500 |
| PLK1 | > 10 000 | > 500 |
| RET | 109 | 55 |
R723 demonstrates selectivity in primary cells
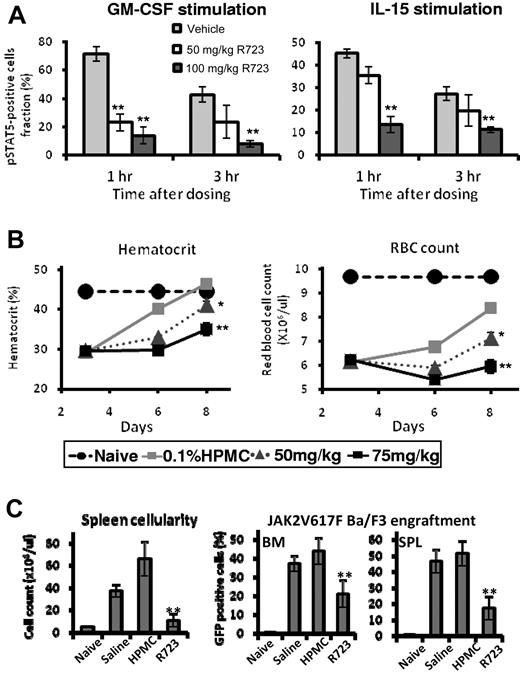
R723 is an orally bioavailable compound demonstrating excellent pharmacokinetic properties in mice, making it a good candidate for in vivo testing (supplemental Figure 3). To assess the effects of R723 on JAK2 and JAK1/JAK3 signaling in vivo, we measured STAT5 phosphorylation in whole blood obtained from R723-treated WT mice (female BALB/c) on ex vivo stimulation of either Gr-1+ granulocytes with GM-CSF or CD8+ T cells with IL-15, respectively. Whereas the GM-CSF–mediated signaling cascade relied exclusively on JAK2, IL-15–dependent stimulation of T cells required both JAK1 and JAK3 activities. Both 50 and 100 mg/kg doses of R723 induced strong inhibition of STAT5 phosphorylation after GM-CSF and, to a lesser extent, IL-15 stimulation, especially when a 50 mg/kg dose of R723 was administered (Figure 1A). We consistently observed a preference for targeting the JAK2-dependent GM-CSF pathway, especially at the 50 mg/kg dose 1 hour after dosing.
R723 shows selectivity and efficacy in mice. (A) Analysis of JAK2- and JAK1/JAK3-dependent STAT5 phosphorylation in primary cells. WT mice (female BALB/c) were orally dosed with vehicle, R723 50 mg/kg, or R723 100 mg/kg. Blood was collected at 1 and 3 hours after dosing and stimulated with either GM-CSF or IL-15. The percentage of pSTAT5-positive Gr-1+ cells with GM-CSF stimulation (left panel) and the percentage of pSTAT5-positive CD8+ cells with IL-15 stimulation (right panel) at each time point are shown. (B) R723 is efficacious in the hemolytic anemia mouse model. Hematocrit (left panel) and RBC count (right panel) changes were examined in mice administered PHZ on days 0 and 1 followed by oral daily administration of R723 or vehicle on days 2-7. Hematocrit and RBC counts of naive mice on day 3 were used as a baseline. (C) NOD/SCID mice injected with Ba/F3-JAK2V617F cells were administered with saline, vehicle (hydroxypropyl methylcellulose), or 50 mg/kg of R723 twice daily. Spleens and BM were harvested 13 days after cell injection. Cell counts per spleen (left panel) and percentage of GFP+ cells (Ba/F3-JAK2V617F) in BM and spleen cells (right panel) are shown. Data are presented as means ± SE. **P < .01; *P < .05.
R723 shows selectivity and efficacy in mice. (A) Analysis of JAK2- and JAK1/JAK3-dependent STAT5 phosphorylation in primary cells. WT mice (female BALB/c) were orally dosed with vehicle, R723 50 mg/kg, or R723 100 mg/kg. Blood was collected at 1 and 3 hours after dosing and stimulated with either GM-CSF or IL-15. The percentage of pSTAT5-positive Gr-1+ cells with GM-CSF stimulation (left panel) and the percentage of pSTAT5-positive CD8+ cells with IL-15 stimulation (right panel) at each time point are shown. (B) R723 is efficacious in the hemolytic anemia mouse model. Hematocrit (left panel) and RBC count (right panel) changes were examined in mice administered PHZ on days 0 and 1 followed by oral daily administration of R723 or vehicle on days 2-7. Hematocrit and RBC counts of naive mice on day 3 were used as a baseline. (C) NOD/SCID mice injected with Ba/F3-JAK2V617F cells were administered with saline, vehicle (hydroxypropyl methylcellulose), or 50 mg/kg of R723 twice daily. Spleens and BM were harvested 13 days after cell injection. Cell counts per spleen (left panel) and percentage of GFP+ cells (Ba/F3-JAK2V617F) in BM and spleen cells (right panel) are shown. Data are presented as means ± SE. **P < .01; *P < .05.
R723 inhibits erythropoiesis in a PHZ-induced hemolytic anemia model
To investigate the ability of R723 to inhibit JAK2 in vivo, we used the acute mouse model based on induction of anemia on PHZ treatment.31 PHZ-induced RBC damage and sequential depletion lead to hyperstimulation of normal and EMH accompanied by transient splenomegaly, followed by quick (7-10 days) recovery to normal hematocrit levels. As expected, 3 days after the first PHZ injection, both hematocrit and RBC values in all groups dropped to an average of 66% and 64% of naive controls, respectively. On days 6 and 8, however, progressive recovery was observed in vehicle control animals, whereas animals administered R723 showed a dose-dependent and significant (P < .05) delay in recovery (Figure 1B), with the 75 mg/kg twice daily dose producing the strongest effect on both parameters (P < .01).
R723 is effective in an acute Ba/F3-JAK2V617F leukemia model
We investigated the effect of R723 in a mouse leukemia model relying on the use of murine Ba/F3 cells expressing JAK2V617F as a driver of cell proliferation. This model is particularly aggressive, with a lethal outcome within 15 days of cell injection in vehicle-treated mice. In the R723-treated group, we observed a small (2 days, 13%) but highly significant improvement in survival (P < .004) compared with the vehicle-treated cohort (supplemental Figure 4). In another study, animals were killed on day 13, before the full disease onset, to evaluate the levels of green fluorescent protein–positive (GFP+) Ba/F3-JAK2V617F cells in the spleen and BM. We observed a significant decrease in spleen cellularity and in GFP+ cells in BM and spleen from R723-treated mice compared with those from untreated or vehicle-treated mice (Figure 1C).
In vitro growth inhibition of JAK2V617F-harboring hematopoietic cells by R723
We investigated the effect of R723 in a murine model of MPN induced by JAK2V617F. H2Kb promoter–controlled JAK2V617F-expressing mice (V617F-TG) show extreme leukocytosis, thrombocytosis, and progressive anemia,9 as well as hepatosplenomegaly with EMH, megakaryocytosis, and fibrosis in the BM. BM cells show constitutive activation of STAT5 and formation of cytokine-independent growth of CFU-E colonies (as is also seen in JAK2V617F-positive MPN patients) and exhibit high mortality compared with WT mice. These features of V617F-TG closely resemble PMF patients and their progression.
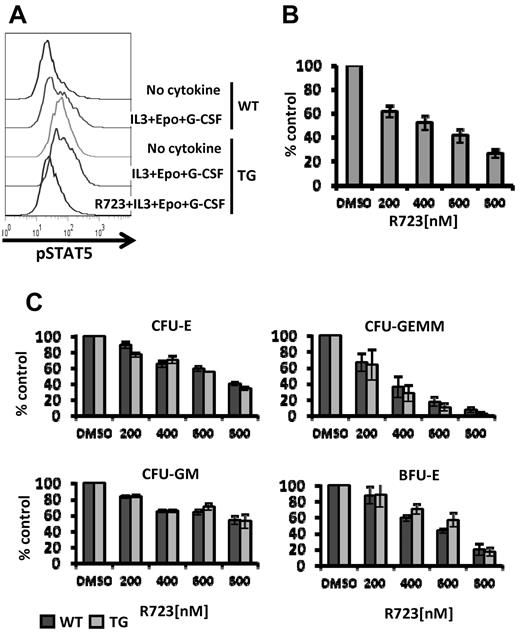
We also investigated the in vitro efficacy of R723 on WT and V617F-TG BM cells by assessing STAT5 activation in Mac-1/Gr-1+ cells. Cells were starved of growth factors for 4 hours and then stimulated with IL-3, EPO, and G-CSF. V617F-TG myeloid cells had more phosphorylated basal levels of STAT5 than WT myeloid cells, and after IL-3 + EPO + G-CSF stimulation, the degree of phosphorylation further increased. In vitro treatment of V617F-TG myeloid cells with R723 resulted in a marked decrease in phosphorylation of STAT5 (Figure 2A). These data indicate that R723 is capable of inhibiting activation of STAT5, the main effector of JAK-STAT signaling, in primary hematopoietic cells expressing JAK2V617F.
R723 inhibits JAK/STAT signaling and growth of JAK2V617F harboring hematopoietic cells. (A) BM cells from WT or V617F-TG mice were harvested and cultured without serum for 3 hours. Cells were incubated with vehicle or R723 for 1 hour, followed by the stimulation with IL-3, EPO, and G-CSF for 15 minutes. Mac-1/Gr-1+ myeloid cells were analyzed for levels of STAT5 phosphorylation by flow cytometry. One representative experiment of 3 is shown. (B) Effects of R723 on EPO-independent CFU-E colonies derived from V617F-TG. BM cells from V617F-TG were cultured in duplicate in methylcellulose culture medium in the absence of EPO with and without R723. The number of CFU-E colonies was counted on day 3. R723 treatment significantly suppressed CFU-E in V617F-TG. Three independent experiments were performed. Data are presented as means ± SE. (C) Effects of R723 on cytokine-dependent BM colonies derived from WT and V617F-TG mice. BM cells from WT and V617F-TG mice were cultured in cytokine-containing methylcellulose with and without R723. The number of CFU-E colonies was counted on day 3 (top left). The numbers of CFU-GEMM (top right), CFU-GM (bottom left), and BFU-E (bottom right) colonies were counted on day 7. R723 inhibited the cytokine-dependent colonies (CFU-E, CFU-GEMM, CFU-GM, and BFU-E) derived from both WT and V617F-TG cells to the same extent. Three independent experiments were performed, each using 1 different WT mouse and 1 different V617F-TG mouse. Data are presented as means ± SE.
R723 inhibits JAK/STAT signaling and growth of JAK2V617F harboring hematopoietic cells. (A) BM cells from WT or V617F-TG mice were harvested and cultured without serum for 3 hours. Cells were incubated with vehicle or R723 for 1 hour, followed by the stimulation with IL-3, EPO, and G-CSF for 15 minutes. Mac-1/Gr-1+ myeloid cells were analyzed for levels of STAT5 phosphorylation by flow cytometry. One representative experiment of 3 is shown. (B) Effects of R723 on EPO-independent CFU-E colonies derived from V617F-TG. BM cells from V617F-TG were cultured in duplicate in methylcellulose culture medium in the absence of EPO with and without R723. The number of CFU-E colonies was counted on day 3. R723 treatment significantly suppressed CFU-E in V617F-TG. Three independent experiments were performed. Data are presented as means ± SE. (C) Effects of R723 on cytokine-dependent BM colonies derived from WT and V617F-TG mice. BM cells from WT and V617F-TG mice were cultured in cytokine-containing methylcellulose with and without R723. The number of CFU-E colonies was counted on day 3 (top left). The numbers of CFU-GEMM (top right), CFU-GM (bottom left), and BFU-E (bottom right) colonies were counted on day 7. R723 inhibited the cytokine-dependent colonies (CFU-E, CFU-GEMM, CFU-GM, and BFU-E) derived from both WT and V617F-TG cells to the same extent. Three independent experiments were performed, each using 1 different WT mouse and 1 different V617F-TG mouse. Data are presented as means ± SE.
We next assessed the effect of R723 on EPO-independent colony formation. R723 inhibited EPO-independent CFU-E growth of BM cells from V617F-TG in a dose-dependent manner. A 5-fold reduction at 800nM R723 was observed (Figure 2B). Cytokine-dependent colony formation (CFU-E, CFU-GEMM, CFU-GM, and BFU-E) was also inhibited by the presence of R723. In all colony types, R723 inhibited colony growth of both V617F-TG and WT cells at the same level (Figure 2C). This agrees reasonably well with the results of a 2-point in vitro kinase assay in which R723 showed an identical inhibition profile for both WT and the V617F mutant of JAK2 (supplemental Table 1).
R723 effectively improves survival, leukocytosis, and EMH in JAK2V617F-induced murine MPN
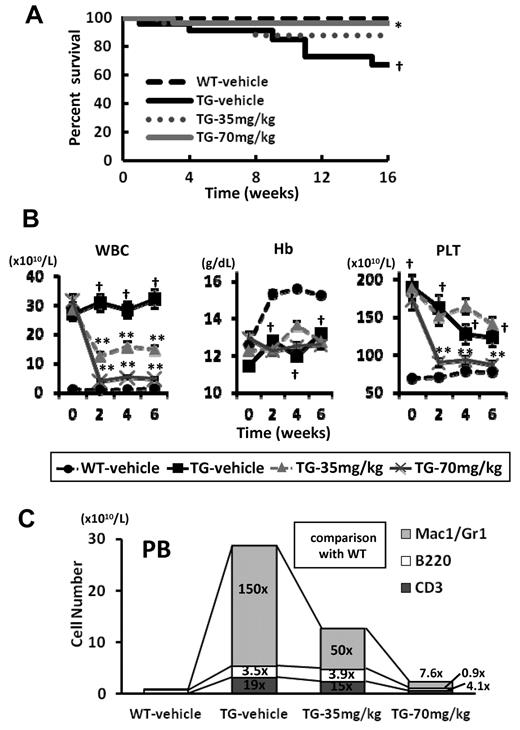
At 12 weeks of age, all V617F-TG mice developed MPN exhibiting leukocytosis, with average white blood cell counts of 29 × 1010/L. V617F-TG mice were divided into 3 groups and treated with R723 by oral gavage at 35 mg/kg twice daily (TG-35mg/kg; n = 30), 70 mg/kg twice daily (TG-70mg/kg; n = 26), or vehicle twice daily (TG-vehicle; n = 23) for 16 weeks. As a control for TG-vehicle, we prepared WT mice treated with vehicle (WT-vehicle; n = 30). During the study, 6 of 23 in the TG-vehicle group and 3 of 30 in the TG-35mg/kg group died, whereas only 1 of 26 mice in the TG-70mg/kg group died (Figure 3A). Therefore, administration of R723 at 70 mg/kg twice daily significantly improved the survival of V617F-TG mice (P < .05).
Survival and changes of PB of V617F-TG mice treated with R723. (A) Kaplan-Meier plot of WT mice treated with vehicle (WT-vehicle) and V617F-TG mice treated with vehicle (TG-vehicle), 35 mg/kg of R723 (TG-35mg/kg), or 70 mg/kg of R723 (TG-70mg/kg) for 16 weeks. There was a statistical difference in survival between the TG-vehicle group and the WT-vehicle group (†P < .01) and between the TG-70mg/kg group and the TG-vehicle group (*P < .05 by log-rank test). (B) Differential blood counts in WT-vehicle and TG-vehicle, TG-35mg/kg, or TG-70mg/kg treated with R723 for 6 weeks. V617F-TG mice showed severe leukocytosis and thrombocytosis at 12 weeks of age compared with age-matched WT mice (†P < .01). The leukocyte and platelet count in V617F-TG mice treated with R723 was significantly reduced compared with the TG-vehicle group (**P < .01). V617F-TG mice had anemia (†P < .01) that was not improved by R723 treatment. Data are presented as means ± SE. (C) Hematopoietic compartment of PB assessed by flow cytometry. At 18 weeks of age in the TG-vehicle group, the Mac-1/Gr-1+ myeloid cells were significantly increased in number and the B220+ B cells and CD3+ T cells were increased to a lesser extent than myeloid cells. R723 treatment for 6 weeks significantly decreased the number of Mac-1/Gr-1+ myeloid cells (P < .01) and mildly decreased the number of B220+ B cells and CD3+ T cells (P < .05). Data are means of 6 mice in each group.
Survival and changes of PB of V617F-TG mice treated with R723. (A) Kaplan-Meier plot of WT mice treated with vehicle (WT-vehicle) and V617F-TG mice treated with vehicle (TG-vehicle), 35 mg/kg of R723 (TG-35mg/kg), or 70 mg/kg of R723 (TG-70mg/kg) for 16 weeks. There was a statistical difference in survival between the TG-vehicle group and the WT-vehicle group (†P < .01) and between the TG-70mg/kg group and the TG-vehicle group (*P < .05 by log-rank test). (B) Differential blood counts in WT-vehicle and TG-vehicle, TG-35mg/kg, or TG-70mg/kg treated with R723 for 6 weeks. V617F-TG mice showed severe leukocytosis and thrombocytosis at 12 weeks of age compared with age-matched WT mice (†P < .01). The leukocyte and platelet count in V617F-TG mice treated with R723 was significantly reduced compared with the TG-vehicle group (**P < .01). V617F-TG mice had anemia (†P < .01) that was not improved by R723 treatment. Data are presented as means ± SE. (C) Hematopoietic compartment of PB assessed by flow cytometry. At 18 weeks of age in the TG-vehicle group, the Mac-1/Gr-1+ myeloid cells were significantly increased in number and the B220+ B cells and CD3+ T cells were increased to a lesser extent than myeloid cells. R723 treatment for 6 weeks significantly decreased the number of Mac-1/Gr-1+ myeloid cells (P < .01) and mildly decreased the number of B220+ B cells and CD3+ T cells (P < .05). Data are means of 6 mice in each group.
V617F-TG mice at 12 weeks of age had severe leukocytosis (Figure 3B). After 2 weeks of R723 treatment, the leukocyte count was reduced to 45% in the TG-35mg/kg group (P < .01) and to 13% in the TG-70mg/kg group (P < .01) compared with the TG-vehicle group, and the effect was maintained until the end of the study (Figure 3B). In V617F-TG mice, not only Mac-1/Gr-1+ myeloid cells, but also B220+ B cells and CD3+ T cells increased in number. Compared with the numbers in WT, the numbers of myeloid cells, B cells, and T cells were increased by 150-fold, 3.5-fold, and 19-fold, respectively (Figure 3C). After 6 weeks of 70 mg/kg R723 treatment in V617F-TG mice, the number of PB myeloid cells, B cells, and T cells reached 7.6-fold, 0.9-fold, and 4.1-fold, respectively, compared with that in WT (Figure 3C). Although R723 treatment reduced the number of all types of PB cells in a dose-dependent manner, the reduction of myeloid cell number was the most significant (P < .01): to less than one-tenth of that of the TG-vehicle group. A reduction in platelet count of 48% was observed in the TG-70mg/kg group (P < .01) compared with the TG-vehicle group (Figure 3B). Because platelet numbers in the TG-vehicle group were reduced gradually in the natural disease course, the difference between the 2 groups disappeared after 9 weeks of treatment. In contrast to the effect on leukocyte and platelet numbers, there was no improvement in anemia in V617F-TG mice treated with R723 (Figure 3B).
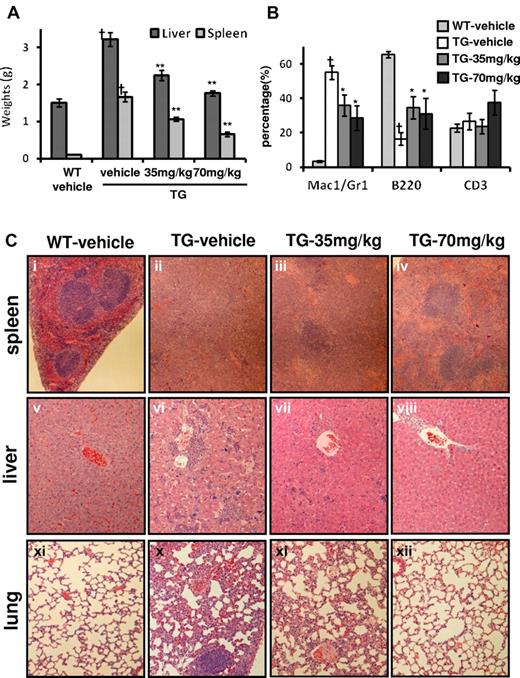
R723 treatment also improved hepatosplenomegaly in V617F-TG mice in a dose-dependent manner (Figure 4A). In the spleen, Mac-1/Gr-1+ myeloid cells associated with EMH were significantly decreased, and B220+ B cells were relatively increased by R723 treatment (Figure 4B). Along with reduction of organ weights and infiltrating myeloid cells, there was also clear evidence of a dose-dependent reduction in histopathology of EMH in the spleen, liver, and lungs from R723-treated V617F-TG mice (Figure 4C). Histopathological analysis of spleens from the TG-vehicle group exhibited complete effacement of normal splenic architecture and invasion of myeloid cells, whereas R723 treatment resulted in a marked reduction of cell invasion and restored architecture in V617F-TG spleens. Changes in the liver and lungs were more dramatic. EMH consisting of infiltrates of maturing myeloid cells and megakaryocytes seen in the TG-vehicle group were reduced in a dose-dependent manner by R723 treatment and almost completely disappeared in the TG-70mg/kg group (Figure 4C). In contrast to the drastic pathological improvement in spleen, liver, and lung (Figure 4C), R723 had little effect on the progression of fibrosis and megakaryocyte hyperplasia in BM (data not shown).
Improvement of hepatosplenomegaly and EMH in V617F-TG mice treated with R723 for 6 weeks. (A) R723 effects on liver and spleen weights after R723 treatment for 6 weeks. Liver and spleen weights in V617F-TG mice treated with vehicle (TG-vehicle) were increased compared with those in WT mice treated with vehicle (WT-vehicle) (†P < .01). R723 treatment in V617F-TG mice significantly reduced organ weights (**P < .01). Data are presented as means ± SE. (B) Hematopoietic compartment of spleen assessed by FACS. The proportion of Mac-1/Gr-1+ myeloid cells significantly increased, and that of B220+ B cells decreased in 18-week-old TG-vehicle mice compared with age-matched WT-vehicle mice (†P < .01). R723 treatment in V617F-TG mice for 6 weeks decreased the proportion of Mac-1/Gr-1+ myeloid cells and increased the proportion of B220+ B cells (*P < .05). The percentage of CD3+ T cells was constant. Data are presented as means ± SE. (C) Histological changes in V617F-TG mice by R723 treatment for 6 weeks. Histology of WT-vehicle and V617F-TG mice treated with vehicle, 35 mg/kg, and 70 mg/kg doses of R723 (TG-vehicle, TG-35mg/kg, TG-70mg/kg, respectively). Cells were stained with H&E. In the spleens of the TG-vehicle mice, the red pulp was expanded with maturing myeloid cells and megakaryocytes and the white pulp was scarce compared with WT-vehicle mice (i-ii). Mice treated with R723 for 6 weeks showed marked reduction of myeloid cell invasion and partially restored architecture (iii-iv). Liver and lung sections from TG-vehicle also displayed EMH (vi and x). Infiltration of myeloid cells disappeared with R723 treatment in V617F-TG mice (vii,viii,xi,xii).
Improvement of hepatosplenomegaly and EMH in V617F-TG mice treated with R723 for 6 weeks. (A) R723 effects on liver and spleen weights after R723 treatment for 6 weeks. Liver and spleen weights in V617F-TG mice treated with vehicle (TG-vehicle) were increased compared with those in WT mice treated with vehicle (WT-vehicle) (†P < .01). R723 treatment in V617F-TG mice significantly reduced organ weights (**P < .01). Data are presented as means ± SE. (B) Hematopoietic compartment of spleen assessed by FACS. The proportion of Mac-1/Gr-1+ myeloid cells significantly increased, and that of B220+ B cells decreased in 18-week-old TG-vehicle mice compared with age-matched WT-vehicle mice (†P < .01). R723 treatment in V617F-TG mice for 6 weeks decreased the proportion of Mac-1/Gr-1+ myeloid cells and increased the proportion of B220+ B cells (*P < .05). The percentage of CD3+ T cells was constant. Data are presented as means ± SE. (C) Histological changes in V617F-TG mice by R723 treatment for 6 weeks. Histology of WT-vehicle and V617F-TG mice treated with vehicle, 35 mg/kg, and 70 mg/kg doses of R723 (TG-vehicle, TG-35mg/kg, TG-70mg/kg, respectively). Cells were stained with H&E. In the spleens of the TG-vehicle mice, the red pulp was expanded with maturing myeloid cells and megakaryocytes and the white pulp was scarce compared with WT-vehicle mice (i-ii). Mice treated with R723 for 6 weeks showed marked reduction of myeloid cell invasion and partially restored architecture (iii-iv). Liver and lung sections from TG-vehicle also displayed EMH (vi and x). Infiltration of myeloid cells disappeared with R723 treatment in V617F-TG mice (vii,viii,xi,xii).
We also evaluated the histopathological toxicity of R723 in the brain, heart, kidney, ovary, testes, and gastrointestinal tract. There were no signs of toxicity related to R723 in any tissue examined at either the 35 mg/kg or the 70 mg/kg dose.
Transplantation of BM cells from V617F-TG causes MPN in WT recipient mice. These mice exhibited granulocytosis, splenomegaly, EMH, and mild BM fibrosis. CD45.2+ BM cells from V617F-TG mice were injected into irradiated recipient WT mice, together with CD45.1+ WT BM cells. Twenty weeks after BM transplantation, we administrated R723 or vehicle by oral gavage at 70 mg/kg twice daily for 4 weeks. As shown in Table 3, R723 treatment decreased the number of leukocytes, especially in Mac-1+ or Gr-1+ myeloid cells, in recipient mice. The number of B or T lymphocytes in the PB was not affected by R723 treatment, nor was the hemoglobin value or platelet number. In recipient mice, the number of CD45.2+ V617F-TG-derived cells decreased, whereas CD45.1+ WT-derived cells remained unchanged by R723 treatment, indicating that R723 selectively inhibited cells harboring V617F JAK2. A 5-fold reduction in spleen weights in R723-treated recipient mice compared with the vehicle-treated recipient mice was also observed. R723 treatment caused a significant reduction of BM fibrosis (supplemental Figure 5), although the degree of myelofibrosis severity in recipient mice was much milder than that in V617F-TG mice.
In vivo effect of R723 on JAK2 V617F–harboring cells
| . | Vehicle (n = 5) . | R723 (n = 5) . | ||
|---|---|---|---|---|
| Pretreatment . | Posttreatment . | Pretreatment . | Posttreatment . | |
| White blood cells, ×103/μL | 19.8 (10.8-28.5) | 19.8 (10.2-31.8) | 15.0 (11.7-25.2) | 10.8 (8.1-15.3)* |
| Mac-1+ or Gr-1+ myeloid, ×103/μL | 10.7 (3.2-19.2) | 11.4 (3.7-22.3) | 7.9 (6.4-20.2) | 4.6 (3.1-9.2)* |
| B220+ B cells, ×103/μL | 2.1 (0.4-9.6) | 2.0 (0.4-9.1) | 1.9 (0.8-5.1) | 2.5 (0.8-3.6) |
| CD4+ or CD8+ T cells, ×103/μL | 4.0 (3.0-7.2) | 2.8 (2.7-7.5) | 4.2 (3.4-4.7) | 3.6 (3.1-4.6) |
| CD45.1+ cells, ×103/μL | 1.5 (0.8-12.3) | 1.6 (0.7-11.2) | 1.5 (0.7-8.1) | 1.3 (0.7-4.4) |
| CD45.2+ cells, ×103/μL | 12.6 (2.6-27.7) | 13.0 (2.2-31.1) | 11.1 (6.9-24.5) | 7.4 (4.3-14.4)* |
| Hemoglobin, g/dL | 11.3 (11.2-13.9) | 11.1 (10.7-14) | 12.5 (11.2-14.5) | 12.9 (10.7-14.3) |
| Platelets, ×1010/L | 61.9 (50.8-81) | 53.2 (14-92.5) | 70.8 (52-119) | 106 (57.2-137) |
| Spleen weight, g | n.d. | 0.20 ± 0.04 | n.d. | 0.04 ± 0.01† |
| . | Vehicle (n = 5) . | R723 (n = 5) . | ||
|---|---|---|---|---|
| Pretreatment . | Posttreatment . | Pretreatment . | Posttreatment . | |
| White blood cells, ×103/μL | 19.8 (10.8-28.5) | 19.8 (10.2-31.8) | 15.0 (11.7-25.2) | 10.8 (8.1-15.3)* |
| Mac-1+ or Gr-1+ myeloid, ×103/μL | 10.7 (3.2-19.2) | 11.4 (3.7-22.3) | 7.9 (6.4-20.2) | 4.6 (3.1-9.2)* |
| B220+ B cells, ×103/μL | 2.1 (0.4-9.6) | 2.0 (0.4-9.1) | 1.9 (0.8-5.1) | 2.5 (0.8-3.6) |
| CD4+ or CD8+ T cells, ×103/μL | 4.0 (3.0-7.2) | 2.8 (2.7-7.5) | 4.2 (3.4-4.7) | 3.6 (3.1-4.6) |
| CD45.1+ cells, ×103/μL | 1.5 (0.8-12.3) | 1.6 (0.7-11.2) | 1.5 (0.7-8.1) | 1.3 (0.7-4.4) |
| CD45.2+ cells, ×103/μL | 12.6 (2.6-27.7) | 13.0 (2.2-31.1) | 11.1 (6.9-24.5) | 7.4 (4.3-14.4)* |
| Hemoglobin, g/dL | 11.3 (11.2-13.9) | 11.1 (10.7-14) | 12.5 (11.2-14.5) | 12.9 (10.7-14.3) |
| Platelets, ×1010/L | 61.9 (50.8-81) | 53.2 (14-92.5) | 70.8 (52-119) | 106 (57.2-137) |
| Spleen weight, g | n.d. | 0.20 ± 0.04 | n.d. | 0.04 ± 0.01† |
Peripheral blood data are presented as medians (range). Spleen weights are presented as means ± SE. The paired data between pretreatment and posttreatment were analyzed with a paired 2-tailed t test. The comparison of spleen weights between vehicle-treated mice and R723-treated mice were analyzed with a 2-tailed t test.
n.d. indicates no data.
P < .05.
P < .01.
The effect of R723 on BM progenitor cells in V617F-TG mice
FACS was performed on BM cells in V617F-TG mice treated with R723 or vehicle. In V617F-TG mice, the proportion of LSK and GMP cells increased (P < .01) and the proportions of CMP, MEP, and MKP cells were comparable to those of the WT mice. Four weeks of R723 treatment in V617F-TG mice normalized the proportion of GMP cells (P < .05). MEP and MKP cells were increased compared with the WT or V617F-TG mice treated with vehicle (P < .01; Figure 5A and supplemental Table 2). Conversely, the LSK cell population was not changed by 4 weeks of R723 treatment. The proportion of BM progenitors was also analyzed by colony formation assay. CFU-GEMM and CFU-GM colonies were increased in number in the BM from the TG-vehicle group compared with that from the WT-vehicle group. The suppression of CFU-GM was observed in V617F-TG by R723 treatment (Figure 5B).
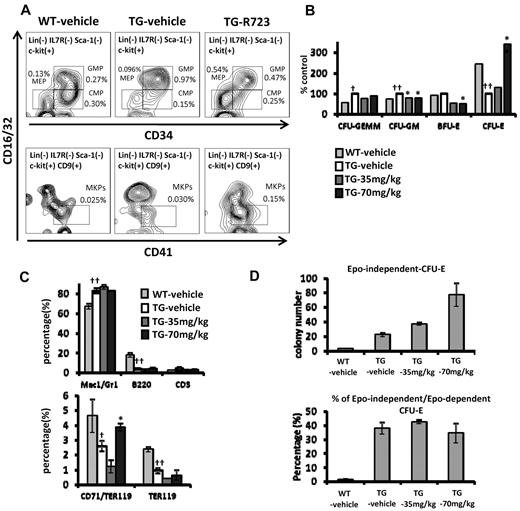
Effect of R723 on BM of V617F-TG mice. (A) BM progenitors in V617F-TG mice and effect of in vivo R723 treatment. V617F-TG mice were dosed orally with R723 at 70 mg/kg or vehicle for 4 weeks. BM cells were collected, and the proportion of CMPs (Lin−Sca-1−cKit+CD34+ FcγRlo), GMPs (Lin−Sca-1−cKit+CD34+FcγRhi), MEPs (Lin−Sca-1−cKit+CD34−FcγR−/lo), and MKPs (Lin−cKit+CD9+FcγRlo CD41+) was determined by FACS. In V617F-TG mice, the proportion of GMPs increased, and those of CMPs, MEPs, and MKPs were comparable to those in WT mice. Four weeks of R723 treatment in V617F-TG mice normalized the proportion of GMPs. MEPs and MKPs were increased compared with WT mice or V617F-TG mice treated with vehicle. These results are representative of 6 independent experiments. (B) Changes in BM progenitor cells after R723 treatment. The numbers of CFU-GEMM and CFU-GM colonies increased and the numbers of CFU-E colonies decreased in the BM from 18-week-old TG-vehicle mice compared with those in the BM from the age-matched WT-vehicle mice (†P < .05; ‡P < .01). R723 treatment for 6 weeks suppressed CFU-GM and BFU-E colonies (*P < .05). In contrast, CFU-E colonies increased in V617F-TG mice after R723 treatment (**P < .01). Data are presented as means ± SE. (C) FACS analysis of BM cells. The percentage of Mac-1/Gr-1+ myeloid cells increased and that of B220+ B cells decreased in 18-week-old V617F-TG mice treated with vehicle (TG-vehicle) compared with the age-matched WT mice treated with vehicle (WT-vehicle) (‡P < .01). R723 treatment for 6 weeks had little effect on the proportion of BM myeloid or B cells in V617F-TG mice (top panel). The proportion of CD71/TER119 double-positive erythroblasts and TER119 single-positive erythroblasts decreased in TG-vehicle mice compared with that in WT-vehicle mice (†P < .05; ‡P < .01). Significant restoration of CD71/TER119-positive erythroblasts was observed in V617F-TG mice after R723 treatment for 6 weeks (*P < .05). TER119 single-positive erythroblasts were not restored by R723 treatment (bottom panel). Data are presented as means ± SE. (D) Changes in EPO-independent CFU-E colonies after in vivo R723 treatment. The number of EPO-independent CFU-E colonies increased in BM cells from 18-week-old TG-vehicle mice compared with that in BM cells from the age-matched WT-vehicle mice (†P < .01). In vivo R723 treatment for 6 weeks increased the number of EPO-independent CFU-E colonies (*P < .01). Data are presented as means ± SE. Whereas EPO-dependent CFU-E colonies increased in V617F-TG mice after in vivo R723 treatment (panel B), the proportion of EPO-independent/EPO-dependent CFU-E colonies remained the same.
Effect of R723 on BM of V617F-TG mice. (A) BM progenitors in V617F-TG mice and effect of in vivo R723 treatment. V617F-TG mice were dosed orally with R723 at 70 mg/kg or vehicle for 4 weeks. BM cells were collected, and the proportion of CMPs (Lin−Sca-1−cKit+CD34+ FcγRlo), GMPs (Lin−Sca-1−cKit+CD34+FcγRhi), MEPs (Lin−Sca-1−cKit+CD34−FcγR−/lo), and MKPs (Lin−cKit+CD9+FcγRlo CD41+) was determined by FACS. In V617F-TG mice, the proportion of GMPs increased, and those of CMPs, MEPs, and MKPs were comparable to those in WT mice. Four weeks of R723 treatment in V617F-TG mice normalized the proportion of GMPs. MEPs and MKPs were increased compared with WT mice or V617F-TG mice treated with vehicle. These results are representative of 6 independent experiments. (B) Changes in BM progenitor cells after R723 treatment. The numbers of CFU-GEMM and CFU-GM colonies increased and the numbers of CFU-E colonies decreased in the BM from 18-week-old TG-vehicle mice compared with those in the BM from the age-matched WT-vehicle mice (†P < .05; ‡P < .01). R723 treatment for 6 weeks suppressed CFU-GM and BFU-E colonies (*P < .05). In contrast, CFU-E colonies increased in V617F-TG mice after R723 treatment (**P < .01). Data are presented as means ± SE. (C) FACS analysis of BM cells. The percentage of Mac-1/Gr-1+ myeloid cells increased and that of B220+ B cells decreased in 18-week-old V617F-TG mice treated with vehicle (TG-vehicle) compared with the age-matched WT mice treated with vehicle (WT-vehicle) (‡P < .01). R723 treatment for 6 weeks had little effect on the proportion of BM myeloid or B cells in V617F-TG mice (top panel). The proportion of CD71/TER119 double-positive erythroblasts and TER119 single-positive erythroblasts decreased in TG-vehicle mice compared with that in WT-vehicle mice (†P < .05; ‡P < .01). Significant restoration of CD71/TER119-positive erythroblasts was observed in V617F-TG mice after R723 treatment for 6 weeks (*P < .05). TER119 single-positive erythroblasts were not restored by R723 treatment (bottom panel). Data are presented as means ± SE. (D) Changes in EPO-independent CFU-E colonies after in vivo R723 treatment. The number of EPO-independent CFU-E colonies increased in BM cells from 18-week-old TG-vehicle mice compared with that in BM cells from the age-matched WT-vehicle mice (†P < .01). In vivo R723 treatment for 6 weeks increased the number of EPO-independent CFU-E colonies (*P < .01). Data are presented as means ± SE. Whereas EPO-dependent CFU-E colonies increased in V617F-TG mice after in vivo R723 treatment (panel B), the proportion of EPO-independent/EPO-dependent CFU-E colonies remained the same.
Mac-1/Gr-1+ myeloid cells in the BM of the TG-vehicle group were increased, and B220+ B cells were decreased compared with that of the WT-vehicle group (Figure 5C). R723 treatment had little effect on the proportion of BM myeloid and B cells in V617F-TG mice.
We then extended our analysis to erythroid-lineage cells. The proportions of CD71/TER119 double-positive early erythroblasts and TER119 single-positive late erythroblasts were decreased in the TG-vehicle group compared with those in the WT-vehicle group (Figure 5C and supplemental Table 2). Significant restoration of CD71/TER119 double-positive early erythroblasts was observed in the TG-70mg/kg group, but TER119 single-positive late erythroblasts were not restored by R723 treatment. CFU-E colonies were decreased in number in the BM from the TG-vehicle group compared with that in the WT-vehicle group. The suppression of BFU-E was observed in V617F-TG mice by R723 treatment; however, the number of CFU-E colonies increased in the TG-70mg/kg group compared with that in the TG-vehicle group (Figure 5B). The number of EPO-independent CFU-E colonies was increased in the V617F-TG group by in vivo R723 treatment compared with that in the TG-vehicle group (Figure 5D). Whereas the number of EPO-dependent CFU-E colonies was increased in V617F-TG mice by in vivo R723 treatment, the proportion of EPO-independent/EPO-dependent CFU-E colonies remained the same.
Discussion
In the present study, R723, a potent, orally bioavailable JAK2-selective inhibitor, was shown to have significant in vitro activity against JAK2V617F. In addition, R723 demonstrated good efficacy and low toxicity in vivo in 3 animal models. Our data indicate that R723 has the potential for effective treatment of V617F-positive MPNs.
R723 was equipotent against both the WT and the V617F mutant-type of JAK2 in biochemical assays and, correspondingly, R723 equally inhibited colony formation of both V617F-TG and WT BM cells. In the PHZ-induced anemia model, R723 was able to significantly delay EPO-driven hematocrit recovery from chemical insult, indicating its in vivo ability to inhibit WT JAK2 kinase in the context of EPO receptor pathway overstimulation. R723 was also effective in 2 JAK2V617F-driven animal models, the Ba/F3-JAK2V617F leukemia model, and in V617F-TG mice. In the aggressive murine leukemia model using Ba/F3-JAK2V617F cells, R723 had clear beneficial effects on disease progression by suppressing the proliferation of tumor cells, which led to an incremental improvement in survival and a decrease in the overall tumor burden in the spleen and BM, although the blood of the R723-treated mice did not inhibit phosphorylation of STAT5 in Ba/F3-JAK2V617F cells completely, only by 30%-40% (supplemental Figure 4 and 1C). The ultimate confirmation of R723 efficacy came from testing it in V617F-TG mice with features closely resembling those seen with human PMF,9 which is often poorly controlled with conventional therapy and the most appropriate target of a new therapeutic approach using a JAK2 inhibitor. V617F-TG mice have shorter lives than WT mice. In our study, the animals died suddenly, precluding detailed histopathologic analysis. Among a small cohort of V617F-TG mice in which limited necropsy was performed, subcutaneous and intestinal hemorrhage and gangrenous bowels were observed, which might have been caused by abdominal vessel obstruction. In addition, the bleeding time of V617F-TG mice was extremely prolonged compared with that of WT mice (data not shown). We therefore speculated that the cause of death was thrombosis and/or hemorrhage. Mullally et al32 and Akada et al33 also reported that thrombotic events might be the cause of V617F-expressing mice. Oral administration of R723 in V617F-TG mice led to the prolongation of survival and profound improvements in leukocytosis, thrombocytosis, and hepatosplenomegaly. These results raise hope for improvements in the treatment of PMF patients. Conversely, anemia, megakaryocyte hyperplasia, and fibrosis in BM were little affected. The effect of R723 on BM fibrosis varied greatly between models. It was not dissolved in V617F-TG mice during R723 treatment, and instead became just as advanced as it was in V617F-TG mice treated with vehicle. R723 treatment caused a significant reduction of myelofibrosis in recipient mice transplanted with V617F-TG BM cells together with WT BM cells (supplemental Figure 5), but the reason for this is not yet clear. Severe progression of myelofibrosis might be resistant to JAK2 inhibition, because the degree of myelofibrosis severity in recipient mice was much milder than that in V617F-TG mice. Interestingly, this outcome closely resembled the results of human clinical trials of other JAK2 inhibitors, in which the main improvements were limited to rapid decreases in spleen size and normalization of leukocyte count without resolution of fibrosis and only marginal improvements in transfusion dependence.25,26 R723 also has effects in recipient mice transplanted with BM cells from V617F-TG mice. In this transplantation model, R723 seemed to prefer inhibiting the growth of cells harboring V617F to WT cells, although R723 had a nearly identical inhibition profile for both WT and V617F mutant JAK2 in the in vitro kinase assay and equally inhibited colony formation of both WT and V617F-TG BM cells.
The JAK2V617F mutation is observed in the majority of PV patients and in approximately half of PMF patients. The effect of the JAK2 mutation on erythropoiesis differs from disease to disease. PV is characterized by erythrocytosis, whereas the main feature of PMF is progressive anemia. The situation was similar in our mouse model. We previously reported 2 lines of V617F-transgenic mice: line 1 transgenic mice exhibiting PV- or ET-resembling features and line 2 transgenic mice (this line was used to evaluate the effect of R723) exhibiting PMF-like diseases such as BM fibrosis, leukoerythroblastosis, and anemia. JAK2V617F knock-in mice resembling human PV were described recently.32 The reason that erythrocytosis or anemia resulted from the same JAK2 mutation both in human diseases and mice models is not clear. We found that some genes were specifically expressed in line 2 transgenic mice (data not shown), and now characterize their roles. These proteins may cooperate with JAK2V617F to influence erythropoiesis.
The effect of R723 on cell components seems to differ by cell lineage. As mentioned previously, R723 did not improve anemia but did effectively improve leukocytosis and thrombocytosis in V617F-TG mice. The frequency of CFU-E and the CD71/TER119 double-positive cells, which represent late erythroid progenitors and erythroblasts,34 respectively, decreased in V617F-TG mice compared with WT mice, and R723 treatment elevated these erythroid progenitors to levels nearly equal to that of WT mice. This improvement was probably due to the ability of R723 to inhibit the abnormal JAK2V617F signal. However, TER119 single-positive cells, which represent late erythroblasts, were still low in V617F-TG mice after R723 treatment. The generation of committed erythroid progenitors did not require EPO, but the terminal maturation from CD71/TER119 double-positive cells to TER119 single-positive cells did require EPO,35 indicating that differentiation depends on JAK2 activation. Because R723 inhibits WT JAK2 as well as JAK2V617F, the inhibition of WT JAK2 by R723 might abrogate the terminal differentiation of erythroblasts to erythrocytes, and this might be the reason that anemia was not improved in V617F-TG mice treated with R723. JAK2V617F-selective inhibitors, which do not inhibit WT JAK2, might overcome this problem.
There are JAK2V617F-positive disease-initiating cells that have long-term, multipotent, and self-renewing activity in the hematopoietic stem cell compartment.36,37 Mullually et al32 reported that the JAK2V617F mutation had nominal effects on the size or function of the LSK compartment critical for MPN initiation and that the JAK2 inhibitor TG101348 failed to eliminate the MPN-initiating population in JAK2V617F knock-in mice, even though the treatment demonstrated histopathological improvement of the erythroid hyperplasia and statistically significant reductions in spleen size. As we found in the present study, the compartment of LSK cells increased in V617F-TG mice and R723 treatment did not suppress them, showing again that the effect of R723 on cell components augmented by JAK2V617F seems to differ by cell lineage. R723 effectively suppresses granulopoiesis induced by JAK2V617F, does not suppress LSK cells expanded by JAK2V617F, and suppresses the abnormal JAK2V617F signals in late erythroid progenitors and erythroblasts and the normal EPO signals in late erythrocytes.
To use a JAK2 inhibitor for the treatment of MPNs, long-term therapy is likely to be required for maintenance of remission, such as in the case of imatinib mesylate for BCR-ABL–positive chronic myeloid leukemia.36 In addition to the standard toxicities, emphasis should be placed on minimization of activity against other kinases, especially JAK1 or JAK3, to prevent immunosuppression associated with prolonged inhibition of JAK3 and possibly JAK1.37,38 R723 demonstrated reasonable selectivity in both in vitro kinase- and cell-based assays, although the selectivity was somewhat different between the 2 assay systems. In cell-based assays, a few fold selectivity was observed when using mouse CTLL-2 or human primary T cells compared with related JAK2 assays. A single oral dose of R723 demonstrated a preference for targeting the JAK2-dependent GM-CSF pathway in granulocytes rather than the JAK1- or JAK3-dependent IL-15 pathway in T cells. Six weeks of oral administration of R723 to V617F-TG mice showed that the inhibitory effect of R723 on T or B lymphocytes was much less than that on myeloid cells. These data demonstrate that R723 has a limited immunosuppressive effect and a potentially favorable clinical safety profile. All JAK2 inhibitors currently used in clinical trials inhibit JAK2 potently, but their off-target profiles are variable.21-24 The clinical implications of the distinct profiles remain to be determined.
In conclusion, we identified a potent inhibitor of JAK2, R723, which showed some selectivity in vitro in biochemical and cell-based assays. R723 demonstrated efficacy in vivo in 3 animal models addressing different aspects of disease progression. In V617F-TG mice, which closely mimic human PMF, R723 significantly improved survival, hepatosplenomegaly, leukocytosis, and thrombocytosis, thus confirming the viability of a targeted therapy approach in managing JAK2V617F-positive MPNs. We conclude that R723 could become a viable option available to PMF, PV, and ET patients who develop resistance to conventional therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank C. Wey, M. Matsushita, T. Shinmori, and K. Tsukura for their technical assistance; Dr N. Lin and Dr T. Kitamura for their critical advice; and Dr J. Diehl and Dr T. Young for critical reading of the manuscript.
This work was supported in part by Grants-in-Aid for Scientific Research (20591137, 21010490, and 20790678) from the Ministry of Education, Science, Sports, and Culture in Japan, and by a Grant-in-Aid from the Tokyo Biochemical Research Foundation, Tokyo, Japan.
Authorship
Contribution: K. Shide and V.M. performed research; V.M., E.T., S.F, C.L., M.G., W.L., J.R., J.M., S.B., J.C., C.L., A.R., S.S., P.P., G.P., A.T., M.D., R.S., D.G.P., and Y.H. contributed to screening and initial characterization of the inhibitor; V.M. and Y.H. guided the screening and initial characterization and wrote the manuscript; T.K., H.K.S., and T.M. analyzed results; and K. Shide and K. Shimoda conceived the research, guided its design, analysis, and interpretation, and wrote the manuscript.
Conflict-of-interest disclosure: V.M., E.T., S.F., C.L., M.G., W.L., J.R., J.M., S.B., J.C., C.L., A.R, S.S., P.P., G.P., A.T., M.D., R.S., D.G.P., and Y.H. are or have been employees of Rigel Pharmaceuticals, Inc. The remaining authors declare no competing financial interests.
Correspondence: Kazuya Shimoda, Department of Gastroenterology and Hematology, Faculty of Medicine, Miyazaki University, 5200 Kihara, Kiyotake, Miyazaki 889-1692, Japan; e-mail: kshimoda@med.miyazaki-u.ac.jp.
References
Author notes
K.S. and V.M. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal