Abstract
The acquired von Willebrand syndrome (AVWS) is a bleeding disorder that is frequently unrecognized or is misdiagnosed as von Willebrand disease. AVWS is characterized by structural or functional defects of von Willebrand factor (VWF) that are secondary to autoimmune, lymphoproliferative or myeloproliferative, malignant, cardiovascular, or other disorders. VWF abnormalities in these disorders can result from (1) antibody-mediated clearance or functional interference, (2) adsorption to surfaces of transformed cells or platelets, or (3) increased shear stress and subsequent proteolysis. Diagnosis can be challenging as no single test is usually sufficient to prove or exclude AVWS. Furthermore, there are no evidence-based guidelines for management. Treatments of the underlying medical condition, including chemo/radiotherapy, surgery, or immunosuppressants can result in remission of AVWS, but is not always feasible and successful. Because of the heterogeneous mechanisms of AVWS, more than one therapeutic approach is often required to treat acute bleeds and for prophylaxis during invasive procedures; the treatment options include, but are not limited to, desmopressin, VWF-containing concentrates, intravenous immunoglobulin, plasmapheresis or recombinant factor VIIa. Here, we review the management of AVWS with an overview on the currently available evidence and additional considerations for typical treatment situations.
Introduction
Managing the acquired von Willebrand syndrome (AVWS) remains a difficult task for the practicing hematologist, despite several reviews published on the pathology, diagnosis, and management of this disorder.1-14 Most reviews have concluded that AVWS is underdiagnosed and sometimes misdiagnosed, probably because of the broad spectrum of its clinical and laboratory features. Recent data suggest that AVWS is becoming more prevalent and that general hematologists are more likely to be referred patients with this condition. After a brief overview of its epidemiology and pathophysiology, we focus on the practical diagnosis and management of AVWS.
Definition
In contrast to inherited von Willebrand disease, the diagnostic category of AVWS includes any qualitative, structural, or functional disorder of VWF that is not inherited and is associated with an increased risk of bleeding. Therefore, a key aspect of the diagnostic workup is the exclusion of a bleeding history in other family members.
Epidemiology
The true prevalence of AVWS has not yet been established. Only 266 cases were reported in the literature from its first description in 196815 until 1999. An additional 186 cases were reported in the International Society on Thrombosis and Hemostasis (ISTH) registry, a retrospective survey published in 2000.16 Budde et al reported 187 cases diagnosed in a German reference laboratory among 5014 plasma samples from patients with bleeding disorders received over a 2-year period.17 Based on these values, AVWS appears to be a relatively uncommon disorder.
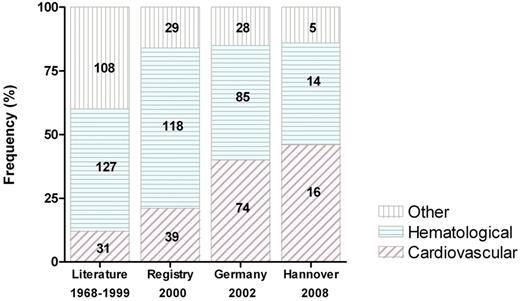
AVWS is usually associated with an underlying disorder. According to the ISTH registry, lymphoproliferative (48%), cardiovascular (21%), myeloproliferative (15%), other neoplastic (5%), and autoimmune disorders (2%) are most frequent.16 Less commonly, AVWS has been described in hypothyroidism, uremia, or without an underlying disorder. In recent cohort studies, an increased association with cardiovascular disorders (40%-46%) was found, possibly reflecting higher awareness among cardiologists and cardiac surgeons (Figure 1).17,18
The changing spectrum of AVWS-associated disorders. Bars represent the frequency of disease groups; absolute numbers are given on each bar. Publication date is given below the bars. Data are from a literature survey (“Literature”),5 a retrospective international survey (“Registry”),16 a German reference laboratory (“Germany”),17 and a retrospective single-center study (“Hannover”).18
The changing spectrum of AVWS-associated disorders. Bars represent the frequency of disease groups; absolute numbers are given on each bar. Publication date is given below the bars. Data are from a literature survey (“Literature”),5 a retrospective international survey (“Registry”),16 a German reference laboratory (“Germany”),17 and a retrospective single-center study (“Hannover”).18
More recently, AVWS has been frequently diagnosed in selected cohorts of patients with aortic valve stenosis (79%)19 and continuous-flow left ventricular assist devices (LVAD, up to 100%).20-22 The latter have received increased attention of late because of the increasing numbers of heart failure patients who are being maintained for prolonged periods while awaiting transplantation, and because of the increased recognition of chronic gastrointestinal bleeds from intestinal arteriovenous malformations in this setting. These data suggest that AVWS is becoming more prevalent than previously assumed, at least in certain groups of patients. According to the age distribution of underlying disorders, AVWS occurs in every age group but is most frequent in the elderly with a median age of 62 years (range, 2-96 years) at the time of diagnosis.16
Pathogenesis
In contrast to acquired hemophilia, which is attributable to neutralizing autoantibodies against factor VIII (FVIII), a variety of pathogenic mechanisms have been proposed to cause structural or functional disturbances of VWF. These include autoantibodies, either interfering with platelet23-28 or collagen binding,29-31 or increasing VWF clearance from the plasma.32 Sequestration of high-molecular-weight (HMW) multimers was demonstrated in patients with hematologic disorders because of adsorption to myeloma cells or platelets, but also in reactive thrombocytosis.33-36 Proteolytic cleavage of VWF can occur after shear stress-induced unfolding,37,38 and AVWS resulting from this mechanism was described in disorders with increased shear stress, in particular aortic valve stenosis19,37,39 and LVAD.20-22 Proteolytic cleavage has also been described in patients with pancreatitis, liver cirrhosis, leukemia, and certain medications.40,41 In hypothyroidism, AVWS seems to result from decreased synthesis of otherwise normal VWF.42
Paths to diagnosis
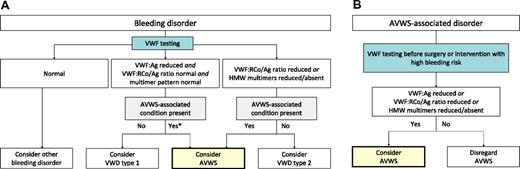
There are 2 main scenarios in which the diagnosis of AVWS should be considered: (1) bleeding patients in whom laboratory testing suggests abnormalities of VWF and (2) patients with known AVWS-associated disorders seeking advice before undergoing procedures associated with a high risk of bleeding (Figure 2).
Pathways to diagnose AVWS according to the reason for testing. (A) Patients tested because of bleeding should also be evaluated for AVWS-associated disorders. If such a disorder is present, consider AVWS if VWF testing demonstrates quantitative or qualitative disorder of VWF. (B) Patients with known AVWS-associated disorders are suggested to undergo testing before surgery, and AVWS should be considered if VWF abnormalities are found.
Pathways to diagnose AVWS according to the reason for testing. (A) Patients tested because of bleeding should also be evaluated for AVWS-associated disorders. If such a disorder is present, consider AVWS if VWF testing demonstrates quantitative or qualitative disorder of VWF. (B) Patients with known AVWS-associated disorders are suggested to undergo testing before surgery, and AVWS should be considered if VWF abnormalities are found.
When we should consider the diagnosis of AVWS in a bleeding patient
von Willebrand disease is considered to be the most frequent hereditary bleeding disorder43 and must be considered in the differential diagnosis of every new bleeding patient who does not have another obvious etiology. Initial laboratory findings typical of von Willebrand disease generally do not allow differentiation between von Willebrand disease and AVWS. Therefore, AVWS must be considered whenever laboratory findings would suggest von Willebrand disease, in particular in the presence of AVWS-associated disorders (Figure 2A). Even if an AVWS-associated disorder is not known, attention must be paid because the disorder may not have been previously identified.
How to differentiate AVWS from von Willebrand disease
Distinguishing AVWS and von Willebrand disease is important because the approaches to treatment can be very different. Points to consider in the differential diagnosis are summarized in Table 1. In most cases, the differentiation is straightforward, although there is no single aspect sufficient on its own. Late onset of bleeding and negative family history should prompt the suspicion of AVWS, but additional workup can be required because mild von Willebrand disease can be asymptomatic for decades and lack a remarkable family history because of its low penetrance. On the other hand, the presence of an AVWS-associated disorder does not prove AVWS because these disorders could be present coincidentally. In uncertain cases, testing of family members, genetic analysis, and assays for VWF–specific antibodies and inhibitors can be helpful.
Differential diagnosis between AVWS and VWD
| Aspect . | In favor of AVWS . | In favor of VWD . | Limitations . |
|---|---|---|---|
| Personal history | Late onset of bleeding Uneventful surgery before onset of bleeding | Early onset of bleeding No uneventful surgery or no previous high-risk situations | Variable penetrance of VWD |
| Family history | Negative | Positive | Variable penetrance of VWD |
| AVWS-associated disorder | Present | Absent | Coincidental presence of highly prevalent disorders (eg, MGUS in the elderly) |
| Laboratory evaluation | Presence of inhibitor or VWF-binding antibodies | VWF gene mutation | Low frequency of detectable inhibitors in AVWS Alloantibodies in rare cases of VWD type 3 |
| Treatment response | Remission after treatment of underlying disorder Response to IVIG (in IgG-MGUS-associated AVWS) Short-lived response to VWF-containing concentrates or desmopressin | Normal recovery and half-life of VWF-containing concentrate Sustained response to desmopressin | Cannot be assessed before treatment |
| Aspect . | In favor of AVWS . | In favor of VWD . | Limitations . |
|---|---|---|---|
| Personal history | Late onset of bleeding Uneventful surgery before onset of bleeding | Early onset of bleeding No uneventful surgery or no previous high-risk situations | Variable penetrance of VWD |
| Family history | Negative | Positive | Variable penetrance of VWD |
| AVWS-associated disorder | Present | Absent | Coincidental presence of highly prevalent disorders (eg, MGUS in the elderly) |
| Laboratory evaluation | Presence of inhibitor or VWF-binding antibodies | VWF gene mutation | Low frequency of detectable inhibitors in AVWS Alloantibodies in rare cases of VWD type 3 |
| Treatment response | Remission after treatment of underlying disorder Response to IVIG (in IgG-MGUS-associated AVWS) Short-lived response to VWF-containing concentrates or desmopressin | Normal recovery and half-life of VWF-containing concentrate Sustained response to desmopressin | Cannot be assessed before treatment |
VWD indicates von Willebrand disease; VWF, von Willebrand factor; MGUS, monoclonal gammopathy of undetermined significance; and IVIG, intravenous immunoglobulin.
Should we actively search for AVWS-associated disorders?
We recommend screening for AVWS-associated disorders in all patients with laboratory findings typical of von Willebrand disease and a negative family history. Careful history taking and physical examination should be sufficient to rule out most AVWS-associated cardiovascular disorders, in particular severe aortic valve stenosis. Likewise, most hematologic, malignant, and autoimmune disorders should be addressed clinically. In addition, serum protein electrophoresis and a complete blood count should be obtained to screen for monoclonal gammopathy and other hematologic disorders, respectively.
Should we consider AVWS in nonbleeding patients with AVWS-associated disorders?
A patient with AVWS may have a negative bleeding history simply because the condition has not existed long enough to become symptomatic. In our own practices, we offer testing to patients with AVWS-associated disorders before major surgery and other interventions with a high risk of bleeding (Figure 2B). Although this is in line with a recommendation by the ISTH,16 the value of testing and treating AVWS in previously asymptomatic patients has not been evaluated by clinical trials. Because AVWS-associated disorders are relatively common in the population, it remains possible that testing might produce many false-positive results and result in unnecessary treatments. Therefore, diagnostic testing in asymptomatic patients should be based on the individual physician's clinical judgment until evidence from clinical studies becomes available.
Diagnostic tests
Routine tests
The initial tests used to assess AVWS are the same as for von Willebrand disease. Figure 3 shows results derived from a single-center cohort study,18 which were similar to other studies and registries in the field. Bleeding time and activated partial thromboplastin time are not very useful. FVIII:C, VWF:Ag, VWF:RCo, and collagen-binding activity (VWF:CB) are sometimes decreased, most frequently in lymphoproliferative disorders.16,18 A reduced function/antigen ratio (VWF:RCo/Ag or VWF:CB/Ag) can indicate structural or functional disorders, even if the absolute activity is within normal limits.
Routine VWF tests in AVWS associated with cardiovascular (CV) and lymphoproliferative (LP) disorders. Data are from a retrospective single-center study.18 Note that all patients with cardiovascular disorders and part of the patients with lymphoproliferative disorders had normal or increased VWF:Ag, VWF:RCo, and VWF:CB. The VWF:CB/Ag ratio was more often decreased than the VWF:RCo/Ag ratio. All patients had a loss or decrease of HMW multimers (not shown). Solid lines indicate the median; and dashed lines indicate the upper limit of detection (400 IU/mL) and the lower limit of normal (50 IU/mL) or the reference range for ratios.
Routine VWF tests in AVWS associated with cardiovascular (CV) and lymphoproliferative (LP) disorders. Data are from a retrospective single-center study.18 Note that all patients with cardiovascular disorders and part of the patients with lymphoproliferative disorders had normal or increased VWF:Ag, VWF:RCo, and VWF:CB. The VWF:CB/Ag ratio was more often decreased than the VWF:RCo/Ag ratio. All patients had a loss or decrease of HMW multimers (not shown). Solid lines indicate the median; and dashed lines indicate the upper limit of detection (400 IU/mL) and the lower limit of normal (50 IU/mL) or the reference range for ratios.
Multimer analysis
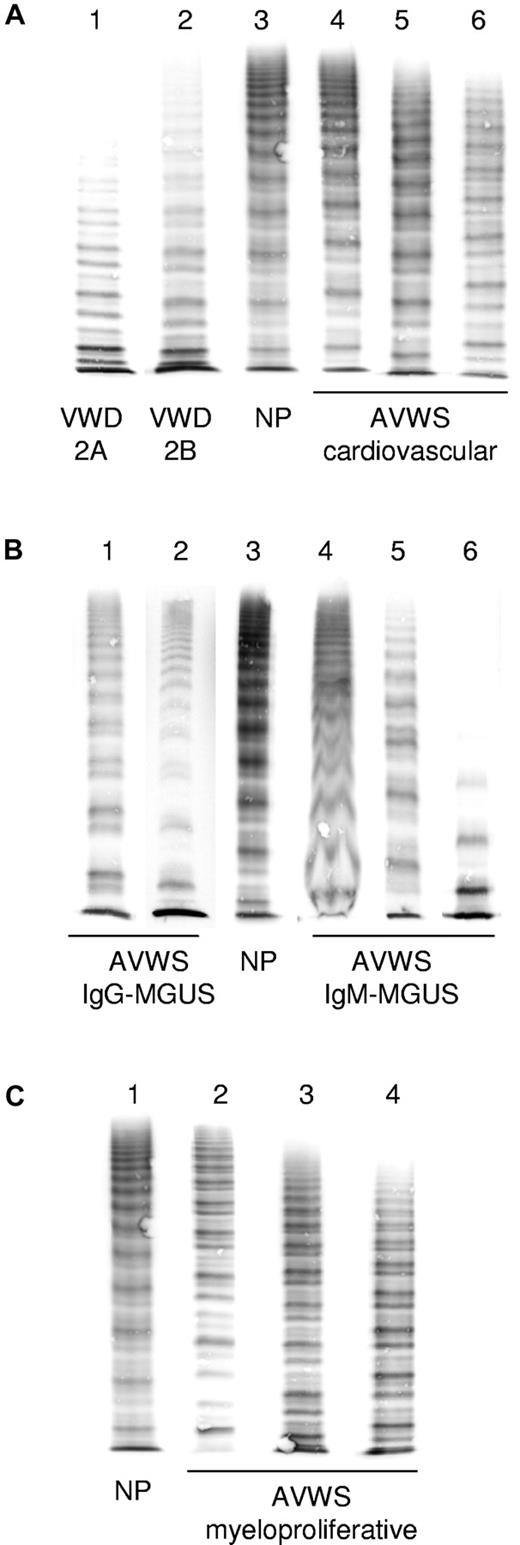
Typical multimer patterns for different disorders are demonstrated in Figure 4. A loss or decrease of HMW multimers can be quantified using densitometry.44 However, these methods are not available in many laboratories and have not yet been standardized. Moreover, preanalytical variables can contribute to artifactual losses of HMW multimers. When used properly, multimer analysis can be a very sensitive tool for detecting structural abnormalities of VWF and typical patterns specific for different disorders can help to distinguish AVWS from von Willebrand disease.17 A decrease of HMW multimers can sometimes be observed in patients with normal VWF:RCo and VWF:CB and even normal VWF:RCo/Ag and VWF:CB/Ag ratios.18,22 We therefore usually obtain VWF:Ag, VWF:RCo, VWF:CB, and multimer analysis in patients with suspected AVWS.
Typical appearance of VWF multimers in AVWS compared with von Willebrand disease. (A) Congenital von Willebrand disease: lane 1 indicates type 2A; lane 2, type 2B; and lane 3 normal plasma (NP). Cardiovascular disorders: lane 4 indicates aortic valve stenosis; lane 5, LVAD; and lane 6, patient on extracorporeal membrane oxygenator (note decrease of HMW multimers). (B) Lymphoproliferative disorders: lanes 1 and 2 indicate IgG-MGUS (note decrease of HMW multimers and relative increase of the lower satellite band within triplets); and lanes 4 to 6, IgM-MGUS (note blurred structure of triplets and loss of HMW multimers in lane 6). (C) Myeloproliferative disorders: lanes 2 to 4 (note variable decrease of HMW multimers and disturbed triplet structure). Lanes have been arranged from different gels and fit for size and staining intensity.
Typical appearance of VWF multimers in AVWS compared with von Willebrand disease. (A) Congenital von Willebrand disease: lane 1 indicates type 2A; lane 2, type 2B; and lane 3 normal plasma (NP). Cardiovascular disorders: lane 4 indicates aortic valve stenosis; lane 5, LVAD; and lane 6, patient on extracorporeal membrane oxygenator (note decrease of HMW multimers). (B) Lymphoproliferative disorders: lanes 1 and 2 indicate IgG-MGUS (note decrease of HMW multimers and relative increase of the lower satellite band within triplets); and lanes 4 to 6, IgM-MGUS (note blurred structure of triplets and loss of HMW multimers in lane 6). (C) Myeloproliferative disorders: lanes 2 to 4 (note variable decrease of HMW multimers and disturbed triplet structure). Lanes have been arranged from different gels and fit for size and staining intensity.
VWF propeptide
The assessment of VWF propeptide (know previously as VWF:Ag II) has been suggested to improve diagnosis of AVWS because it represents a marker of VWF biosynthesis. An increased propeptide/VWF:Ag ratio reflects accelerated clearance of VWF from the plasma. However, the same has been found in a subset of patients with von Willebrand disease type 1 indicating accelerated clearance of VWF as a reason for their condition.45,46 Therefore, propeptide/VWF:Ag ratio may not always discriminate between AVWS and von Willebrand disease and cannot be recommended for routine use at present time.
Autoantibodies
Autoantibodies play a role in the pathogenesis of some patients with AVWS, in particular those with lymphoproliferative disorders. The presence of autoantibodies appears to be associated with a more severe bleeding tendency.18,47 In a minority of patients, inhibitory (neutralizing) antibodies can be detected in mixing studies of VWF:RCo or VWF:CB. In contrast to acquired hemophilia, where FVIII inhibitors are virtually always detectable with standard laboratory assays, the frequency of inhibitor detection is low in AVWS. This does not necessarily mean that autoantibodies are absent and might reflect the presence of non-neutralizing autoantibodies that accelerate VWF clearance from the circulation without inhibiting measurable functions of the protein.32 Moreover, inhibitors are sometimes saturated in complexes with VWF, preventing detection unless the complex is dissociated by heating or other methods.27 Non-neutralizing VWF-binding antibodies can be detected by enzyme-linked immunosorbent assay and have been reported to occur in patients with lymphoproliferative but also other underlying disorders.18,48 However, no standardized assays are available yet for detecting those. Plasma-derived VWF contains ABO blood group antigen and should not be used as an antigen for enzyme-linked immunosorbent assay to detect autoantibodies because the presence of isoagglutinins may cause false-positive results. Recombinant human VWF expressed in cultured animal cells is currently under investigation as a reagent that may potentially resolve this issue.
General treatment options
The treatment goals in AVWS are (1) to control acute bleeds, (2) to prevent bleeding in high-risk situations, and (3) to obtain long-term remission. The strategies used to obtain these goals depend on the underlying disease mechanisms (Table 2). Whenever possible, treatment should address the underlying disorder, thereby aiming to treat AVWS as well. Unfortunately, treatment of the underlying disorder is not always possible, and obtaining partial remission of the underlying disorder does not always result in an improvement of the bleeding symptoms typical of AVWS. Therefore, additional treatments as listed in Table 2 are usually required to control or prevent bleeding. The available evidence for efficacy and safety of the commonly used hemostatic treatments is summarized as follows.
Therapeutic options according to underlying disorder
| Underlying disorder . | Causal treatment . | Additional treatment options . |
|---|---|---|
| Autoimmune disorders | ||
| Systemic lupus erythematosus | Steroids, cyclophosphamide | IVIG (only IgG-MGUS or anti-VWF IgG), plasmapheresis, antifibrinolytics, VWF-containing concentrate, rFVIIa |
| Lymphoproliferative disorders | ||
| MGUS | Usually untreated | |
| Lymphoma, multiple myeloma | Chemotherapy according to entity | |
| Cardiovascular | ||
| Aortic valve stenosis and other anomalies with increased shear stress | Corrective surgery | VWF-containing concentrate, antifibrinolytics |
| Dysfunctional heart valve prosthesis, LVAD | Corrective surgery if applicable | Reduce or withdraw anticoagulation, VWF-containing concentrate |
| Myeloproliferative neoplasia | ||
| Essential thrombocythemia | Cytoreductive therapy, chemotherapy, or stem cell transplantation in case of progression | |
| Polycythemia vera | Phlebotomy, cytoreductive therapy, chemotherapy, or stem cell transplantation in case of progression | Withdraw aspirin (if applicable), desmopressin, antifibrinolytics, VWF-containing concentrate |
| Chronic myeloid leukemia | Tyrosine kinase inhibitors, stem cell transplantation |
| Underlying disorder . | Causal treatment . | Additional treatment options . |
|---|---|---|
| Autoimmune disorders | ||
| Systemic lupus erythematosus | Steroids, cyclophosphamide | IVIG (only IgG-MGUS or anti-VWF IgG), plasmapheresis, antifibrinolytics, VWF-containing concentrate, rFVIIa |
| Lymphoproliferative disorders | ||
| MGUS | Usually untreated | |
| Lymphoma, multiple myeloma | Chemotherapy according to entity | |
| Cardiovascular | ||
| Aortic valve stenosis and other anomalies with increased shear stress | Corrective surgery | VWF-containing concentrate, antifibrinolytics |
| Dysfunctional heart valve prosthesis, LVAD | Corrective surgery if applicable | Reduce or withdraw anticoagulation, VWF-containing concentrate |
| Myeloproliferative neoplasia | ||
| Essential thrombocythemia | Cytoreductive therapy, chemotherapy, or stem cell transplantation in case of progression | |
| Polycythemia vera | Phlebotomy, cytoreductive therapy, chemotherapy, or stem cell transplantation in case of progression | Withdraw aspirin (if applicable), desmopressin, antifibrinolytics, VWF-containing concentrate |
| Chronic myeloid leukemia | Tyrosine kinase inhibitors, stem cell transplantation |
VWF indicates von Willebrand factor.
Desmopressin
This synthetic analog of vasopressin can be used to prevent and control bleeding in some patients with AVWS. It is usually administered in doses of 0.3 μg/kg over 30 minutes once or twice daily. In the ISTH registry, an overall success rate of 32% was reported. However, success rates varied according to the underlying disorder: they were low in cardiovascular (10%) and myeloproliferative (21%), but higher in autoimmune (33%), lymphoproliferative (44%), and other neoplastic disorders (75%).16 In the only prospective clinical trial, performed in 10 patients with monoclonal gammopathy of undetermined significance (MGUS), all VWF measurements improved after 30 minutes but returned close to baseline after 4 hours.49 We therefore closely monitor VWF:Ag and VWF:RCo, when desmopressin is used for prophylaxis and treatment of bleeds. Caution must be exercised in patients with cardiovascular disorders and in the elderly to prevent fluid overload and hyponatremia, which are the most common adverse effects of desmopressin.
VWF-containing concentrates
Several plasma-derived concentrates containing VWF can be used for replacement therapy. In our practice, we use starting doses between 30 and 100 VWF:RCo units/kg depending on the patient's residual activity, severity of bleeding, and presence of inhibitors. Similar to desmopressin, the half-life of infused VWF can be very short, in particular in patients with MGUS or inhibitors.49 Close monitoring of the clinical response and VWF measurements are needed for tailoring doses and dosing intervals.
Recombinant factor VIIa
Use of this hemostatic agent has been reported in AVWS and in von Willebrand disease patients, particularly those who develop alloantibodies against VWF. It is usually administered with a dose of 90 μg/kg (range, 40-150 μg/kg) and for a median of 3 doses (range, 1-54 doses).50 Treatment is usually effective with responses reported in 96% of patients. Adverse events appear to be uncommon; myocardial infarction was reported in one patient with type 2A von Willebrand disease.51 The rate of thromboembolic complications is low in hemophilia patients receiving recombinant factor VIIa, but it is currently unknown whether this is holds for patients with AVWS or von Willebrand disease. Caution should be exerted, particularly in elderly patients and those at risk of thromboembolism.
Antifibrinolytics
The currently available antifibrinolytic drugs are the lysine analogs ϵ-aminocaproic acid (50-60 mg/kg every 4-6 hours), tranexamic acid (20-25 mg/kg every 8-12 hours), and 4-aminomethylbenzoic acid (50-100 mg every 6-8 hours). These drugs can be administered orally, intravenously, or topically. They bind to plasminogen and plasmin, thereby inhibiting binding to fibrin. Antifibrinolytics increase clot strength in some in vitro models of hemostasis.52 They are primarily used as adjunct therapies together with desmopressin or VWF-containing concentrates for surgery and bleeding in areas of high fibrinolytic activity (ie, the gastrointestinal and urogenital tracts). For minor bleeds in these areas, treatment with antifibrinolytics alone may be sufficient. Caution must be exerted in patients with macroscopic hematuria because of the risk of obstruction by clots formed in the urinary tract.
IVIG
The effectiveness of intravenous immunoglobulin (IVIG) was demonstrated in an open-label crossover study in patients with AVWS associated with MGUS of the IgG class (IgG-MGUS). Doses of 1 g/kg per day were used for 2 days.49 An increase of VWF measurements and shortening of the bleeding time were observed on the day after the second infusion, reaching a maximum after 4 days and slowly returning to baseline within 21 days. IVIG was not effective in AVWS patients with MGUS of the IgM class (IgM-MGUS).
Plasmapheresis
Plasmapheresis can be used to deplete autoantibodies and paraproteins of any immunoglobulin class and has been reported in patients with AVWS resulting from IgM-MGUS.5 Fresh frozen plasma should be used, instead of albumin, to prevent depletion of fibrinogen and other coagulation factors, which might provoke bleeding in patients with AVWS. In case of severe bleeding, restoration of VWF can be accelerated by the concurrent replacement with VWF-containing concentrate or desmopressin.
Specific approaches according to underlying disorders
Lymphoproliferative and autoimmune disorders
Lymphoproliferative disorders account for a significant proportion of cases with AVWS, both in the literature (30%) and in the ISTH registry (48%).16 Other immune disorders, such as systemic lupus erythematosus, mixed connective tissue disease, and GVHD, have been reported less frequently. In a prospective study, 8 of 113 patients (7%) with lymphoproliferative disorders were diagnosed with AVWS, and 4 of 8 demonstrated clinical bleeding.47 In another series, 8 of 11 patients with AVWS resulting from monoclonal gammopathy presented with bleeding before diagnosis, and the risk of new bleeding during follow-up was 11% per year.18 It should be noted, however, that bleeding may not only result from AVWS. In patients with Waldenström disease or IgM-MGUS, bleeding may also result from hyperviscosity.
How to manage acute bleeds.
Desmopressin or VWF-containing concentrates typically result in short-lived improvements of VWF.49 Therefore, these treatments require close monitoring and may sometimes be ineffective. Patients with IgG autoantibodies and paraproteins usually respond well to IVIG. This has been shown directly for IgG-MGUS49 but in our experience is similar in patients with lymphomas or multiple myeloma who have IgG paraproteins. Improvement of VWF measurements is typically observed after 12 to 72 hours and lasts for several days or weeks. IVIG has been recommended for second-line treatment in patients unresponsive to desmopressin or VWF-containing concentrates53 but in our experience can be the better choice for severe and recurrent bleeds because of the longer lasting effect. Because IVIG is not immediately effective, simultaneous use of desmopressin or VWF-containing concentrates can be necessary in the first few days. Patients with paraproteins of the IgM class do not respond to IVIG.49 Plasmapheresis combined with VWF-containing concentrates has been reported to be effective.54 In addition, antifibrinolytics can be helpful, particularly for mucocutaneous bleeds.
How to induce long-term remission.
Treatment of the underlying disorder should be considered whenever possible. MGUS usually remains untreated in asymptomatic patients. If it becomes symptomatic because of AVWS, an effective long-term therapy would be highly warranted but unfortunately has not been established. Chemotherapy and steroids are usually ineffective, apparently because these drugs cannot eradicate slowly proliferating plasma cell clones. Rituximab has also been used without success in one reported case.55 Another case report suggests that the proteasome inhibitor bortezomib could be effective.56 At this time, however, IVIG (for IgG-MGUS) and plasmapheresis (for IgM-MGUS) remain the only treatment options for most patients. For patients with B-cell lymphomas or multiple myeloma, successful chemotherapy (combined with rituximab if appropriate) can result in remission of AVWS with an overall success rate of 35% to 70%.18,47 Steroids, other immunosuppressive drugs, and IVIG (for IgG VWF-binding antibodies) should be considered in autoimmune disorders.
Cardiovascular disorders
AVWS associated with cardiovascular disorders is increasingly recognized. Publications in the field have mainly focused on aortic valve stenosis and more recently on LVAD, but it should be noted that a broad range of congenital and acquired cardiac defects have been reported in association with AVWS, including dysfunctional valve prostheses, endocarditis, septal defects, and others.16-18
Bleeding while on antithrombotic drugs: should we consider AVWS?
Bleeding in patients with cardiovascular disorders who are concurrently treated with anticoagulants or antiplatelet drugs is usually attributed only to the medication. However, coincidence of hemorrhagic disorders can compound the risk of bleeding during treatment with antithrombotic drugs. For example, patients with blood group O have a higher risk of bleeding during vitamin K antagonist treatment compared with non-O blood groups, probably because of lower VWF:Ag and FVIII:C.57 No study has specifically addressed the role of AVWS in patients bleeding during antithrombotic therapy, but we suggest screening for AVWS in patients with unusual or frequent hemorrhage.
What are the characteristic features of AVWS associated with cardiovascular disorders?
In this group of patients, the decrease of HMW multimers is the result of increased shear stress and subsequent proteolysis. Most commonly, VWF:Ag, VWF:RCo, and VWF:CB are normal or even increased.16-22 The VWF:RCo/Ag and VWF:CB/Ag ratio is often, but not always, reduced.16,18,19,22 In some patients, a loss or decrease of HMW multimers is the only laboratory abnormality indicating AVWS. It is not generally accepted that reduced HMW multimers alone, without a disproportional decrease of VWF:RCo or VWF:CB, can explain bleeding. On the other hand, a striking incidence of hemorrhage was reported in patients with severe aortic valve stenosis (21% during 6 months before surgery)19 and LVAD (31% during a median follow-up of 28 months).22 Virtually all of these patients exhibit a decrease of HMW multimers, but only 44% to 79% of them have a reduced VWF:cord blood/Ag ratio.18,19,22 Moreover, the frequency of bleeding is not related to the level of VWF:Ag or VWF:RCo.21 For these reasons, we currently consider a lack of HWM multimers alone to be a risk factor for bleeding, but further studies are needed to provide direct evidence.
What are the preferred treatment modalities?
Treatment should aim to correct the cardiac defect whenever possible. Removal of LVAD has been reported to result in a remission of AVWS.22 Likewise, valve replacement for aortic valve stenosis usually results in an improvement of AVWS, although recurrence was noted in some patients, in particular those with prosthesis malfunction.19 In some patients, frequent and severe bleeding resulting from AVWS may even be considered a sufficient indication for surgical correction, even if the cardiac defect would not require surgery on its own. The risk of future bleeds and the potential benefit of correction must, however, be carefully weighed against the risks of the procedure.
Other treatment modalities are limited and based on anecdotal evidence. Antifibrinolytics may be considered, in particular for mucocutaneous bleeding, although the thromboembolic risk remains a concern in high-risk patients.58 Desmopressin has not been very successful in this setting, with response rates of 10%.16 Moreover, we do not use this agent in patients with congestive heart failure, coronary artery disease, or advanced age (≥ 65 years). Published response rates of VWF-containing concentrates ranged between 10% and 70%,16,18 possibly reflecting different dosing regimens and concentrate characteristics with regard to VWF content and multimer quality.
How to manage antithrombotic therapy in bleeding patients with AVWS.
We usually withdraw antithrombotic medication in actively bleeding patients. Reversal of vitamin K antagonist or antiplatelet therapy should be used in emergency situations according to published guidelines.59,60 In patients who experienced profuse or frequent bleeding, we consider to reduce or even withhold an otherwise indicated antithrombotic therapy in the long term. The thromboembolic risk of bileaflet mechanical aortic valve prosthesis appears to be low enough that anticoagulation can be reduced or withheld in patients with bleeding disorders. However, the risk-benefit assessment can be much more difficult in high-risk situations (eg, in mitral valve prostheses or LVAD). A few published reports suggest that withdrawal of antithrombotic therapy can be appropriate.61,62 Until evidence-based guidelines are available, the competing risks of bleeding and thrombosis must be balanced carefully for the individual patient, and experience should be collected in prospective registries.
Myeloproliferative neoplasia
AVWS associated with myeloproliferative neoplasia (MPN) primarily results from adsorption of VWF to transformed blood cells, in particular platelets. It has been reported in essential thrombocythemia and polycythemia vera, but also in chronic myeloid leukemia, primary myelofibrosis, and sometimes in acute leukemia. In a prospective study by Mohri et al,47 AVWS was diagnosed in 14 of 125 (11%) consecutive patients with MPN.
What determines the risk of bleeding?
Although risk factors for bleeding in MPN have not been well described, most reviews concluded that a very high platelet count (> 1500/nL) is a significant risk factor. Among 100 patients with “hemorrhagic thrombocythemia,” the platelet count was 2016 ± 1070/nL.63 Accordingly, there is an inverse relationship between the platelet count and the VWF:RCo/Ag or VWF:CB/Ag ratio in patients with MPN.64 However, in the prospective series by Mohri et al,47 the median platelet count of patients with MPN and AVWS was only 638/nL (range, 120-1305/nL).47 It therefore appears likely that additional risk factors, such as platelet function defects, contribute to bleeding. The relationship between platelet count, AVWS, platelet function defects, and the risk of bleeding deserves further study. For the time being, we suggest that bleeding patients with MPN be screened for both AVWS and platelet function defects.
What determines the risk of thrombosis?
Thrombosis is a challenging problem because bleeding patients with MPN are also at increased risk for thrombosis, which might be further increased by measures to improve hemostasis. Indeed, the overall frequency of thrombosis in MPN (6.6 per 100 patient-years) appears to be higher than the risk of major bleeding (0.33 per 100 patient-years).65 Thrombotic events are more often arterial than venous. Known risk factors are advanced age (≥ 60 years) and previous thromboembolic events.65,66 Leukocytosis,67 increased marrow fibrosis,68 and the JAK2 V617F mutation69 have also been reported as risk factors in essential thrombocythemia. An association between the platelet count and the risk of thrombosis is not well established. Cytoreductive therapy consistently lowered the risk of thrombosis in several clinical trials. In essential thrombocythemia patients on hydroxyurea, the remaining risk of thrombosis was associated with age, previous thrombosis, and leukocytosis (> 10/nL), but not with the platelet count.70
How to treat bleeding patients with MPN.
Cytoreductive therapy was reported to result in remission of AVWS in 12 of 14 patients with MPN and should be considered in bleeding patients.47 Usually, treatment is not immediately effective and additional treatments can be required in actively bleeding patients. In theory, desmopressin should be a preferred treatment because newly released VWF from endothelial cells contains very large HMW multimers, larger than those contained in plasma-based concentrates. However, desmopressin can fail in situations of acute bleeding because of tachyphylaxis or if endothelial storage pools have already been mobilized by different mechanisms. The reported success rate in patients with MPN has been 21%.16 VWF-containing concentrates have not been reported to result in higher success rates.16 However, in our experience, higher doses (eg, 50 IU/kg 2 or 3 times daily) sometimes result in better treatment results. Alternatively, recombinant factor VIIa may be considered for severe bleeding refractory to other treatment modalities.
How to address thrombotic risks in these patients.
There is a consensus that cardiovascular risk factors should be addressed. Statins have also been recommended.71 Primary prophylaxis with aspirin is generally recommended in polycythemia vera and essential thrombocythemia unless contraindicated. The main contraindications are intolerance and bleeding. Known AVWS in nonbleeding patients can be considered a relative contraindication. In asymptomatic patients with very high platelet counts (> 1500/nL), where AVWS appears to be particularly common, we usually wait with the initiation of aspirin until cytoreductive therapy has lowered the platelet count to < 1000/nL.
If thromboembolic events occur in patients with asymptomatic AVWS, we usually administer antithrombotic drugs in the same way as in the general population. This includes antiplatelet drugs for arterial events and anticoagulants for venous events. In case of concurrent bleeding and thrombosis, the choice of drugs and dosing always is an individual decision. We try to avoid potentially thrombogenic medications, and provide as much antithrombotic therapy as needed and tolerated. For deep vein thrombosis, compression therapy is important. In case of clinically significant pulmonary embolism, temporary inferior vena cava filters can be considered.
In conclusion, AVWS is a significant under-recognized bleeding disorder occurring in several different clinical situations. The diagnostic approach to AVWS remains a challenge to the practicing physician because of the variable clinical presentation and the many different tests that have to be obtained to prove or rule out the diagnosis. Management requires consideration of the underlying disorder and pathogenic mechanisms. We have reviewed here the relatively limited evidence that is available from registries, cohort studies, and a few clinical trials, and also shared our personal experience on how to manage difficult situations. Research in this area has been limited by a number of difficulties, including the prolonged follow-up required, the many confounding factors, and increasingly documented clinical and biologic heterogeneity. Because prospective clinical studies are difficult to organize in this small and heterogeneous group of patients, we encourage physicians to report their experience in the ongoing AVWS registry commenced by the ISTH (www.IntReAVWS.com).
Authorship
Contribution: A.T. wrote the first draft that underwent several rounds of revision by all authors; and all authors developed the concept of the article and approved the final manuscript.
Conflict-of-interest disclosure: A.T. has received research grants, lecture fees or honoraria for consultancy, and membership on advisory boards from Baxter, Bayer, Biotest, CSL Behring, Leo Pharma, Novo Nordisk, and Pfizer. A.B.F. received lecture fees and honoraria for consultancy and membership on advisory boards from Baxter, CSL Behring, Kedrion, Grifols, LFB, and Octapharma. The remaining authors declare no competing financial interests.
Correspondence: Andreas Tiede, Hannover Medical School, Department of Hematology, Hemostasis, Oncology and Stem Cell Transplantation, Feodor Lynen Strasse 5, 30625 Hannover, Germany; e-mail: tiede.andreas@mh-hannover.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal